Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors. It currently ranks fifth among malignant
tumors, accounting for >626,000 cases of cancer annually and
ranks third among tumor-related deaths, with 600,000 mortality
cases per year (1–2). HCC is prevalent in China, accounting
for 55% of new cancer cases and ranks second only to lung cancer
regarding tumor-related mortality. Since early symptoms are not
apparent in HCC, the majority of patients are admitted to hospital
at a later stage, with liver cirrhosis being the presenting symptom
in 85–95% of the cases. The success rate of radical surgical
resection is low (<20%) and the recurrence rate following
radical resection is 35–50%. For the majority of advanced HCC
patients with a poor prognosis, conservative treatment methods are
the primary choice (3).
Consequently, there is a need to improve treatment efficacy for
these patients.
Human fibroblast growth factor receptor 2 (FGFR2),
which is the Bek oncogene expression product, has a high affinity
for a variety of FGFs, including fibroblast growth factor 7 (FGF7)
(4). A previous study demonstrated
that expression of FGF and FGFR was increased in HCC patients
(5). Harimoto et al
(9) also demonstrated that FGFR2
plays an important role in HCC differentiation, with the expression
of FGFR2 being 4.7 times higher in poorly differentiated HCCs. A
high expression of FGFR2 is closely associated with the incidence
of portal vein tumor thrombosis, increased α-fetoprotein levels and
shortened overall survival rates and disease-free survival periods,
suggesting the significance of FGFR2 in HCC development. In
addition, targeted FGFR2 treatment exhibited therapeutic effects in
related studies. Bai et al (10) investigated the role of FGFR2
signaling in vivo and in vitro using GP369, which is
an FGFR2-IIIb-specific antibody and the results indicated that
GP369 inhibited FGFR2 ligand phosphorylation and its relative
protein expression in downstream signaling pathways, thus
suppressing FGFR2-induced cell proliferation. Abnormal FGFR2
signaling is crucial for tumorigenesis and tumor development and
GP369 exhibited potential therapeutic value for patients with
abnormal FGFR2 signal activation. Therefore, FGFR2 may be used as
an effectively adverse indicator for HCC prognosis and as a new
molecular target in therapeutic treatment strategies.
A previous study from our research group (11) demonstrated that the FGFR2 inhibitor
Ki23057, combined with chemotherapeutics, may exert obvious
synergistic effects on the high-expressing FGFR2 gastric carcinoma
drug-resistant cell lines OCUM-2M/SN38, OCUM-2M/PTX and
OCUM-2M/VP16, by downregulating the expression of the excision
repair cross-complementary gene 1 (ERCC1). This provided evidence
that ERCC1 is a target gene in the downstream pathway regulated by
FGFR2; however, the detailed mechanism has not been elucidated. In
combination with their receptors, FGFs are able to phosphorylate
intracellular tyrosine residues on target proteins and accordingly
activate signaling pathways through a variety of intracellular
signal transduction molecules. The FGF-induced downstream cascade
includes the PKC, Ras/Raf/MEK/ERK, JAK/STAT and PI3K pathways
(12). ERCC1 is considered to be a
closely related downstream target gene of ERK1/2, since ERCC1 mRNA
and protein levels may be inhibited by the ERK inhibitor U0126
(13). We hypothesized that FGFR2,
specifically binding with FGF7, may regulate ERCC1 gene expression
through the ERK signaling pathway. This study aimed to verify the
hypothesis that the expression of the ERCC1 gene is associated with
FGFR2-mediated ERK1/2 signaling, through the upregulation of FGF7
and the downregulation of FGFR2 using shRNA gene silencing in
HepG2/OXA cell models.
Materials and methods
Cell culture
Human HCC cell line HepG2
Human HCC HepG2 cells were conventionally cultured
in a high-glucose Dulbecco’s modified Eagle’s medium (Gibco BRL,
Carlsbad, CA, USA) supplemented with 100 U/ml penicillin, 100
μg/ml streptomycin and 10% fetal bovine serum (Hangzhou
Sijiqing Biotec Co., Zhejiang, China) at 37°C in a 5%
CO2 incubator. Confluent culture flasks were subcultured
by using 0.25% trypsin (Guge Biology Co., Wuhan, China) and were
used in experiments after reaching 70–80% confluence.
HepG2/OXA-resistant cell line
An oxaliplatin (OXA)-resistant subline was
established by discontinuously exposing parental HepG2 cells to a
high-OXA concentration (25 μM) medium over the course of one
year until the resulting cells were able to grow exponentially in a
medium containing 1 μM OXA. The drug-resistant HepG2/OXA
cell line (HepG2/OXA) in a logarithmic growth phase was
conventionally cultured in a culture medium containing 1
μmol/l OXA (Sigma Aldrich, St. Louis, MO, USA). Prior to
protein lysis, HepG2/OXA cells were subcultured for three
generations in OXA-free medium.
Short hairpin RNA for Bek gene (Bek
shRNA) cell transfection in HepG2 and HepG2/OXA cells
Cell transfections were performed using Bek shRNA
plasmid (h) (sc-29218-SH kit; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), according to the manufacturer’s instructions.
HepG2/OXA and HepG2 cells were cultured in 6-well culture plates
and were then supplemented with antibiotic-free growth medium
containing fetal bovine serum, until they reached 60–80%
confluence. Using a sample injector, shRNA plasmid DNA solution
(solution A) was directly added into the diluted shRNA plasmid
transfection reagent (solution B). The mixture was gently
triturated and then incubated at room temperature for 30 min. The
samples were rinsed twice with 2 ml shRNA transfection medium to
absorb the matrix and 0.8 ml shRNA plasmid transfection medium was
added to each well. A 200-μl drop of shRNA plasmid and
DNA/shRNA plasmid transfection reagent mixture was added and cells
were incubated at a 37°C incubator for 8 h, followed by incubation
with 1 ml growth medium containing twice the concentration of
normal serum (twice normal culture medium) and antibiotics for an
additional 24 h. After a 48-h transfection, the culture medium was
discarded and cells were incubated in a medium containing 2
μg/ml puromycin dihydrochloride (sc-108071, Santa Cruz
Biotechnology Inc.). The screening medium was replenished every 2
days until cells could be stably cultured. Cells were then rinsed
twice with phosphate-buffered saline and lysed in 300 μl of
1X electrophoresis buffer solution, with gentle agitation and
trituration prior to SDS-PAGE gel electrophoresis.
Experimental grouping
HepG2, HepG2/T, HepG2/OXA and HepG2/OXA/T cells were
conventionally cultured in culture flasks. After reaching 60%
confluence, cells were incubated with a culture medium containing a
final concentration of 5 ng/ml FGF7 (Santa Cruz Biotechnology) for
24 h. Cells were trypsinized and lysed and the protein
concentration was measured with the BCA method and adjusted to 1
μg/μl protein percentage. Cells cultured in FGF7-free
culture medium were used as a control.
Western blot assay for FGFR2, ERCC1,
p-ERK1/2 and β-actin expression
The expression levels of FGFR2, ERCC1, p-ERK1/2 and
β-actin were determined with western blot analysis on NC membranes
(Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA). p-ERK1/2
rabbit anti-monoclonal antibody (sc-16982-R) and ERCC1 mouse
monoclonal antibody were purchased from Santa Cruz Biotechnology
Inc. FGFR2 mouse monoclonal antibody (MAB684) was purchased from
R&D Systems, Inc. (Minneapolis, MN, USA). Secondary antibody
staining was performed using Dylight™ 800-labeled anti-rat IgG
(H+L) and anti-rabbit IgG (H+L) antibodies (Gaithersburg
Biotechnology, Gaithersburg, MD, USA).
Statistical analysis
Data were expressed as the means ± standard
deviation (SD) and differences between groups were compared by
one-way analysis of variance using SPSS17.0 statistical analysis
software (SPSS, Inc., Chicago, IL, USA), whereas intragroup
comparisons were performed with the Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
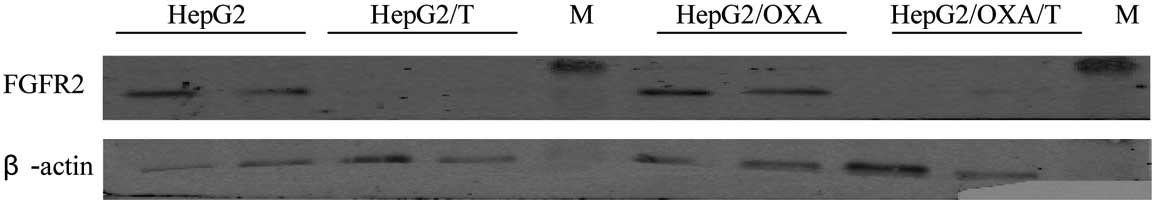
Identification of Bek
shRNA-transfected cells
HepG2 and HepG2/OXA cells transfected with Bek shRNA
were stably grown in a screening medium containing puromycin.
Western blot analysis identified a significant decrease in the
expression of FGFR2 protein, indicating the successful
downregulation of the protein (Fig.
1).
Expression of FGFR2 protein in HepG2,
HepG2/OXA, HepG2/T and HepG2/OXA/T cells
Compared to the parental HepG2 cells, FGFR2
expression in HepG2/OXA cells was significantly increased (Fig. 1; P<0.05). After HepG2 and
HepG2/OXA cells were transfected with Bek shRNA, FGFR2 protein
expression was significantly decreased in HepG2/T and HepG2/OXA/T
cells (Table I; P<0.01). After
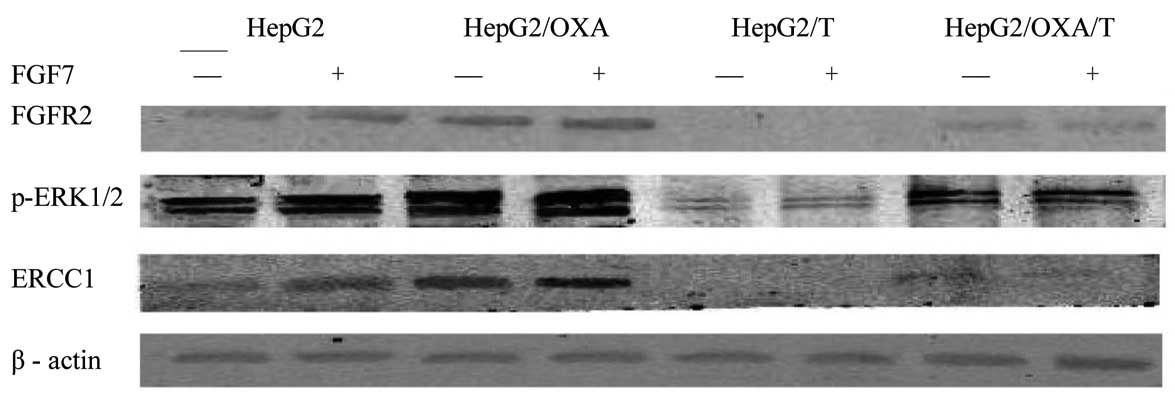
cells were stimulated with FGF7, FGFR2 expression was increased in
the untransfected cells; however, these differences were not
statistically significant compared to the unstimulated control
counterparts (P>0.05; Table I,
Fig. 2).
 | Table I.FGFR2 protein expression
concentrations in HepG2, HepG2/OXA, HepG2/T and HepG2/OXA/T cells
(n=5). |
Table I.
FGFR2 protein expression
concentrations in HepG2, HepG2/OXA, HepG2/T and HepG2/OXA/T cells
(n=5).
| Groups | HepG2 | HepG2/OXA | HepG2/T | HepG2/OXA/T |
|---|
| Control |
0.7228±0.0230c,d | 0.9566±0.0457a | 0.0108±0.0061a | 0.1040±0.0170c |
| FGF7 | 0.7818±0.0821 | 1.1156±0.1117b | 0.0112±0.0033 | 0.1070±0.0181 |
Expression of pERK1/2 protein in
HepG2, HepG2/OXA, HepG2/T and HepG2/OXA/T cells
Compared to the parental HepG2 cells, pERK1/2
expression in HepG2/OXA cells was significantly increased
(P<0.01). Following transfection with Bek shRNA, the pERK1/2
protein expression in HepG2/T and HepG2/OXA/T cells was
significantly decreased (P<0.01), compared to the untransfected
controls. Following stimulation with FGF7 in HepG2 and HepG2/OXA
cells, the expression of pERK1/2 was upregulated and the
differences were statistically significant (P=0.00034 and 0.04359,
respectively). In HepG2/T and HepG2/OXA/T cells, FGF7 stimulation
did not significantly affect pERK1/2 (P>0.05; Table II, Fig.
2).
 | Table II.pERK1/2 protein expression
concentrations in HepG2, HepG2/OXA, HepG2/T and HepG2/OXA/T cells
(n=5). |
Table II.
pERK1/2 protein expression
concentrations in HepG2, HepG2/OXA, HepG2/T and HepG2/OXA/T cells
(n=5).
| Groups | HepG2 | HepG2/OXA | HepG2/T | HepG2/OXA/T |
|---|
| Control |
1.4270±0.0807c,d | 1.9368±0.0918a | 0.1390±0.0351a | 0.4846±0.0719c |
| FGF7 | 1.7596±0.0953a | 2.1040±0.1263b | 0.1576±0.0325 | 0.5056±0.0360 |
ERCC1 protein expression in HepG2,
HepG2/OXA, HepG2/T and HepG2/OXA/T cells
ERCC1 expression in HepG2/OXA cells increased
significantly compared to the parental HepG2 cells (P<0.01).
Following HepG2 and HepG2/OXA cell transfection with Bek shRNA,
ERCC1 protein expression in HepG2/T and HepG2/OXA/T cells was
significantly decreased (P<0.01). FGF7 stimulation upregulated
the expression of ERCC1 in the HepG2/OXA cells and, to a lesser
extent, in HepG2 cells, with statistically significant differences
(P=0 and 0.02436, respectively). FGF7 stimulation upregulated ERCC1
expression in HepG2/T and HepG2/OXA/T cells; however, the
difference was not statistically significant (P>0.05; Table III, Fig.
2).
 | Table III.ERCC1 protein expression
concentrations in HepG2, HepG2/OXA, HepG2/T and HepG2/OXA/T cells
(n=5). |
Table III.
ERCC1 protein expression
concentrations in HepG2, HepG2/OXA, HepG2/T and HepG2/OXA/T cells
(n=5).
| Groups | HepG2 | HepG2/OXA | HepG2/T | HepG2/OXA/T |
|---|
| Control group |
0.4328±0.0548c,d | 1.5498±0.0709a | 0.0138±0.0054b | 0.1382±0.0277c |
| FGF7 | 1.1100±0.0841a | 1.745±0.1403b | 0.0116±0.0050 | 0.1120±0.0305 |
Discussion
ERCC1 is the key rate-limiting enzyme in the
nucleotide excision repair (NER) pathway and is closely associated
with the progression of HCC and drug resistance. Fautrel et
al (5) reported an increase in
ERCC1 expression in fibrotic compared to normal liver tissue and
the expression of ERCC1 protein concentration was significantly
increased in HCC tissue concomitant with hepatic fibrosis, compared
to HCC tissue without fibrosis. These findings suggest that ERCC1
plays an important role in liver fibrosis and HCC. ERCC1 may
predict the sensitivity of platinum-based chemotherapeutic drugs
and has been widely used in the treatment of lung cancer in
large-scale phase III clinical trials. Consequently, lung cancer
patients with high ERCC1 expression levels should avoid the use of
platinum-based chemotherapeutic drugs, which has been formulated in
the NCCN guidelines.
In HCC, the DNA repair pathway and its rate-limiting
enzyme ERCC1 were highly expressed in HCC cell lines, even without
chemotherapeutic stimulation. Ueda et al (6) reported that ≤33% of HCC tissues
presented with a high expression of ERCC1, which was associated
with cisplatin resistance. Our experimental findings indicated that
in the OXA-resistant HCC cell line HepG2/OXA, the expression of
ERCC1 was significantly increased compared to that in the parental
cell line HepG2. This finding is inconsistent with previous
experimental results (14). This
difference may indicate that xeroderma pigmentosum group C (XPC),
rather than ERCC1, determines the DNA damage repair in the HepG2
cell lines and the discrepancies may be due to the selection of
drugs used to induce DNA damage (7). Further investigation is required to
elucidate the mechanism of regulation of ERCC1 expression, which
may contribute to the development of platinum-resistant drugs for
the reversal of HCC and to the enhancement of the efficacy of HCC
treatment.
In view of the mechanisms that regulate the
expression of ERCC1, the present study demonstrated that, since the
ERCC1 promoter region may bind to a variety of transcription
factors, numerous external factors may induce the expression of
ERCC1. However, the specific molecular mechanisms may vary in
different types of tumor cells. Youn et al (15) reported that cisplatin-resistant
cells may upregulate the transcription factor AP1 through the H-ras
oncogene and increase ERCC1 expression. Wilson et al
(16) observed that the
transcription factor Ets-1 regulates ERCC1 expression in ovarian
cancer cells and Ets-1 and c-Fos exert synergistic effects
(17). Our previous study results
demonstrated that in the drug-resistant gastric cancer cell lines
OCUM-2M/SN38, OCUM-2M/PTX and OCUM-2M/VP16, Ki23057 monoclonal
antibody targeting of FGFR2 downregulated ERCC1 expression,
indicating that ERCC1 is an FGFR2 downstream-regulated target gene
(11). In the present study, it was
demonstrated that, compared to parental HepG2 cells, FGFR2
expression in HepG2/OXA cells was significantly increased.
Following shRNA gene silencing, FGFR2 was significantly decreased
and the ERCC1 protein expression in HepG2/T and HepG2/OXA/T cells
was also significantly inhibited, suggesting that ERCC1 is another
FGFR2 downstream-regulated target gene. However, additional
investigations are required to completely elucidate its
function.
FGFR2 is the product of the Bek oncogene expression
product and a transmembrane tyrosine kinase receptor with a high
affinity for a variety of FGFs (8).
Studies have demonstrated (13)
that ERCC1 in HCC cells is the downstream target of MEK/ERK and
PI3K. When exposed to epidermal growth factor, NER activity and
ERCC1 expression increase in normal liver and HCC cells in
conjunction with ERK1/2 and the EFG-mediated activation of ERCC1
may be inhibited by U0126 and ERK1/2 siRNA silencing. Furthermore,
basically expressed ERCC1 may be inhibited by a PI3K inhibitor,
FKBP12, rapamycin-associated protein shRNA or rapamycin-targeting
PI3K kinase (18). In this study,
we observed that the multidrug resistant cell line HepG2/OXA and
its parental cell line HepG2 exhibited high expression levels of
FGFR2 and ERCC1, as well as a significant increase in ERK1/2.
Following shRNA silencing of FGFR2, accompanied by a decrease in
phosphorylated ERK1/2 expression, ERCC1 expression was
significantly inhibited, indicating that the ERK1/2 pathway plays
an important role in the regulation of FGF7/FGFR2-mediated ERCC1
expression.
In summary, the FGFR2-mediated ERK1/2 signaling
pathway is critical for the regulation of ERCC1 expression. We
investigated the FGFR2-mediated ERK1/2 signaling pathway in a
broader attempt to identify new pathways for ERCC1 regulation,
inhibition of DNA injury repair and the reinforcement of platinum
drugs.
Acknowledgements
This study was supported by grants
from the National Natural Sciences Foundation of China (no.
81001067), the Ministry of Science and Technology International
Cooperation Project (no. S2010GR0991) and the AstraZeneca Special
Research Foundation for Targeted Therapy of Wu Jieping Medical
Foundation (no. 320.6700.09068)
References
|
1.
|
Llovcet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
2.
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Katoh M: Cancer genomics and genetics of
FGFR2 (Review). Int J Oncol. 33:233–237. 2008.PubMed/NCBI
|
|
5.
|
Fautrel A, Andrieux L, Musso O, Boudjema
K, Guillouzo A and Langouët S: Overexpression of the two nucleotide
excision repair genes ERCC1 and XPC in human hepatocellular
carcinoma. J Hepatol. 43:288–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ueda S, Shirabe K, Morita K, Umeda K,
Kayashima H, Uchiyama H, Soejima Y, Taketomi A and Maehara Y:
Evaluation of ERCC1 expression for cisplatin sensitivity in human
hepatocellular carcinoma. Ann Surg Oncol. 18:1204–1211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wu Q, Beland FA, Chang CW and Fang JL: XPC
is essential for nucleotide excision repair of zidovudine-induced
DNA damage in human hepatoma cells. Toxicol Appl Pharmacol.
251:155–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Gauglhofer C, Sagmeister S, Schrottmaier
W, et al: Up-regulation of the fibroblast growth factor 8 subfamily
in human hepatocellular carcinoma for cell survival and
neoangiogenesis. Hepatology. 53:854–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Harimoto N, Taguchi K, Shirabe K, Adachi
E, Sakaguchi Y, Toh Y, Okamura T, Kayashima H, Taketomi A and
Maehara Y: The significance of fibroblast growth factor receptor 2
expression in differentiation of hepatocellular carcinoma.
Oncology. 78:361–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Bai A, Meetze K, Vo NY, et al: GP369, an
FGFR2-IIIb-specific antibody, exhibits potent antitumor activity
against human cancers driven by activated FGFR2 signaling. Cancer
Res. 70:7630–7639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Qiu H, Yashiro M, Zhang X, Miwa A and
Hirakawa K: A FGFR2 inhibitor, Ki23057, enhances the
chemosensitivity of drug-resistant gastric cancer cells. Cancer
Lett. 307:47–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Katoh M and Katoh M: FGF signaling network
in the gastrointestinal tract (Review). Int J Oncol. 29:163–168.
2006.PubMed/NCBI
|
|
13.
|
Ko JC, Su YJ, Lin ST, Jhan JY, Ciou SC,
Cheng CM and Lin YW: Suppression of ERCC1 and Rad51 expression
through ERK1/2 inactivation is essential in emodin-mediated
cytotoxicity in human non-small cell lung cancer cells. Biochem
Pharmacol. 79:655–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ko JC, Tsai MS, Kuo YH, et al: Modulation
of Rad51, ERCC1, and thymidine phosphorylase by emodin result in
synergistic cytotoxic effect in combination with capecitabine.
Biochem Pharmacol. 81:680–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Youn CK, Kim MH, Cho HJ, Kim HB, Chang IY,
Chung MH and You HJ: Oncogenic H-Ras up-regulates expression of
ERCC1 to protect cells from platinum-based anticancer agents.
Cancer Res. 64:4849–4857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wilson LA, Yamamoto H and Singh G: Role of
the transcription factor Ets-1 in cisplatin resistance. Mol Cancer
Ther. 3:823–832. 2004.PubMed/NCBI
|
|
17.
|
Logan SK, Garabedian MJ, Campbell CE and
Werb Z: Synergistic transcriptional activation of the tissue
inhibitor of metalloproteinases-1 promoter via functional
interaction of AP-1 and Ets-1 transcription factors. J Biol Chem.
271:774–782. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Andrieux LO, Fautrel A, Bessard A,
Guillouzo A, Baffet G and Langouët S: GATA-1 is essential in
EGF-mediated induction of nucleotide excision repair activity and
ERCC1 expression through ERK2 in human hepatoma cells. Cancer Res.
67:2114–2123. 2007. View Article : Google Scholar : PubMed/NCBI
|