1. Introduction
Colorectal cancer (CRC) is currently the third most
common malignancy worldwide. Radical resection is curative for only
~50% of the patients (1), whereas
for the majority of patients with advanced-stage or metastatic
disease, or for those who cannot be treated with radical resection,
chemotherapy is the main treatment of choice (2,3). The
survival rate of patients with metastatic CRC has significantly
improved with the application of molecularly-targeted drugs, such
as oxaliplatin.
Oxaliplatin, a diaminocyclohexane platinum compound,
interrupts the replication and transcription of DNA (4). Oxaliplatin is the third generation of
platinum drugs after cisplatin and carboplatin and is effective in
the treatment of CRC, particularly CRC that is resistant to
5-fluorouracil (5,6). Oxaliplatin may also be effective for
the treatment of tumors that do not respond adequately to cisplatin
and carboplatin, as well as drug-resistant tumors. Oxaliplatin acts
synergistically with other anticancer drugs, such as fluorouracil,
topoisomerase inhibitors and microtubule inhibitors (7,8).
Satisfactory clinical results have also been
achieved with the combined application of oxaliplatin and
molecular-targeted drugs, such as bevacizumab and cetuximab,
administered intravascularly, with a median survival time of 30
months in the majority of the patients and of >3 years in
certain patients (9). Although a
number of studies indicated that the combined application of
oxaliplatin with other chemotherapeutics and molecular-targeted
drugs may achieve good clinical results in the treatment of CRC,
the associated toxicity and side effects, such as neurotoxicity,
cardiotoxicity, gastrointestinal reactions, hemorrhage and
hypersensitivity, may outweigh the benefits of the treatment
(10–14).
The nature of the active species generated in
vivo, uptake, efflux, intracellular trafficking or insufficient
diffusion in tumor tissues, resulting in decreased curative effects
and increased toxicity for certain chemotherapeutic agents
(15). Oxaliplatin therapy based on
a simple vesicular delivery system may reduce the potential side
effects, target specific organs and improve the therapeutic
effects.
2. Liposomes as anticancer drug
carriers
Over the last few decades, liposomes have been
widely accepted as agent nanocarriers. Liposomes are small,
spherical artificial vesicles that consist of cholesterol and
natural non-toxic phospholipids. Due to their size,
biocompatibility and hydrophobic and hydrophilic properties,
liposomes are promising drug delivery systems. Liposomes have a
phospholipid bilayer structure that is compatible with cell
membranes (16); therefore, they
are among the most effective drug carriers into cells, with
slow-releasing and targeting characteristics and the ability to
reduce side effects (17,18). Drugs coated in liposomes are slowly
released through infiltration or degradation of liposomes, leading
to a reduction in the metabolism and excretion of drugs by the body
and prolonged time of action. Liposomes as exogenous substances may
be devoured by macrophages; however, liposomal drugs administered
intravenously may selectively act on the mononuclear macrophage
system (19,20). The drugs delivered by
surface-modified liposomes escape being taken up by the endodermis
system, act specifically on target organs, increase drug
concentration in these organs and improve the therapeutic effects,
while reducing toxicity (21,22).
In addition, drugs insulated by bilayer liposomes are stable;
therefore, surface-modified liposomes exhibit advantages in the
treatment of a number of diseases, particularly cancer.
The toxicity of drugs coated by ordinary liposomes
may be reduced; however, the therapeutic effects are severely
affected as the drugs lose their bioactivity. Previous studies
demonstrated that different types of liposomes may be obtained
based on liposome modifiers (23,24)
and modified liposomes may be more effective drug delivery
systems.
Liposomes are broadly divided into the following 3
groups according to their different properties:
Long-circulating liposomes (stealth
liposomes)
The surface conformation of the phospholipid bilayer
structure is modified by adding gangliosides or a polyethylene
glycol (PEG) derivative possessing a flexible chain that occupies
the space immediately adjacent to the liposome surface, tends to
exlcude other macromolecules from this space (25,26),
and prevent blood plasma opsonins binding to the liposome surface.
Consequently, PEG decreases the recognition of liposomes by the
mononuclear phagocyte system and enables liposomes to remain stable
in the circulation and exhibit a prolonged half-life (27,28).
This type of liposome has been applied in clinical practice and
achieved satisfactory effects in individualized treatment, such as
treatment for hepatocellular carcinoma with doxorubicin liposomes
and ovarian carcinoma with paclitaxel liposomes (29–31).
Active targeting liposomes
Liposomes targeting antibodies, peptides, glycoside
residues, hormones and receptors. The ligands are constructed on
the phospholipid bilayer structure (32–37);
thus, the liposomes are able to identify and migrate to the target
organ and release the anticancer agent.
Liposomes with special properties
This type of liposomes includes pH-sensitive,
thermosensitive, magnetic and positive liposomes (38–41).
There are several types of liposomes; however, there are currently
no uniform standards regarding their application and these
liposomes should be selected according to the different treatment
or experimental requirements.
3. PEG-liposomes with enhanced permeability
and retention (EPR) effect
It is crucial to investigate PEG-liposomes with EPR
effect, as the EPR effect of tumors on macromolecules is a common
phenomenon. Previous studies reported that new vessel formation is
the basis of solid tumor growth (42,43).
Compared to normal tissues, capillaries in tumor tissues exhibit
the following characteristics: irregular wall structure, dilated
lumen, defective wall and loosely arranged endothelial cells
(44), incomplete lymphangiogenesis
and defective lymphatic return. Therefore, these abnormalities may
result in the penetration of macromolecules and lipid granules from
the lumen into the surrounding tissues, which is referred to as the
EPR of solid tumor tissues. The pathological characteristics of
solid tumors may enable the macromolecular anticancer drugs to
achieve a highly distributed concentration in tumor tissues
(45,46).
Currently available evidence indicates that
liposomes accumulate in solid tumor tissues and efficiently inhibit
tumor growth (47,48), which is associated with the EPR
effect. Due to the increased permeability of the solid tumor
vessels to macromolecules and the incomplete lymphatic clearance,
the lipid granules may remain in the tumor tissues for weeks or
even months (49). Long-circulating
liposomes, immune liposomes and liposomes with special properties
may increase the drug cumulative effect in tumor tissues due to
their active organ targeting (50,51).
4. PEG-liposomal oxaliplatin for the
treatment of CRC
Regular liposomes have a low encapsulation
efficiency and poor stability. Long-circulating liposomes modified
by PEG are more stable in the plasma and have a longer circulation
time and relatively lower toxicity (52). Moreover, our previous in
vitro study demonstrated the easy coherence of PEG-liposomes to
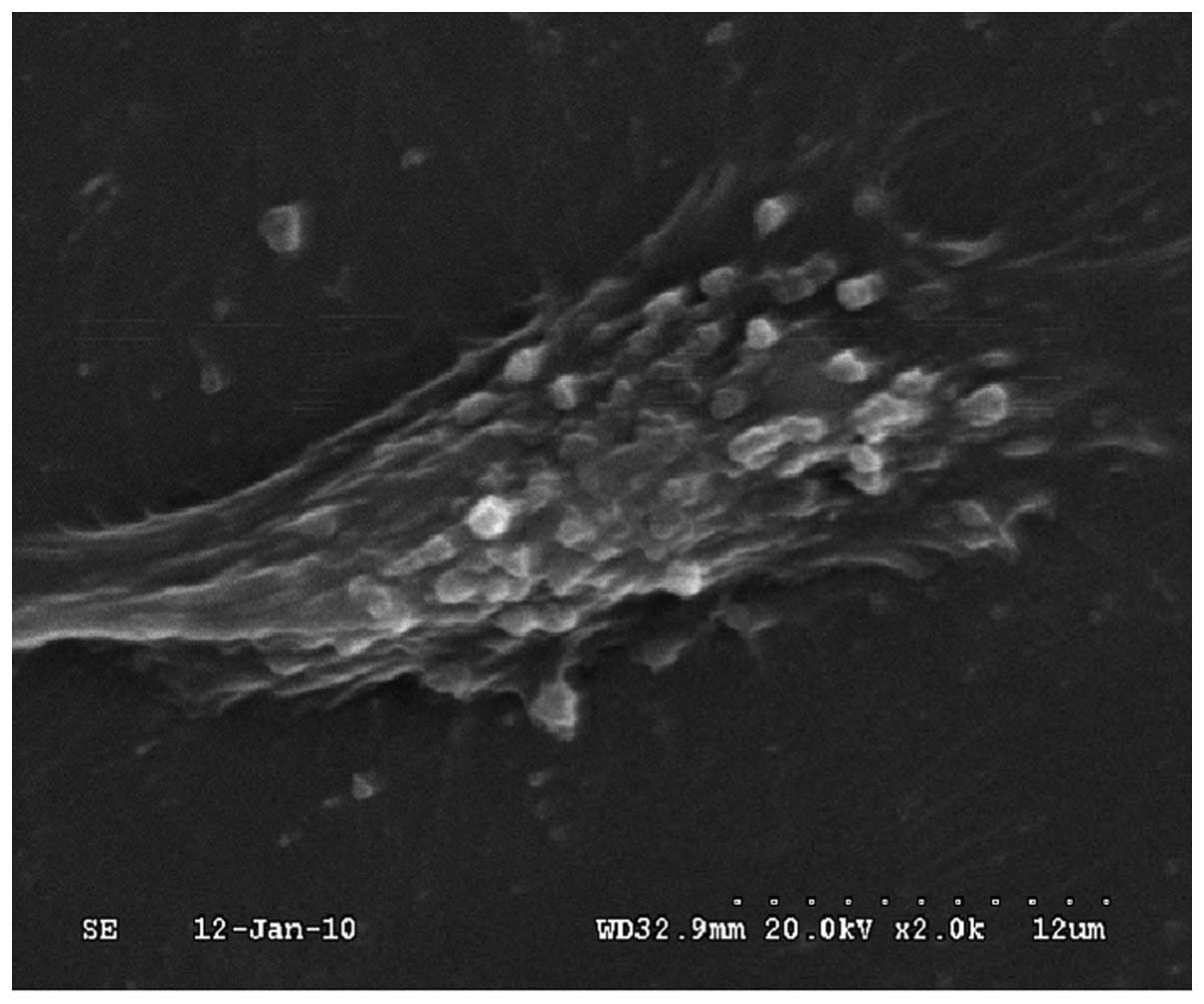
cells (Fig. 1), their
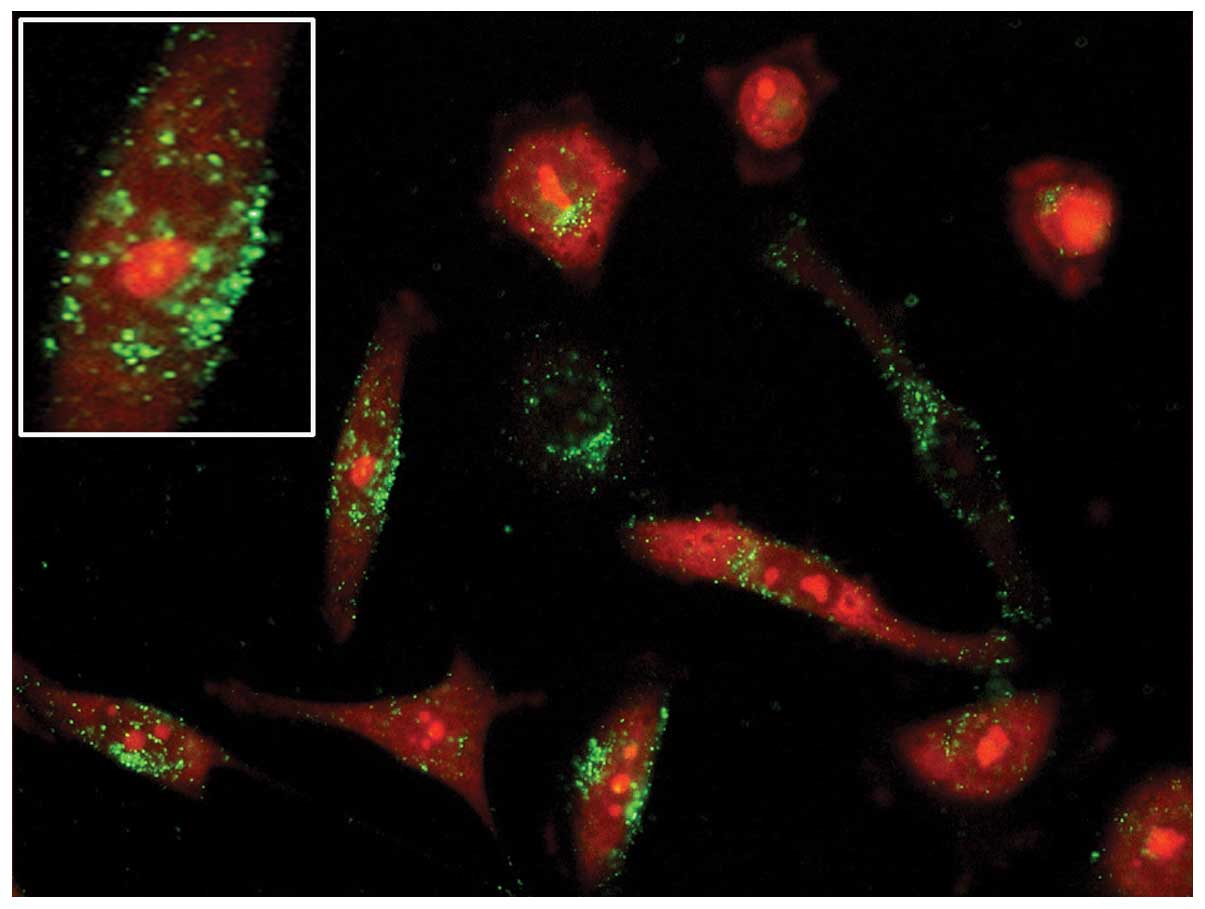
internalization and their subsequent intracellular route (Fig. 2). However, it is the size of the
particles that determines the entry pathway (53).
As oxaliplatin has a different antineoplastic
spectrum and no cross-resistance with cisplatin, it exerts a good
curative effect on advanced CRC. Liposome studies on oxaliplatin
and its derivatives are attracting increasing attention,
particularly regarding liposomes modified by PEG. The surface
modification of PEG-liposomes with specific ligands, such as
monoclonal antibodies, peptides, folic acid and transferrin, may
further improve the active targeting efficiency of liposomes
(25,30,54).
Considering the water solubility of oxaliplatin, the
low encapsulation efficiency of liposomes is the main concern. A
previous study reported that the encapsulation efficiency of
oxaliplatin liposomes was ~30% (55), whereas PEG-liposomal oxaliplatin
prepared with the film dispersion method by Zalba et al
(56) exhibited an encapsulation
efficiency of ≤35%. Liposomes prepared by optimizing the
preparation technique, as described by Liu et al (57), exhibited an encapsulation efficiency
of ≤69.1%. Our previous study demonstrated that the encapsulation
efficiency of PEG-liposomal oxaliplatin was ~58% (58). These differences in the
encapsulation efficiency may be associated with the different
preparation techniques.
The action time of oxaliplatin coated with liposomes
was significantly prolonged and its toxicity against normal cells
was significantly reduced. High concentrations of oxaliplatin were
obtained in the cytoplasm and then combined with nuclear DNA as
>95% of PEG-liposomal oxaliplatin was internalized by CRC cells
(59). Treatments for CRC with
PEG-liposomal oxaliplatin are currently at the research phase. Doi
et al (60) investigated the
therapeutic effect of PEG-liposomal oxaliplatin in a mouse CRC
model and demonstrated that PEG-liposomal oxaliplatin exerted a
significant inhibitory effect on tumors compared to free
oxaliplatin (>50%), with an increased drug content in tumors.
Jain et al (61) coated
oxaliplatin with hyaluronic acid-chitosan, administered the drug to
nude mice bearing TH29 colorectal tumor xenografts and found that
the drug concentration in the tumor tissues reached a peak value 24
h after administration. Radioisotope scanning revealed that the
liposomes had accumulated in the colorectal tumor 24 h after
administration.
Abu Lila et al (62) recently reported a higher cumulative
distribution effect of PEG-liposomal oxaliplatin in colorectal
tumor tissues through a comparative study of CRC, lung cancer and
melanoma. Different types of tumor cells can take up different
amounts of drug-carrying liposomes, indicating that the
permeability of different tumor vessels is a factor affecting tumor
localization and the antitumor effects of drug-carrying liposomes
(63). In our previous experiment,
oxaliplatin was coated with DSPE-PEG2000-modified liposomes and the
PEG-liposomes exerted a significant antitumor effect in vivo
and in vitro (51,64). Further investigations revealed that
Fas/Fas ligand and the caspase pathway may be involved in the
apoptosis-inducing effects of PEG-liposomal oxaliplatin on CRC
cells (65).
Tumors are unable to grow without vessels and
capillaries are the foundation of tumor survival. Taking advantage
of the properties of PEG-liposomes may allow drugs to migrate to
the target organ by constructing a vascular-targeting substance,
such as vascular endothelial growth factor (VEGF) and VEGF
monoclonal antibody peptides, on the surface of liposomes (66). Therefore, the preparation of
PEG-liposomal oxaliplatin is of great clinical significance.
5. Conclusion
Oxaliplatin exerts a good curative effect on CRC,
fully embodying the advantages of platinum drugs. However, there is
a need to reduce the toxic side effects of oxaliplatin. As a novel
type of drug carrier, liposomes exhibit good targeting properties,
slow-releasing potential, high stability and low toxicity following
surface modification. The active targeting modifications are
significant for altering the biological distribution of antitumor
agents, reducing or reversing the multidrug resistance of tumor
cells and improving the efficiency of anticancer drugs. Further
studies investigating the effects of PEG-liposomal oxaliplatin on
CRC are required to establish the advantages of its application in
clinical practice.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of China (no. 81172295).
References
|
1
|
Spolverato G, Ejaz A, Azad N and Pawlik
TM: Surgery for colorectal liver metastases: The evolution of
determining prognosis. World J Gastrointest Oncol. 5:207–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alberts SR, Sargent DJ, Nair S, et al:
Effect of oxaliplatin, fluorouracil, and leucovorin with or without
cetuximab on survival among patients with resected stage III colon
cancer: a randomized trial. JAMA. 307:1383–1393. 2012. View Article : Google Scholar
|
|
3
|
Garcia-Foncillas J and Diaz-Rubio E:
Progress in metastatic colorectal cancer: growing role of cetuximab
to optimize clinical outcome. Clin Transl Oncol. 12:533–542. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiseman LR, Adkins JC, Plosker GL, et al:
Oxaliplatin: a review of its use in the management of metastatic
colorectal cancer. Drugs Aging. 14:459–475. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simpson D, Dunn C, Curran M and Goa KL:
Oxaliplatin: a review of its use in combination therapy for
advanced metastatic colorectal cancer. Drugs. 63:2127–2156. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang DY, Li Y, Liu JH, et al: Efficacy and
tolerance of maintenance therapy in patients with incurable
advanced colorectal cancer. J Southern Med Uni. 33:1815–1818.
2013.(In Chinese).
|
|
7
|
Brodowicz T, Ciuleanu TE, Radosavljevic D,
et al: FOLFOX4 plus cetuximab administered weekly or every second
week in the first-line treatment of patients with KRAS wild-type
metastatic colorectal cancer: a randomized phase II CECOG study.
Ann Oncol. 24:1769–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Douillard JY, Oliner KS, Siena S, et al:
Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal
cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Messersmith WA, Jimeno A, Jacene H, et al:
Phase I trial of oxaliplatin, infusional 5-fluorouracil, and
leucovorin (FOLFOX4) with erlotinib and bevacizumab in colorectal
cancer. Clin Colorectal Cancer. 9:297–304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McWhinney SR, Goldberg RM and McLeod HL:
Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 8:10–16.
2009. View Article : Google Scholar
|
|
11
|
Ochenduszko SL and Krzemieniecki K:
Targeted therapy in advanced colorectal cancer: more data, more
questions. Anticancer Drugs. 21:737–748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cortejoso L, Garcia MI, Garcia-Alfonso P,
et al: Differential toxicity biomarkers for irinotecan- and
oxaliplatin-containing chemotherapy in colorectal cancer. Cancer
Chemother Pharmacol. 71:1463–1472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Francia R, Siesto RS, Valente D, et al:
Pharmacogenomics panel test for prevention toxicity in patient who
receive fluoropirimidine/oxaliplatin-based therapy. Eur Rev Med
Pharmacol Sci. 16:1211–1217. 2012.PubMed/NCBI
|
|
14
|
Hoff PM, Saad ED, Costa F, et al:
Literature review and practical aspects on the management of
oxaliplatin-associated toxicity. Clin Colorectal Cancer. 11:93–100.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olszewski U and Hamilton G: A better
platinum-based anticancer drug yet to come? Anticancer Agents Med
Chem. 10:293–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patil YP and Jadhav S: Novel methods for
liposome preparation. Chem Phys Lipids. 177:8–18. 2014. View Article : Google Scholar
|
|
17
|
Jain RL and Shastri JP: Study of ocular
drug delivery system using drug-loaded liposomes. Int J Pharm
Investig. 1:35–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suntres ZE: Liposomal antioxidants for
protection against oxidant-induced damage. J Toxicol.
2011:1524742011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pagano RE and Weinstein JN: Interactions
of liposomes with mammalian cells. Annu Rev Biophys Bioeng.
7:435–468. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yefimova SL, Kurilchenko IY, Tkacheva TN,
et al: Comparative study of dye-loaded liposome accumulation in
sensitive and resistant human breast cancer cells. Exp Oncol.
34:101–106. 2012.PubMed/NCBI
|
|
21
|
Saffari M, Shirazi HF, Oghabian MA, et al:
Preparation and in-vitro evaluation of an antisense-containing
cationic liposome against non-small cell lung cancer: a comparative
preparation study. Iran J Pharm Res. 12(Suppl): 3–10.
2013.PubMed/NCBI
|
|
22
|
Preiss MR and Bothun GD:
Stimuli-responsive liposome-nanoparticle assemblies. Expert Opin
Drug Deliv. 8:1025–1040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rangger C, Helbok A, Sosabowski J, et al:
Tumor targeting and imaging with dual-peptide conjugated
multifunctional liposomal nanoparticles. Int J Nanomedicine.
8:4659–4671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Zhang J, Wang DK, et al: Anti-tumor
activity of folate receptor targeting docetaxel-loaded
membrane-modified liposomes. Acta Pharma Sinica. 48:1142–1147.
2013.(In Chinese).
|
|
25
|
Nag OK and Awasthi V: Surface engineering
of liposomes for stealth behavior. Pharmaceutics. 5:542–569. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Noble GT, Stefanick JF, Ashley JD, et al:
Ligand-targeted liposome design: challenges and fundamental
considerations. Trends Biotechnol. 32:32–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Immordino ML, Dosio F and Cattel L:
Stealth liposomes: review of the basic science, rationale, and
clinical applications, existing and potential. Int J Nanomedicine.
1:297–315. 2006.PubMed/NCBI
|
|
28
|
Akbarzadeh A, Rezaei-Sadabady R, Davaran
S, et al: Liposome: classification, preparation, and applications.
Nanoscale Res Lett. 8:1022013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Allen TM and Cullis PR: Liposomal drug
delivery systems: from concept to clinical applications. Adv Drug
Deliv Rev. 65:36–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samad A, Sultana Y and Aqil M: Liposomal
drug delivery systems: an update review. Curr Drug Deliv.
4:297–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cattel L, Ceruti M and Dosio F: From
conventional to stealth liposomes: a new frontier in cancer
chemotherapy. Tumori. 89:237–249. 2003.PubMed/NCBI
|
|
32
|
Smith-Jones PM, Vallabhajosula S, Navarro
V, et al: Radiolabeled monoclonal antibodies specific to the
extracellular domain of prostate-specific membrane antigen:
preclinical studies in nude mice bearing LNCaP human prostate
tumor. J Nucl Med. 44:610–617. 2003.
|
|
33
|
Yan Z, Zhan C, Wen Z, et al:
LyP-1-conjugated doxorubicin-loaded liposomes suppress lymphatic
metastasis by inhibiting lymph node metastases and destroying tumor
lymphatics. Nanotechnology. 22:4151032011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brignole C, Marimpietri D, Gambini C, et
al: Development of Fab’ fragments of anti-GD(2) immunoliposomes
entrapping doxorubicin for experimental therapy of human
neuroblastoma. Cancer Lett. 197:199–204. 2003.
|
|
35
|
Yang Y, Yan Z, Wei D, et al:
Tumor-penetrating peptide functionalization enhances the
anti-glioblastoma effect of doxorubicin liposomes. Nanotechnology.
24:4051012013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan Z, Wang F, Wen Z, et al:
LyP-1-conjugated PEGylated liposomes: a carrier system for targeted
therapy of lymphatic metastatic tumor. J Control Release.
157:118–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishida O and Maruyama K: Transferrin
conjugated PEG-liposomes as intracellular targeting carrier for
tumor therapy. Jpn J Clin Med. 56:657–662. 1998.(In Japanese).
|
|
38
|
Rane S and Prabhakar B: Optimization of
paclitaxel containing pH-sensitive liposomes by 3 factor, 3 level
box-behnken design. Indian J Pharm Sci. 75:420–426. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dicheva BM and Koning GA: Targeted
thermosensitive liposomes: an attractive novel approach for
increased drug delivery to solid tumors. Expert Opin Drug Deliv.
11:83–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Linemann T, Thomsen LB, Jardin KG, et al:
Development of a novel lipophilic, magnetic nanoparticle for in
vivo drug delivery. Pharmaceutics. 5:246–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alinaghi A, Rouini MR, Johari Daha F, et
al: The influence of lipid composition and surface charge on
biodistribution of intact liposomes releasing from
hydrogel-embedded vesicles. Int J Pharm. 459:30–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iversen PO: Angiogenesis and hematological
malignancies. J Norw Med Assoc. 123:3198–3200. 2003.(In
Norwegian).
|
|
43
|
Bisacchi D, Benelli R, Vanzetto C, et al:
Anti-angiogenesis and angioprevention: mechanisms, problems and
perspectives. Cancer Detect Prev. 27:229–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abdollahi A and Folkman J: Evading tumor
evasion: current concepts and perspectives of anti-angiogenic
cancer therapy. Drug Resist Updat. 13:16–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Waite CL and Roth CM: Nanoscale drug
delivery systems for enhanced drug penetration into solid tumors:
current progress and opportunities. Crit Rev Biomed Eng. 40:21–41.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Prabhakar U, Maeda H, Jain RK, et al:
Challenges and key considerations of the enhanced permeability and
retention effect for nanomedicine drug delivery in oncology. Cancer
Res. 73:2412–2417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Taurin S, Nehoff H and Greish K:
Anticancer nanomedicine and tumor vascular permeability; Where is
the missing link? J Control Release. 164:265–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Greish K: Enhanced permeability and
retention (EPR) effect for anticancer nanomedicine drug targeting.
Methods Mol Biol. 624:25–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maeda H, Bharate GY and Daruwalla J:
Polymeric drugs for efficient tumor-targeted drug delivery based on
EPR-effect. Eur J Pharm Biopharm. 71:409–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Karn PR, Cho W and Hwang SJ: Liposomal
drug products and recent advances in the synthesis of supercritical
fluid-mediated liposomes. Nanomedicine (Lond). 8:1529–1548. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang C, Liu HZ, Lu WD, et al:
PEG-liposomal oxaliplatin potentialization of antitumor efficiency
in a nude mouse tumor-xenograft model of colorectal carcinoma.
Oncol Rep. 25:1621–1628. 2011.PubMed/NCBI
|
|
52
|
Nakamura H, Doi Y, Abu Lila AS, et al:
Sequential treatment of oxaliplatin-containing PEGylated liposome
together with S-1 improves intratumor distribution of subsequent
doses of oxaliplatin-containing PEGylated liposome. Eur J Pharm
Biopharm. Dec 17–2013.(Epub ahead of print). View Article : Google Scholar
|
|
53
|
Rejman J, Oberle V, Zuhorn IS and Hoekstra
D: Size-dependent internalization of particles via the pathways of
clathrin- and caveolae-mediated endocytosis. Biochem J.
377:159–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hood RR, Shao C, Omiatek DM, et al:
Microfluidic synthesis of PEG- and folate-conjugated liposomes for
one-step formation of targeted stealth nanocarriers. Pharm Res.
30:1597–1607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Abu Lila AS, Doi Y, Nakamura K, et al:
Sequential administration with oxaliplatin-containing PEG-coated
cationic liposomes promotes a significant delivery of subsequent
dose into murine solid tumor. J Control Release. 142:167–173.
2010.
|
|
56
|
Zalba S, Navarro I, Troconiz IF, et al:
Application of different methods to formulate PEG-liposomes of
oxaliplatin: evaluation in vitro and in vivo. Eur J Pharm Biopharm.
81:273–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu XP, Geng DQ, Xu HX, et al: Research on
the preparation of oxaliplatin liposome. J Wuhan Univ Technol.
30:50–53. 2008.
|
|
58
|
Yang C, Liu HZ, Fu ZX and Lu WD:
Oxaliplatin long-circulating liposomes improved therapeutic index
of colorectal carcinoma. BMC Biotechnology. 11:212011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tippayamontri T, Kotb R, Paquette B, et
al: Cellular uptake and cytoplasm/DNA distribution of cisplatin and
oxaliplatin and their liposomal formulation in human colorectal
cancer cell HCT116. Invest New Drugs. 29:1321–1327. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Doi Y, Okada T, Matsumoto H, et al:
Combination therapy of metronomic S-1 dosing with
oxaliplatin-containing polyethylene glycol-coated liposome improves
antitumor activity in a murine colorectal tumor model. Cancer Sci.
101:2470–2475. 2010. View Article : Google Scholar
|
|
61
|
Jain A, Jain SK, Ganesh N, et al: Design
and development of ligand-appended polysaccharidic nanoparticles
for the delivery of oxaliplatin in colorectal cancer. Nanomedicine.
6:179–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Abu Lila AS, Matsumoto H, Doi Y, et al:
Tumor-type-dependent vascular permeability constitutes a potential
impediment to the therapeutic efficacy of liposomal oxaliplatin.
Eur J Pharm Biopharm. 81:524–531. 2012.PubMed/NCBI
|
|
63
|
Abu Lila AS, Ichihara M, Shimizu T, et al:
Ex-vivo/in-vitro anti-polyethylene glycol (PEG) immunoglobulin M
production from murine splenic B cells stimulated by PEGylated
liposome. Biol Pharm Bull. 36:1842–1848. 2013.PubMed/NCBI
|
|
64
|
Yang C, Liu HZ and Fu ZX: Effects of
PEG-liposomal oxaliplatin on apoptosis, and expression of Cyclin A
and Cyclin D1 in colorectal cancer cells. Oncol Rep. 28:1006–1012.
2012.PubMed/NCBI
|
|
65
|
Yang C, Liu HZ and Fu ZX: PEG-liposomal
oxaliplatin induces apoptosis in human colorectal cancer cells via
Fas/FasL and caspase-8. Cell Biol Int. 36:289–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wicki A, Rochlitz C, Orleth A, et al:
Targeting tumor-associated endothelial cells: anti-VEGFR2
immunoliposomes mediate tumor vessel disruption and inhibit tumor
growth. Clin Cancer Res. 18:454–464. 2012. View Article : Google Scholar : PubMed/NCBI
|