Introduction
The insulin-producing tumoral BRIN-BD11 cell line
was produced by electrofusion of NEDH rat islet B cells with
immortal RINm5F cells (1). The
insulin-secreting BRIN-BD11 cell line has provided a model for the
study of pancreatic B-cell functions, including glucose, amino acid
and hypotonicity-induced insulin secretion (1–7),
expression and the role of the adenosine triphosphate
(ATP)-sensitive potassium channels (8), the electrogenic
Na+-HCO3− cotransporter NBCe1
(9), plasma membrane
Ca2+-ATPase (10),
aquaglyceroporin 7 (11) and the
malate-aspartate NADH shuttle (12). BRIN-BD11 cells express glucose
transporter-2 and display an improved metabolic response to glucose
(13). In view of the latter
observations, the uptake of D-glucose and its non-metabolized
analog, 3-O-methyl-D-glucose, as well as the effects of
cytochalasin B were investigated in these cells in the present
study.
Materials and methods
Materials
L-[1-14C]glucose,
3-O-[14C]-methyl-D-glucose (labelled with 14C
in the methyl group) and D-[U-14C]glucose were purchased
from PerkinElmer, Inc. (Boston, MA, USA). Cytochalasin B,
L-glucose, 3-O-methyl-D-glucose and RPMI-1640 medium were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Ca2+-
and-Mg2+-free Hank’s balanced salt solution (HBSS) and
trypsin-EDTA were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA).
The BRIN-BD11 cells that were kindly provided by
Professors R. Beauwens and R. Crutzen (Laboratory of Molecular
Physiology, Brussels Free University, Brussels, Belgium) were grown
at 37°C in a humidified incubator, with an atmosphere of 5%
CO2 in air, and cultured in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum, 50 IU/ml
penicillin and 50 μg/ml streptomycin (Invitrogen Life
Technologies). The D-glucose (Sigma-Aldrich) and L-glutamine
concentrations of the culture medium were 11.1 and 2.0 mM,
respectively.
The BRIN-BD11 cells were gently washed with 5 ml
Ca2+- and-Mg2+-free HBSS for 30 sec at room
temperature (20°C) prior to being detached from the tissue culture
flask with 3 ml trypsin-EDTA (0.05%) solution. Subsequent to being
washed with culture medium, the cells were counted and suspended in
Krebs-Ringer bicarbonate buffer (1.5–2.0×106 cells/ml)
(14).
Methods
In order to take measurements of the net uptake of
3-O-[14C]-methyl-D-glucose (1.0 μCi/ml) and
D-[U-14C]glucose (1.0 μCi/ml) by the BRIN-BD11 cells, 50
μl cell suspension (50–75×103 cells) was mixed with 50
μl of a bicarbonate- and HEPES-buffered salt-balanced medium
containing bovine serum albumin (1 mg/ml), 2.0 mM L-glucose, 16.7
mM D-glucose or 3-O-methyl-D-glucose in the absence or presence of
cytochalasin B (40.0 μM). The cells were incubated for 5–30 min at
37°C in incubation medium containing tritiated water
(3HOH) (4.2 μCi/ml; New England Nuclear, Boston, MA,
USA). For evaluation of the extracellular space and the total water
space, 50×103 cells were also incubated for 5–30 min at
37°C in 0.1 ml of a bicarbonate- and HEPES-buffered salt-balanced
medium containing L-[1-14C]glucose (1.3 μCi/ml) and
3HOH (4.2 μCi/ml). Following incubation, 0.15 ml of a
mixture of dibutylphthalate and di-isononylphthalate (10:3, v/v;
Sigma-Aldrich) was added to each polyethylene tube (Beckman
Coulter, Fulterton, CA, USA). This was then centrifuged for 3 min
at 5,000 × g. The bottom of the tube (polyethylene Bechman
microfuge tube) containing the cell pellet was then cut, placed in
a counting vial containing 5.0 ml of scintillation fluid (ICN
Biomedicals, Costa Mesa, CA, USA) and, after mixing, examined for
its radioactive content in a double channel
(14C/3H) beta counter (TRI-CARB 2810 TR,
PerkinElmer). Following correction for the blank value found under
the same experimental conditions in the absence of cells, the
results were expressed as nl/103 cells.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean together with either the number of individual
determinations (n) or the degree of freedom (df). The statistically
significant differences (P<0.05) between the mean values was
assessed by Student’s t-test.
Results
Measurement of 3HOH space in
the presence/absence of cytochalasin B
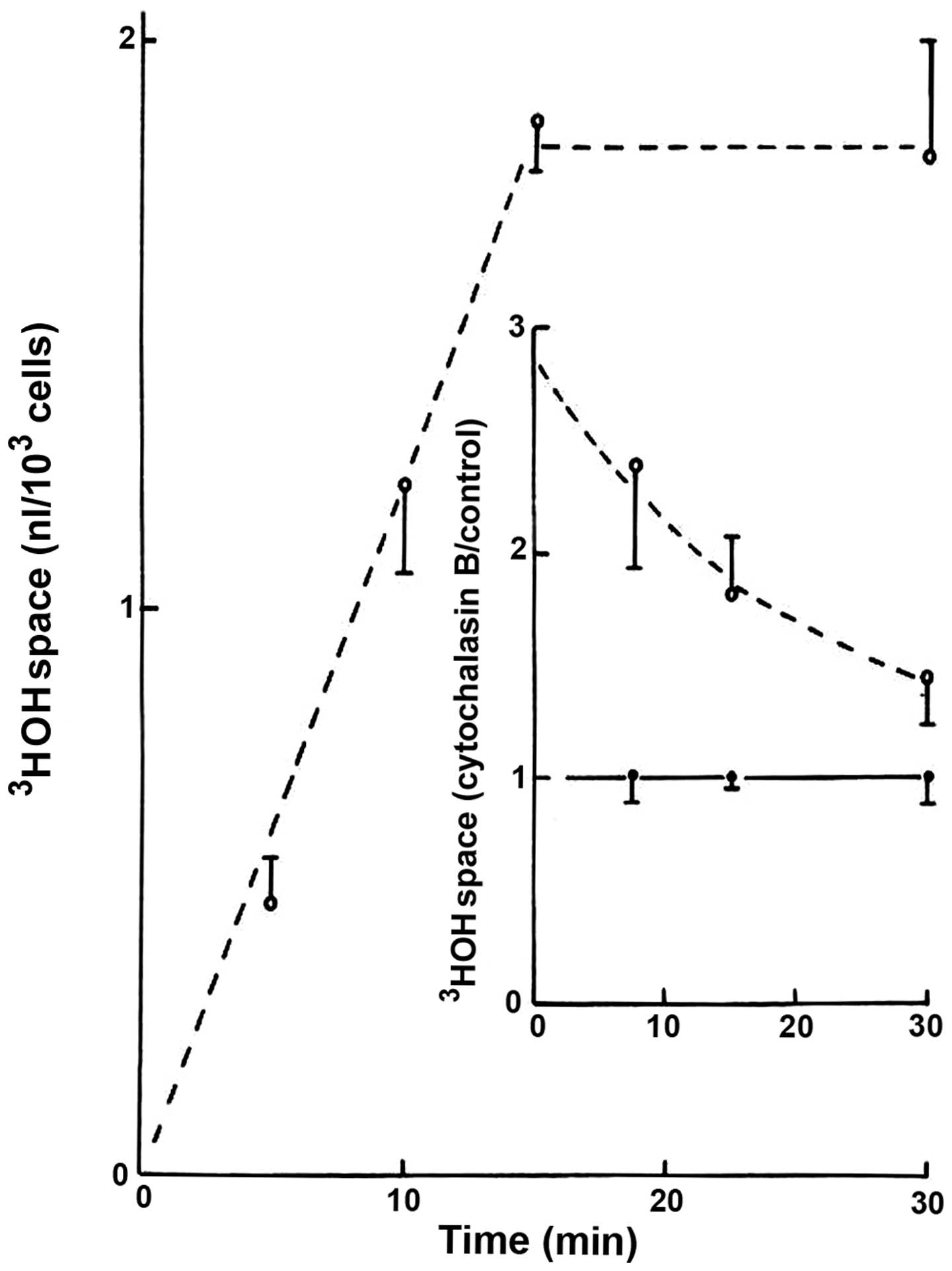
In the absence of cytochalasin B, the apparent
distribution space of 3HOH progressively increased over
the first 15 min of incubation, reaching a steady-state averaging
1.82±0.15 nl/103 cells (n=18; Fig. 1). The presence of cytochalasin B (20
μM) increased the 3HOH space, which then gradually
decreased in an exponential manner during incubation (Fig. 1). Thus, a negative correlation
coefficient (r=−0.9873) was found between the length of incubation
and the logarithmic values for the cytochalasin B-induced increase
in 3HOH space, expressed relative to the mean control
values recorded at the same time of incubation in the same
experiments. As indicated from such logarithmic values, the time
required to provoke a 50% decrease in the response to cytochalasin
B was ~850 sec, and therefore no significant difference between the
results recorded in the presence/absence of cytochalasin B was
observed after the 30-min incubation period.
The extracellular space, as indicated from the
distribution space of L-[1-14C]glucose, used as an
extracellular marker, averaged over 10–30 min of incubation
0.84±0.13 nl/103 cells (n=18), representing 43.8±3.5%
(n=18) of the total 3HOH space. In the absence of
cytochalasin B, the intracellular space, as indicated from the
paired difference between the distribution space of 3HOH
and that of L-[1-14C]glucose, averaged 1.11±0.20
nl/103 cells (n=18).
Pooling all available control data collected after
10–30 min of incubation, the paired ratio between the distribution
space of either 3-O-[14C]-methyl-D-glucose or
D-[U-14C]glucose (8.3 mM each) and that of
3HOH averaged to 62.7±6.4% (n=26). As summarized in
Table I, there was no significant
difference (df, 24; P>0.5) between the mean paired ratio for the
distribution space of 3-O-[14C]-methyl-D-glucose or
D-(U-14C)glucose and that of 3HOH. Moreover,
as determined from data collected in each individual experiment,
the difference between the mean paired ratio for the distribution
space of either 3-O-[14C]-methyl-D-glucose or
D-[U-14C]glucose and that of 3HOH, as well as
the difference between the mean paired ratio for the distribution
space of L-[1-14C]glucose and that of 3HOH,
did not differ significantly from one another (P>0.87) compared
with the results recorded with either D-glucose or its
non-metabolized analogue; and in both cases, yielded mean values
significantly different from zero (P<0.02). Furthermore, as
previously observed in intact pancreatic islets (15), the intracellular space accessible to
D-glucose or its non-metabolized analog in the BRIN-BD11 cells
represented approximately half of the total intracellular
space.
 | Table IMean control values recorded in the
absence of cytochalasin B. |
Table I
Mean control values recorded in the
absence of cytochalasin B.
| Variables | Incubation time,
min | Mean control
values |
|---|
| 3HOH
space, nl/103 cells | 15–30 | 1.82±0.15 (n=18) |
|
L-[1-14C]glucose space,
nl/103 cells | 10–30 | 0.84±0.13 (n=18) |
|
L-[1-14C]glucose
space/3HOH space, % | 10–30 | 43.8±3.5 (n=18) |
| 3HOH space
minus L-[1-14C]glucose space, nl/103
cells | 10–30 | 1.11±0.20 (n=18) |
|
3-O-[14C]-methyl-D-glucose
space/3HOH space, % | 10–30 | 65.9±9.7 (n=16) |
|
D-[U-14C]-D-glucose
space/3HOH space, % | 10–30 | 57.5±6.1 (n=10) |
|
3-O-[14C]-methyl-D-glucose
space/3HOH space minus | | |
|
L-[1-14C]glucose
space/3HOH space, % | 10–30 | 18.6±7.1 (df=24) |
|
D-[U-14C]-D-glucose
space/3HOH space minus | | |
|
L-[1-14C]glucose
space/3HOH space, % | 10–30 | 20.6±7.4 (df=16) |
Effects of cytochalasin B
The paired ratio between the distribution space of
either 3-O-[14C]-methyl-D-glucose or
D-[U-14C]glucose (8.3 mM each) and that of
3HOH averaged over 5–30 min of incubation in the absence
of cytochalasin B 67.1±7.3% (n=21). As expected, this was
significantly higher (P>0.01) compared with the paired value
between the distribution space of L-[1-14C]glucose and
3HOH in the absence of cytochalasin B. The latter mould
metabolite decreased the paired ratio between the distribution
space of either 3-O-[14C]-methyl-D-glucose or
D-[U-14C]glucose and that of 3HOH
(P<0.02). Thus, relative to the control values for such a ratio
recorded in the absence of cytochalasin B (100.0±5.5%; n=21), the
measurements made in the presence of cytochalasin B averaged to
83.2±3.2% (n=21). It should be stressed that the mean values for
the latter variable were virtually identical in all cases,
averaging to 84.1±4.6 (n=3) and 84.3±2.3% (n=3), after 10 and 30
min of incubation, respectively, in the presence of
3-O-[14C]-methyl-D-glucose, and to 83.4±10.7 (n=5),
82.8±6.7 (n=5) and 82.4±6.5% (n=5) after 5, 15 and 30 min of
incubation, respectively, in the presence of
D-[U-14C]glucose.
Discussion
The time-related increase in the 3HOH
space provoked by cytochalasin B in the BRIN-BD11 cells was an
expected finding. In ductal cells from submandibular salivary
glands, no significant difference (df, 18; P>0.25) in such a
space is observed after 20 min of incubation in the absence or
presence of cytochalasin B (unpublished data). Similarly, in acinar
cells from the same salivary gland, the 3HOH space
measured after 20 min of incubation in the presence of cytochalasin
B averages to 102.5±8.3% (n=15; P>0.85) of the mean
corresponding control values recorded within the same experiments
(100.0±9.8%; n=15) (unpublished data). The findings observed in the
BRIN-BD11 cells suggest that cytochalasin B transiently affects the
access of 3HOH (and either
3-O-[14C]-methyl-D-glucose or
D-[U-14C]glucose) to the intracellular space.
The present study demonstrated that cytochalasin B
inhibits the uptake of D-glucose or its non-metabolized analog by
BRIN-BD11 cells, which is in accordance with recent observations in
acinar and ductal cells of the rat submandibular salivary glands.
For instance, in the acinar cells the paired ratio between the
distribution space of either 3-O-[14C]-methyl-D-glucose
or D-[U-14C]glucose and that of 3HOH averaged
after 20 min of incubation 83.4±3.6% (n=18) of the mean
corresponding control values. The latter finding is virtually
identical to that found in the BRIN-BD11 cells.
The present findings concerning the cytochalasin
B-induced inhibition of either
3-O-[14C]-methyl-D-glucose or
D-[U-14C]glucose uptake by the BRIN-BD11 cells is in
accordance with the findings of a previous study on the inhibition
of D-glucose uptake, utilization, oxidation, glucose-stimulated
lactate output and D-glucose conversion to acidic metabolites by
the mould metabolite in rat-isolated pancreatic islets (16). The data listed in Table II, which were computed from primary
data provided in a previous study (17), document that the relative magnitude
of the inhibitory action of cytochalasin B, used at the same
concentration as in the present study, on D-glucose catabolism, as
determined by three distinct metabolic criteria
(D-[5-3H]glucose conversion to 3HOH and
D-[U-14C]glucose conversion to both
14CO2 and radioactive acidic metabolites),
was in purified islet B cells comparable to those recorded in the
present study for the uptake of D-glucose and its non-metabolized
analog by BRIN-BD11 cells. This analogy reinforces the view that
the primary site of action of cytochalasin B on the handling of
D-glucose concerns hexose transport across the plasma membrane. The
data summarized in Table II
further illustrates two additional characteristics with regard to
the effect of cytochalasin B on glucose handling by islet cells.
First, in either intact islets or dispersed islet cells, the
relative magnitude of the inhibitory action of cytochalasin B
progressively decreased as the extracellular concentration of
D-glucose increased from 2.8 to 8.3 and 16.7 mM. Expressed relative
to the corresponding control values (no cytochalasin B), the
experimental results recorded in the presence of the mould
metabolite averaged 66.5±4.0 (n=27), 73.2±2.5 (n=46) and 84.0±3.0%
(n=75) at 2.8, 8.3 and 16.7 mM D-glucose, respectively. Second, at
the same D-glucose concentration (2.8 or 16.7 mM), the relative
magnitude of the inhibitory action of cytochalasin B was less
pronounced in purified islet B cells than either intact islet or
dispersed islet cells (17,18). Thus, the percentage inhibition
recorded in the purified B cells represented 44.8±18.0% (n=103;
P<0.008) of the mean corresponding value found at the same
D-glucose concentration in intact islets and/or dispersed islet
cells (100.0±9.7%; n=102). Such a difference coincides with the
fact that purified B cells are much less sensitive to the
inhibitory action of cytochalasin B on D-glucose catabolism
compared with non-B islet cells (17).
 | Table IIEffects of cytochalasin B on D-glucose
metabolism in rat islets, dispersed islet cells and purified islet
B cells. |
Table II
Effects of cytochalasin B on D-glucose
metabolism in rat islets, dispersed islet cells and purified islet
B cells.
| D-glucose, % (n) |
|---|
|
|
|---|
| Cell type | 2.8 mM | 8.3 mM | 16.7 mM |
|---|
| Control (no
cytochalasin B) |
| Islets | - | 100.0±3.2 (48) | 100.0±2.4 (46) |
| Dispersed islet
cells | 100.0±6.2 (26) | - | 100.0±5.7 (26) |
| B cells | 100.0±4.9 (49) | - | 100.0±5.0 (55) |
| Cytochalasin B (21
μM) |
| Islets | - | 73.2±2.5 (46) | 83.9±2.4 (46) |
| Dispersed islet
cells | 66.5±4.0 (27) | - | 84.2±3.6 (29) |
| B cells | 87.4±6.7 (49) | - | 91.8±4.7 (54) |
In conclusion, the present study extends to
BRIN-BD11 cells the knowledge that cytochalasin B impairs the
uptake of D-glucose and that of one of its non-metabolized analogs.
The identity of the glucose transporter(s) affected by cytochalasin
B requires further investigation.
Acknowledgements
This study was supported by the Belgian Foundation
for Scientific Medical Research (grant no. 3.4520.07) and by a
grant from the European Commission (collaborative project VIBRANT
228933: In Vivo Imaging of Beta cell Receptors by Applied Nano
Technology).
References
|
1
|
McClenaghan NH, Barnett CR, Ah-Sing E, et
al: Characterization of a novel glucose-responsive
insulin-secreting cell line, BRIN-BD11, produced by electrofusion.
Diabetes. 45:1132–1140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McClenaghan NH, Barnett CR, O’Harte FP and
Flatt PR: Mechanisms of amino acid-induced insulin secretion from
the glucose-responsive BRIN-BD11 pancreatic B-cell line. J
Endocrinol. 151:349–357. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salgado AP, Pereira FC, Seiça RM, et al:
Modulation of glucose-induced insulin secretion by cytosolic redox
state in clonal beta-cells. Mol Cell Endocrinol. 154:79–88. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dixon G, Nolan J, McClenaghan N, Flatt PR
and Newsholme P: A comparative study of amino acid consumption by
rat islet cells and the clonal beta-cell line BRIN-BD11 - the
functional significance of L-alanine. J Endocrinol. 179:447–454.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miguel JC, Patterson S, Abdel-Wahab YH,
Mathias PC and Flatt PR: Time-correlation between membrane
depolarization and intracellular calcium in insulin secreting
BRIN-BD11 cells: studies using FLIPR. Cell Calcium. 36:43–50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beauwens R, Best L, Markadieu N, et al:
Stimulus-secretion coupling of hypotonicity-induced insulin release
in BRIN-BD11 cells. Endocrine. 30:353–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Crutzen R, Louchami K, Carpentier
YA, Sener A and Malaisse WJ: Direct effects of eicosapentaenoic and
docosahexaenoic acids on phospholipid and triglyceride fatty acid
pattern, glucose metabolism, 86rubidium net uptake and insulin
release in BRIN-BD11 cells. Endocrine. 35:438–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chapman JC, McClenaghan NH, Cosgrove KE,
et al: ATP-sensitive potassium channels and efaroxan-induced
insulin release in the electrofusion-derived BRIN-BD11 beta-cell
line. Diabetes. 48:2349–2357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bulur N, Virreira M, Soyfoo MS, et al:
Expression of the electrogenic
Na+-HCO3−-cotransporter NBCe1 in
tumoral insulin-producing BRIN-BD11 cells. Cell Physiol Biochem.
24:187–192. 2009.
|
|
10
|
Kamagate A, Sener A, Courtois P, Malaisse
WJ and Herchuelz A: Effects of plasma membrane
Ca2-ATPase overexpression upon D-glucose metabolism in
insulin-producing BRIN-BD11 cells. Biosci Rep. 28:251–258.
2008.
|
|
11
|
Delporte C, Virreira M, Crutzen R,
Louchami K, Sener A, Malaisse WJ and Beauwens R: Functional role of
aquaglyceroporin 7 expression in the pancreatic beta-cell line
BRIN-BD11. J Cell Physiol. 221:424–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bender K, Maechler P, McClenaghan NH,
Flatt PR and Newsholme P: Overexpression of the malate-aspartate
NADH shuttle member Aralar1 in the clonal beta-cell line BRIN-BD11
enhances amino-acid-stimulated insulin secretion and cell
metabolism. Clin Sci (Lond). 117:321–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rasschaert J, Flatt PR, Barnett CR,
McClenaghan NH and Malaisse WJ: D-glucose metabolism in BRIN-BD11
islet cells. Biochem Mol Med. 57:97–105. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malaisse WJ, Maggetto C, Leclercq-Meyer V
and Sener A: Interference of glycogenolysis with glycolysis in
pancreatic islets from glucose-infused rats. J Clin Invest.
91:432–436. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malaisse WJ: On the track to the
beta-cell. Diabetologia. 44:393–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levy J, Herchuelz A, Sener A,
Malaisse-Lagae F and Malaisse WJ: Cytochalasin B-induced impairment
of glucose metabolism in islets of Langerhans. Endocrinology.
98:429–437. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jijakli H, Zhang HX, Dura E, Ramirez R,
Sener A and Malaisse WJ: Effects of cytochalasin B and D upon
insulin release and pancreatic islet cell metabolism. Int J Mol
Med. 9:165–172. 2002.PubMed/NCBI
|
|
18
|
Courtois P, Sener A and Malaisse WJ:
Impairment by cytochalasin B of the inhibitory action of
D-mannoheptulose upon D-glucose metabolism in rat pancreatic
islets. Int J Mol Med. 5:385–388. 2000.PubMed/NCBI
|