Introduction
Essential hypertension (EH), as a polygenic disease
with a high prevalence rate, is considered to develop from a
complex interaction of diverse environmental conditions with
multiple genetic factors in which the underlying genetic mechanism
remains unknown (1,2).
A number of studies have been conducted to
investigate the genetic components of EH. The genes involved in
classical systems, including Renin-angiotensin-aldosterone system
(RAAS), the central nervous system and the vascular-endothelial
system, have been previously focused on (3,4) and
the homocysteine (Hcy) pathway is an emerging target. The
methylenetetrahydrofolate reductase (MTHFR) C677T mutation
is one of the most common gene polymorphisms. Mapped to chromosomal
region 1p36.3, the MTHFR gene spans a 2.2-kb length with 11
exons and 10 introns. The product, known as MTHFR, is a
critical enzyme in Hcy metabolism (5). The C677C to T mutation in the
catalyzing region of the MTHFR gene may induce the
displacement of alanine by valine. This change may lead to the
thermolability of the enzyme and the inhibition of MTHFR
activity, thus decreasing the transformation from
5,10-methyltetrahydrofolate to 5-methyltetrahydrofolate, which acts
as a co-substrate for the conversion from Hcy to methionine
(6). The level of plasma Hcy, as a
result, increases and may lead to certain pathological changes,
including vascular endothelial injury, vascular smooth muscle
proliferation and nitric oxide production inhibition (7), which may lead to the development of
hypertension, as well as other diseases (8–11).
Studies on the MTHFR C677T gene polymorphism
and EH association have been extensively performed previously, but
the results obtained remain controversial. In 2003, a study
conducted in Spain suggested that the MTHFR TT genotypes can
predict the development of EH in males, and the association may be
mediated by the elevation of the Hcy level (12). In 2008 and 2012, Lin et al
(13) and Yin et al
(14) investigated the association
among the Chinese population in the Taichung and Guangxi areas,
respectively, and the studies indicated that the MTHFR 677C
to T genotypes may be significant risk factors for EH. However, in
2012, a case-control study regarding the association between four
Hcy pathway gene variants, including MTHFR 677T and EH in
Caucasians, indicated no significant association (15). More contrary results were found in a
Japanese study by Nakata et al (16), which showed that the risk of
hypertension significantly increased in subjects with the 677CC
genotype, whereas the 677T gene polymorphism contributed to lower
blood pressure.
Therefore, considering that the results of the
studies carried out in the same ethnicity and different populations
vary widely, the present meta-analysis of 10,415 subjects was
conducted from 27 eligible studies to obtain a comprehensive
conclusion on this association. The study demonstrated the
association between the MTHFR C677T gene polymorphism and EH
in the whole population. The association in different subgroup
populations with reasonable clarification of the heterogeneity was
shown clearly.
Materials and methods
Publication search and inclusion
criteria
Electronic databases, including PubMed, Web of
Science, China National Knowledge Infrastructure, WanFang and WeiPu
were searched for relevant studies written in English or Chinese
using the keywords: ‘Methylenetetrahydrofolate reductase or
MTHFR’, ‘polymorphism’ and ‘hypertension’ (the last search
was updated on April 1, 2013). Included studies were required to
meet the following major criteria: i) Case-control or
cross-sectional studies investigating the association between the
MTHFR C677T polymorphism and EH; ii) present genotype counts
of the patient and control groups; iii) diagnosis of EH patients
based on the criteria of systolic blood pressure ≥140 mmHg or
diastolic blood pressure ≥90 mmHg. Secondary hypertension was
excluded; and (iv) for overlapping studies, the studies with the
larger sample size were included.
Data extraction
The following data from each eligible study were
independently extracted in duplicate by two investigators: First
author’s name, publication year, population origin, genotype counts
of cases and controls, study design, sample size, genotyping
method, male percentage and age of patients and controls. The two
extractions were compared and any discrepancy was resolved through
discussion until a consensus was reached.
Statistical analysis
In the current meta-analysis, the association
between the MTHFR C677T gene polymorphism and EH was
revealed and analyzed by the odds ratio (OR) corresponding to the
95% confidence interval (CI) between cases and controls under five
genetic models, which were the allelic (T/C), dominant (TT +
CT/CC), recessive (TT/CT + CC), homozygote (TT/CC) and heterozygote
models (CT/CC).
Between-study heterogeneity was estimated by
χ2-based Q analysis, and significance was set at
P<0.05. The variation caused by heterogeneity was also evaluated
by the inconsistency index, I2 (I2=0–25%, no
heterogeneity; 25–50%, moderate; 50–75%, large; and 75–100%,
extreme heterogeneity). The DerSimonian and Laird random-effects
model was applied whether or not heterogeneity among studies was
observed. The Z test was used to determine the pooled OR, and
significance was set at P<0.05. In addition, to examine the
degree to which an individual study affects the overall estimate,
sensitivity analyses were conducted by removing one study at a time
and analyzing the change of the pooled effect. To explore the
potential sources of heterogeneity, a random-effects
meta-regression was performed. Additionally, subgroup analyses were
implemented subsequent to stratifying the included studies
according to population origin and study design.
Fisher’s exact test was used to assess the
Hardy-Weinberg equilibrium (HWE). Potential publication bias was
estimated by Begg’s funnel plot together with Egger’s linear
regression test. Statistical analysis was performed using STATA
11.0 (StataCorp, College Station, TX, USA).
Results
Search results and study
characteristics
A total of 53 potentially relevant papers were
obtained by the literature search, of which 27 met the inclusion
criteria (8,12–37).
Of the 26 excluded studies, four studies were reviews or
meta-analyses, five were overlapping studies, six lacked controls
groups, three did not provide genotype counts, two were unrelated
to MTHFR C677T and the EH risk and four studies deviated
from the HWE. One family study and one prospective cohort study
were also excluded (Fig. 1). The
present meta-analysis involved 5,418 EH patients and 4,997 controls
of Asian (Chinese, Japanese, Indian and Turks) and Caucasian
(Australian, South Welsh, Argentinean, Austrian, Polish, Spanish
and Czech) origin. Specifically, 6,188 subjects of 15 studies were
Chinese from various parts of China, including Beijing, Tianjin,
Shanghai, Fujian, Shenzhen, Yunnan, Ningxia, Guangxi, Mongolia,
Xinjiang and Taiwan (Table I).
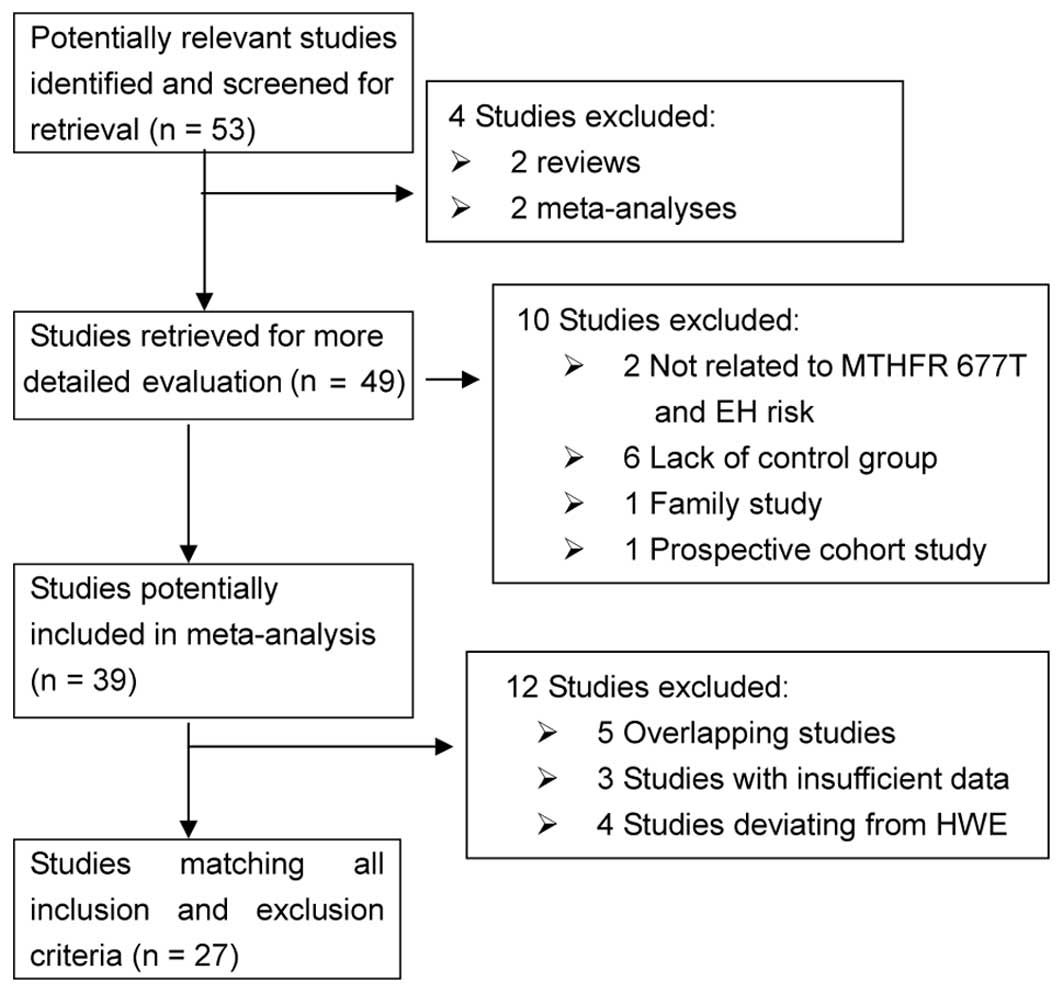 | Figure 1Flow diagram of the study selection
process in the meta-analysis. Electronic databases, including
PubMed, Web of Science, China National Knowledge Infrastructure
(CNKI), WanFang and WeiPu, were searched for relevant studies that
were written in English or Chinese, using the keywords
‘methylenetetrahydrofolate reductase or MTHFR’, ‘polymorphism’ and
‘hypertension’. A total of 53 potentially relevant studies were
obtained by the literature search (last study was updated on April
1, 2013), of which 27 met the inclusion criteria. Of the 26
excluded studies, four were reviews or meta-analyses, five were
overlapping studies, six lacked a controls group, three did not
provide genotype counts, two were unrelated to MTHFR 677T and
essential hypertension (EH) risk and four studies deviated from the
Hardy-Weinberg equilibrium (HWE). One family study and one
prospective cohort study were also excluded. |
 | Table ICharacteristics of 27 included
studies of the association between the methylenetetrahydrofolate
reductase C677T gene polymorphism and essential hypertension
(EH). |
Table I
Characteristics of 27 included
studies of the association between the methylenetetrahydrofolate
reductase C677T gene polymorphism and essential hypertension
(EH).
| First author
(Refs.) | Year | Origin | Case | Control | EH/control | Study design | Geno-typing | Male, % | EH age, years | Control age,
years |
|---|
|
|
|---|
| CC | CT | TT | CC | CT | TT |
|---|
| Yin (14) | 2012 | Chinese | 244 | 358 | 68 | 322 | 309 | 51 | 670/682 | PB
case-control | PCR-RFLP | 0.494 |
M49.9±16.2
F48.3±15.8 |
M48.8±11.8
F47.1±11.2 |
| Zhang (30) | 2012 | Chinese | 128 | 53 | 8 | 117 | 41 | 7 | 189/165 | PB
case-control | PCR-RFLP | 0.729 | 50.3±9.7 | 49.9±7.7 |
| Cao (31) | 2012 | Chinese | 65 | 105 | 53 | 49 | 68 | 30 | 223/147 | HB
case-control | PCR-RFLP | 0.608 | 78.9±7.1 | 74.5±8.2 |
| Liu (17) | 2011 | Chinese | 58 | 70 | 27 | 74 | 47 | 19 | 155/140 | HB
case-control | PCR-RFLP | 0.695 | 44.1±11.3 | 41.7±11.4 |
| Liu (18) | 2011 | Chinese | 54 | 59 | 33 | 61 | 39 | 12 | 146/112 | HB
case-control | PCR-RFLP | 0.531 | 48.3±16.0 | 45.1±16.2 |
| Cai (19) | 2009 | Chinese | 77 | 44 | 9 | 31 | 7 | 1 | 130/39 | HB
case-control | PCR-RFLP | - | Total 61.8±9.8 | |
| Luo (20) | 2008 | Chinese | 260 | 151 | 31 | 138 | 51 | 6 | 442/195 | HB
case-control | PCR-RFLP | 0.538 | 62.8±10.9 | 62.0±10.3 |
| Lin (13) | 2008 | Chinese | 19 | 27 | 4 | 73 | 44 | 6 | 50/123 | HB
case-control | PCR-RFLP | 0.584 | 60.1±10.8 | 59.0±8.7 |
| Xing (21) | 2007 | Chinese | 202 | 309 | 184 | 182 | 222 | 105 | 695/509 | HB
case-control | PCR-RFLP | 0.489 | 48.9±12.6 | 48.5±13.1 |
| Tang (22) | 2007 | Chinese | 139 | 93 | 20 | 138 | 51 | 6 | 252/195 | HB
case-control | PCR-RFLP | 0.539 | 63.0±12.8 | 61.0±10.2 |
| Li (32) | 2006 | Chinese | 18 | 6 | 2 | 21 | 7 | 2 | 26/30 | HB
case-control | PCR-RFLP | 0.536 | 60.3±11.3 | 55.5±13.5 |
| Hu (33) | 2006 | Chinese | 55 | 39 | 16 | 61 | 42 | 12 | 110/115 | PB
case-control | Sequenom | 0.458 | 56.7±10.6 | 55.3±10.3 |
| Liu (34) | 2005 | Chinese | 29 | 45 | 26 | 31 | 50 | 19 | 100/100 | PB
case-control | PCR-RFLP | 0.500 | 67.2±4.2 | 66.3±4.4 |
| Wang (23) | 2002 | Chinese | 17 | 51 | 37 | 14 | 23 | 9 | 105/46 | HB
case-control | PCR-RFLP | 0.165 | 59.0~73.0 | 56.0~62.0 |
| Zhan (28) | 2000 | Chinese | 44 | 68 | 15 | 62 | 84 | 24 | 127/170 | PB
case-control | PCR-RFLP | 0.468 | 57.9±9.9 | 50.6±10.5 |
| Hui (35) | 2007 | Japanese | 83 | 129 | 49 | 104 | 123 | 44 | 261/271 | PB
case-control | RTFQ-PCR | 0.662 | 51.1±5.6 | 51.5±8.6 |
| Lwin (29) | 2006 | Japanese | 39 | 58 | 19 | 64 | 117 | 38 | 116/219 | PB
cross-sectional | PCR-RFLP | 1.000 | Total 53.1±8.9 | |
| Nakata (16) | 1998 | Japanese | 63 | 91 | 19 | 65 | 83 | 36 | 173/184 | HB
case-control | PCR-RFLP | - | - | - |
| Markan (25) | 2007 | Indian | 105 | 40 | 8 | 105 | 28 | 0 | 153/133 | HB
case-control | PCR-RFLP | 0.531 | 47.7±12.4 | 46.2±10.8 |
| Ilhan (24) | 2008 | Turk | 36 | 32 | 10 | 72 | 26 | 2 | 78/100 | HB
case-control | RTFQ-PCR | 0.573 | 57.2±10.0 | 57.5±11.1 |
| Fowdar (15) | 2012 | Australian | 170 | 174 | 33 | 175 | 183 | 35 | 377/393 | PB
case-control | PCR-RFLP | 0.452 | 63.1±10.9 | 61.0±10.5 |
| Ng (26) | 2009 | South Welsh | 14 | 14 | 10 | 40 | 32 | 8 | 38/80 | PB
case-control | PCR-RFLP | 0.432 | 66.0~83.0 | 65.0~90.0 |
| Fridman (27) | 2008 | Argentine | 15 | 21 | 4 | 39 | 38 | 9 | 40/86 | HB
cross-sectional | PCR-RFLP | 0.468 | Total
45.2±16.8 | |
| Tylicki (36) | 2005 | Austrian,
Polish | 40 | 39 | 11 | 42 | 38 | 10 | 90/90 | HB
case-control | PCR-RFLP | 0.367 | 59.4±1.3 | 52.1±1.6 |
| Heux (8) | 2004 | Australia | 87 | 125 | 35 | 105 | 119 | 25 | 247/249 | HB
case-control | PCR-RFLP | 0.562 | Total
55.0±11.0 | |
| Rodriguez (12) | 2003 | Spanish | 83 | 115 | 34 | 95 | 100 | 20 | 232/215 | PB
case-control | PCR-RFLP | 0.734 | 58.6±9.0 | 57.5±8.1 |
| Benes (37) | 2001 | Czech | 73 | 93 | 27 | 86 | 106 | 17 | 193/209 | HB
case-control | PCR-RFLP | 0.761 | 55.6±11.5 | 54.9±11.6 |
Pooled effect under different genetic
models
Among the 27 included studies, 14 separate studies
showed a significant association between the MTHFR 677T gene
polymorphism and EH risk (8,12–14,17–26),
whereas the remaining 13 studies did not (15,16,27–37).
Through meta-analysis, a significant association was revealed
between the MTHFR 677T gene polymorphism and EH under the
allelic (OR, 1.32; 95% CI, 1.20–1.45; P=0.000), dominant (OR, 1.39;
95% CI, 1.25–1.55; P=0.000), recessive (OR, 1.38; 95% CI,
1.18–1.62; P=0.000), homozygote (OR, 1.59; 95% CI, 1.32–1.92;
P=0.000), and heterozygote (OR, 1.32; 95% CI, 1.20–1.45; P=0.000)
genetic models (Figs. 2–5 and Table
II). The overall pooled effect remained almost unchanged
following the sensitivity analysis, which indicated the robustness
of the results in the meta-analysis.
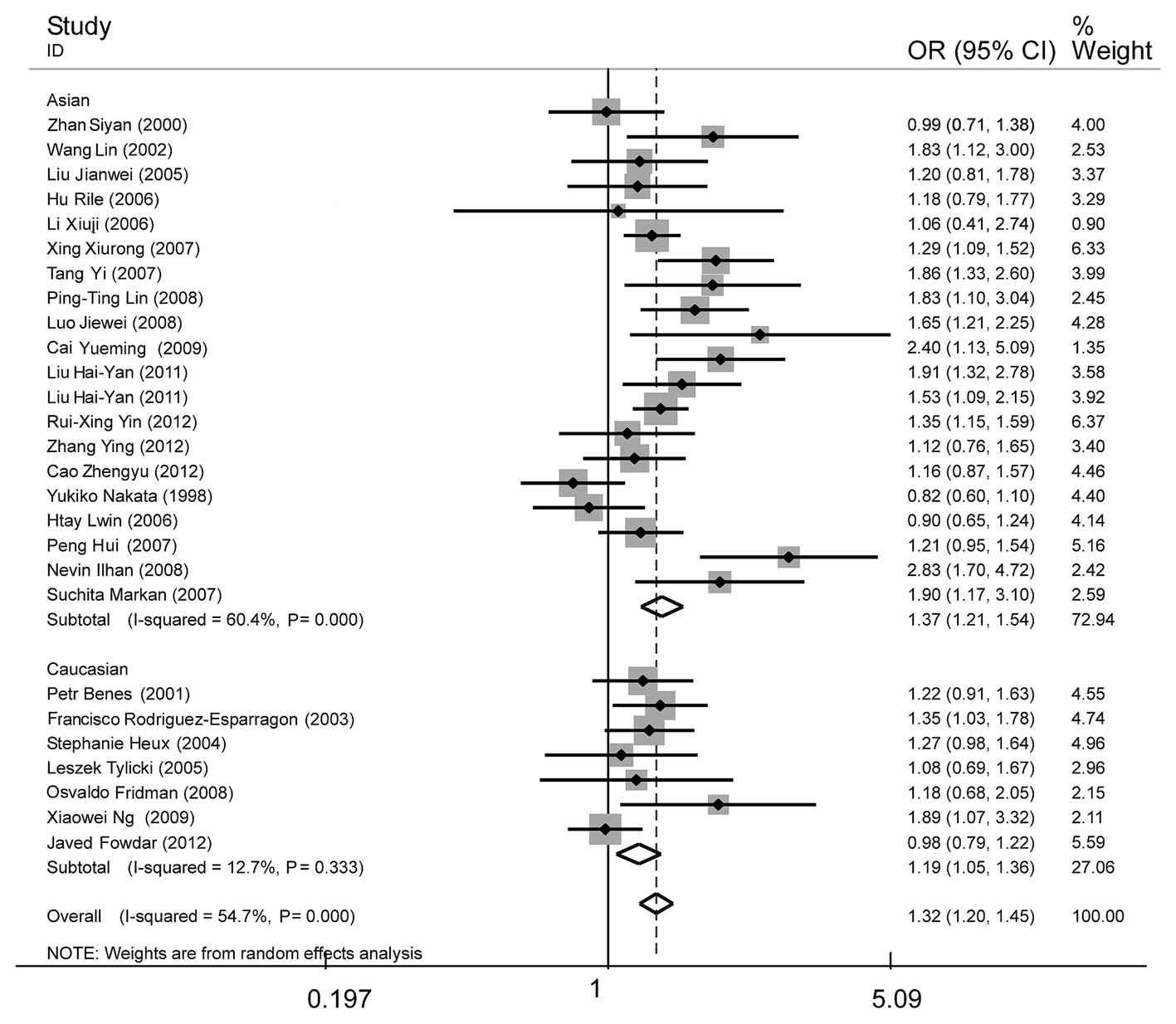 | Figure 2Forest plot of essential hypertension
associated with the MTHFR C677T gene polymorphism under the
allelic model (T/C). A significant association was revealed in the
whole population (OR, 1.32; 95% CI, 1.20–1.45), Asian subgroup
(OR=1.37; 95% CI, 1.21–1.54) and Caucasian subgroup (OR=1.19; 95%
CI, 1.05–1.36). The heterogeneity test was significant in the whole
population (I2=54.7%, P=0.000) and Asian subgroup
(I2=60.4%, P=0.000), whereas the Caucasian subgroup
showed no significant heterogeneity (I2=12.7%, P=0.333).
MTHFR, methylenetetrahydrofolate reductase; OR, odds ratio; CI,
confidence interval. |
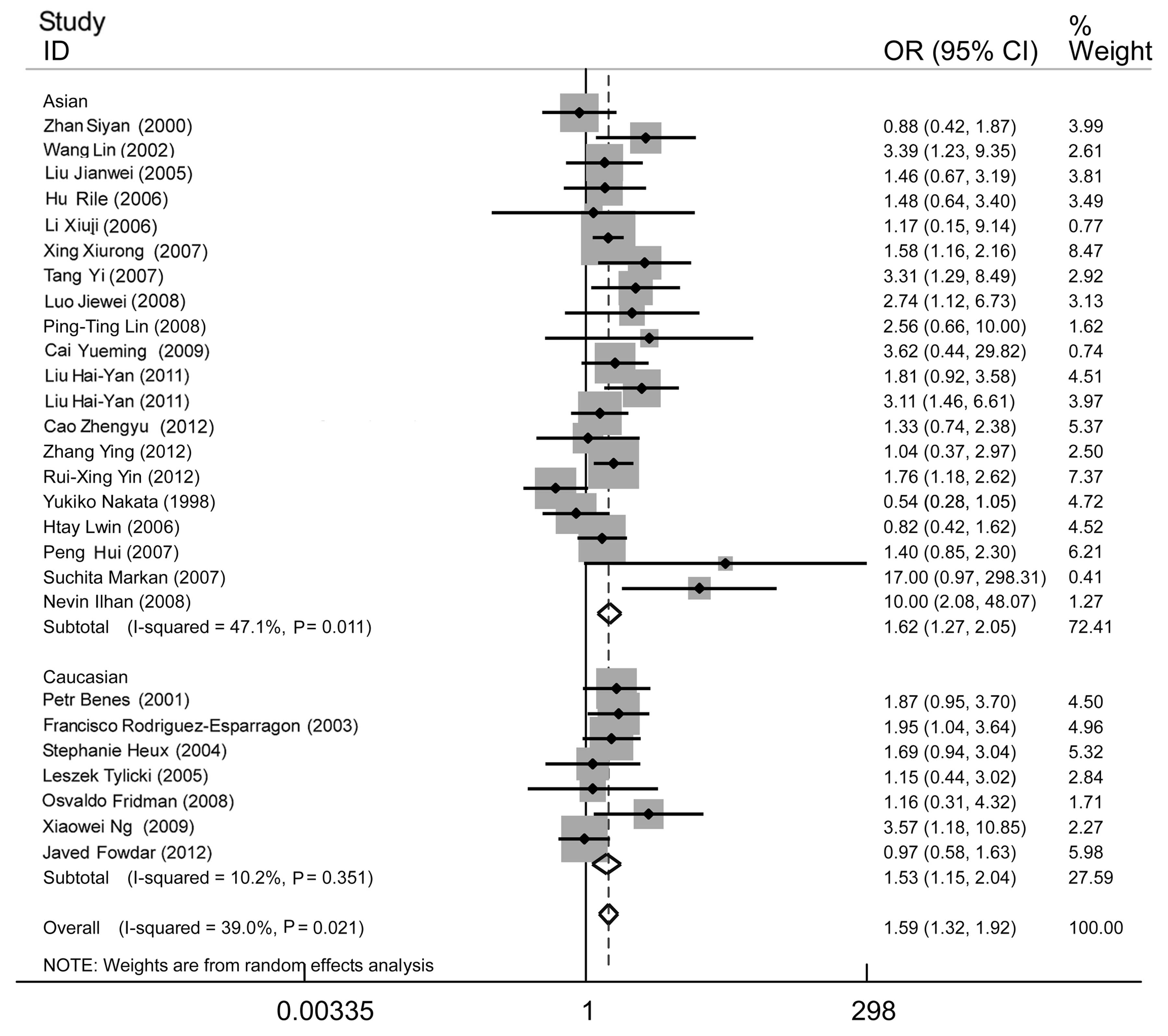 | Figure 5Forest plot of essential hypertension
associated with the MTHFR C677T gene polymorphism under the
homozygote model (TT/CC). A significant association was revealed in
the whole population (OR, 1.59; 95% CI, 1.32–1.92), Asian subgroup
(OR, 1.62; 95% CI, 1.27–2.05) and Caucasian subgroup (OR, 1.53; 95%
CI, 1.15–2.04). The heterogeneity test was significant in the whole
population (I2=39.0%, P=0.021) and Asian subgroup
(I2=47.1%, P=0.011), whereas the Caucasian subgroup
showed no significant heterogeneity (I2=10.2%, P=0.351).
MTHFR, methylenetetrahydrofolate reductase; OR, odds ratio; CI,
confidence interval. |
 | Table IISummary of the meta-analysis results
of the association between the MTHFR C677T gene polymorphism
and essential hypertension under the different genetic models. |
Table II
Summary of the meta-analysis results
of the association between the MTHFR C677T gene polymorphism
and essential hypertension under the different genetic models.
| Genetic model | Group | Pool OR (95%
CI) | P-value | Heterogeneity | Studies number | Case size, n | Control size,
n |
|---|
|
|---|
| I2,
% | P-value |
|---|
| Allelic | Whole
population | 1.32
(1.20–1.45) | 0.000a | 54.7 | 0.000a | 27 | 5418 | 4997 |
| Asian | 1.37
(1.21–1.54) | 0.000a | 60.4 | 0.000a | 20 | 4201 | 3675 |
| Caucasian | 1.19
(1.05–1.36) | 0.007a | 12.7 | 0.333 | 7 | 1217 | 1322 |
| Dominant | Whole
population | 1.39
(1.25–1.55) | 0.000a | 35.6 | 0.036a | 27 | 5418 | 4997 |
| Asian | 1.47
(1.28–1.68) | 0.000a | 39.9 | 0.034a | 20 | 4201 | 3675 |
| Caucasian | 1.19
(1.02–1.40) | 0.031a | 0.0 | 0.654 | 7 | 1217 | 1322 |
| Recessive | Whole
population | 1.38
(1.18–1.62) | 0.000a | 29.2 | 0.079 | 27 | 5418 | 4997 |
| Asian | 1.37
(1.12–1.68) | 0.002a | 37.6 | 0.047a | 20 | 4201 | 3675 |
| Caucasian | 1.42
(1.10–1.84) | 0.008a | 2.6 | 0.406 | 7 | 1217 | 1322 |
| Homozygote | Whole
population | 1.59
(1.32–1.92) | 0.000a | 39.0 | 0.021a | 27 | 5418 | 4997 |
| Asian | 1.62
(1.27–2.05) | 0.000a | 47.1 | 0.011a | 20 | 4201 | 3675 |
| Caucasian | 1.53
(1.15–2.04) | 0.004a | 10.2 | 0.351 | 7 | 1217 | 1322 |
| Heterozygote | Whole
population | 1.32
(1.20–1.45) | 0.000a | 9.7 | 0.321 | 27 | 5418 | 4997 |
| Asian | 1.40
(1.25–1.56) | 0.000a | 12.7 | 0.296 | 20 | 4201 | 3675 |
| Caucasian | 1.13
(0.96–1.34) | 0.152 | 0.0 | 0.878 | 7 | 1217 | 1322 |
A subsequent subgroup analysis stratified by
ethnicity was conducted in which a significant association between
the MTHFR C677T gene polymorphism and EH was revealed in the
Asian and Caucasian subgroups. The association was evident in all
genetic models in the Asian subgroup (allelic: OR, 1.37; 95% CI,
1.21–1.54; P=0.000; dominant: OR, 1.47; 95% CI, 1.28–1.68; P=0.000;
recessive: OR, 1.37; 95% CI, 1.12–1.68; P=0.002; homozygote: OR,
1.62; 95% CI, 1.27–2.05; P=0.000; and heterozygote: OR, 1.40; 95%
CI, 1.25–1.56; P=0.000), whereas in the Caucasian subgroup an
association was apparent under all models, except the heterozygote
model (allelic: OR, 1.19; 95% CI, 1.05–1.36; P=0.007; dominant: OR,
1.19; 95% CI, 1.02–1.40; P=0.031; recessive OR, 1.42; 95% CI,
1.10–1.84; P=0.008; homozygote: OR, 1.53; 95% CI, 1.15–2.04;
P=0.004; and heterozygote: OR, 1.13; 95% CI, 0.96–1.34; P=0.152).
The association detected in the Asian group was stronger compared
to the Caucasian group under all genetic models, with the exception
of the recessive model (Figs.
2–5 and Table II).
Heterogeneity analysis
The heterogeneity test was significant under the
allelic (I2=54.7%, P=0.000), dominant
(I2=35.6%, P=0.036) and homozygote (I2=39%,
P=0.021) models. Following the subgroup analysis, there was no
significant heterogeneity observed in the Caucasian subgroup,
whereas it remained apparent in the Asian subgroup (Figs. 2–5
and Table II). A further subgroup
analysis in the Asian group was considered by stratification of
Chinese, Japanese and others (Indian and Turk). Heterogeneity was
not evident in any subgroup under the different genetic models,
indicating that population origin may be one explanation for the
between-study heterogeneity (Figs.
6–7 and Table III).
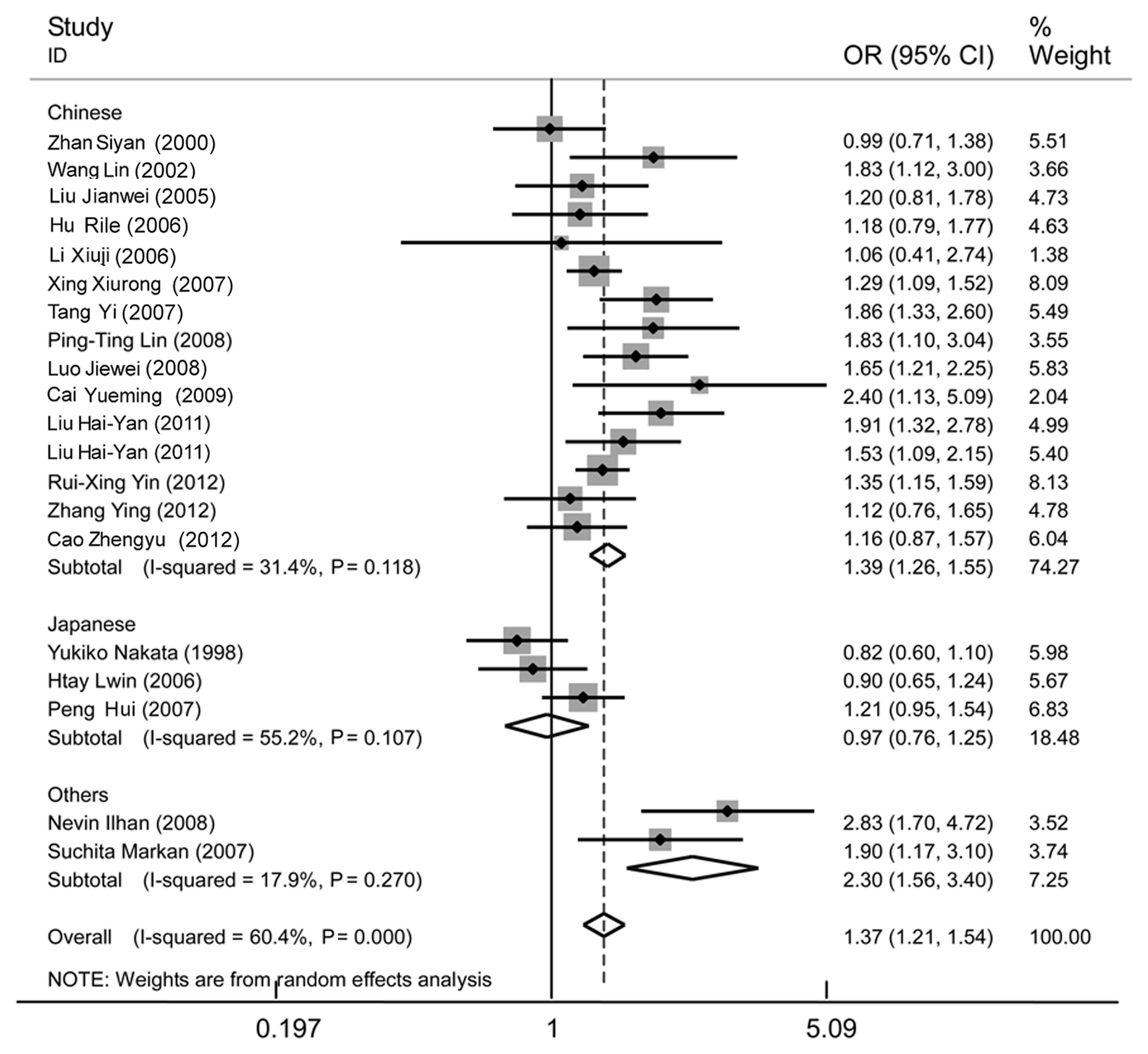 | Figure 6Forest plot of essential hypertension
associated with the MTHFR C677T gene polymorphism in the
Asian population under the allelic model (T/C) stratified by
different populations, including Chinese, Japanese and others
(Indian and Turk). A significant association was revealed in the
Asian population (OR, 1.37; 95% CI, 1.21–1.54), as well as the
Chinese (OR, 1.39; 95% CI, 1.26–1.55) and the others (OR, 2.3; 95%
CI, 1.56–3.40) subgroups. No significant association was found in
the Japanese subgroup (OR, 0.97; 95% CI, 0.76–1.25). A significant
heterogeneity was detected in the Asian group (I2=60.4%,
P=0.000), but no longer existed in the Chinese
(I2=31.4%, P=0.118), Japanese (I2=55.2%,
P=0.107) and others (I2=17.9%, P=0.270) subgroup,
through subgroup analysis. MTHFR, methylenetetrahydrofolate
reductase; OR, odds ratio; CI, confidence interval. |
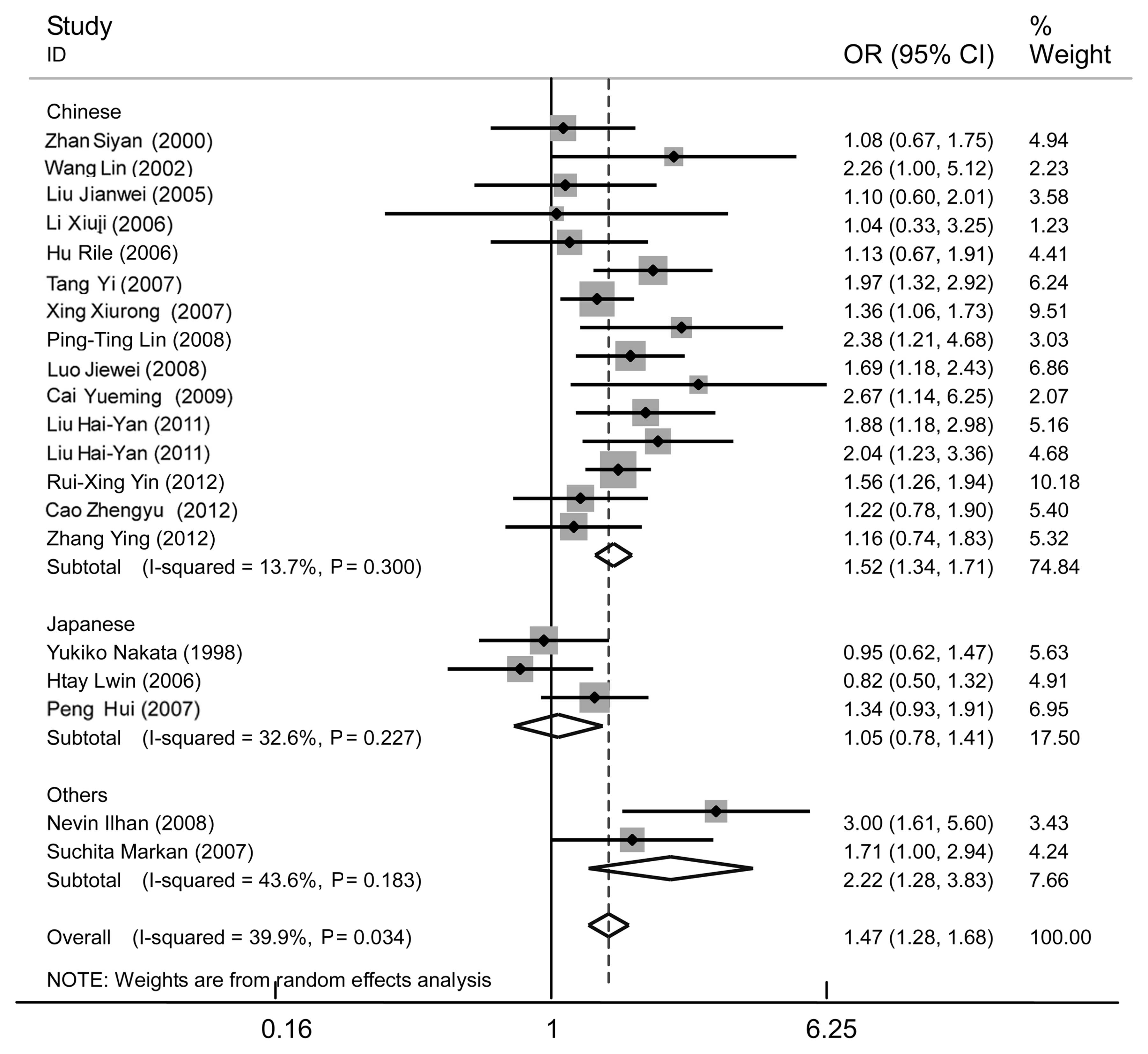 | Figure 7Forest plot of essential hypertension
associated with the MTHFR C677T gene polymorphism in the
Asian population under the dominant model (TT + CT/CC) stratified
by different populations; Chinese, Japanese and others (Indian and
Turk). A strong association was revealed in the Asian population
(OR, 1.47; 95% CI, 1.28–1.68), as well as the Chinese (OR, 1.52;
95% CI, 1.34–1.71) and others (OR, 2.22; 95% CI, 1.28–3.83)
subgroups. No significant association was found in the Japanese
subgroup (OR, 1.05; 95% CI, 0.78–1.41). Moderate heterogeneity was
apparent in the Asian population (I2=39.9%, P=0.034),
whereas no significant heterogeneity was detected in the Chinese
(I2=13.7%, P=0.300), Japanese (I2=32.6%,
P=0.227) and others (I2=43.6%, P=0.183) subgroups.
MTHFR, methylenetetrahydrofolate reductase; OR, odds ratio; CI,
confidence interval. |
 | Table IIISummary of the subgroup analysis
results under different genetic models in different Asian
populations. |
Table III
Summary of the subgroup analysis
results under different genetic models in different Asian
populations.
| Genetic model | Group | Pool OR (95%
CI) | P-value | Heterogeneity | Studies number | Case size, n | Control size,
n |
|---|
|
|---|
| I2,
% | P-value |
|---|
| Allelic | Chinese | 1.39
(1.26–1.55) | 0.000a | 31.4 | 0.118 | 15 | 3420 | 2768 |
| Japanese | 0.97
(0.76–1.25) | 0.838 | 55.2 | 0.170 | 3 | 550 | 674 |
| Others (Indian and
Turk) | 2.30
(1.56–3.40) | 0.000a | 17.9 | 0.270 | 2 | 231 | 233 |
| Dominant | Chinese | 1.52
(1.34–1.71) | 0.000a | 13.7 | 0.300 | 15 | 3420 | 2768 |
| Japanese | 1.05
(0.78–1.41) | 0.755 | 32.6 | 0.227 | 3 | 550 | 674 |
| Others (Indian and
Turk) | 2.22
(1.28–3.83) | 0.004a | 43.6 | 0.183 | 2 | 231 | 233 |
| Recessive | Chinese | 1.45
(1.24–1.70) | 0.000a | 0.0 | 0.737 | 15 | 3420 | 2768 |
| Japanese | 0.85
(0.51–1.40) | 0.523 | 60.3 | 0.081 | 3 | 550 | 674 |
| Others (Indian and
Turk) | 8.58
(2.20–33.53) | 0.002a | 0.0 | 0.632 | 2 | 231 | 233 |
| Homozygote | Chinese | 1.71
(1.44–2.03) | 0.000a | 0.0 | 0.512 | 15 | 3420 | 2768 |
| Japanese | 0.88
(0.50–1.56) | 0.668 | 61.8 | 0.073 | 3 | 550 | 674 |
| Others (Indian and
Turk) | 11.30
(2.85–44.79) | 0.001a | 0.0 | 0.742 | 2 | 231 | 233 |
The further subgroup analysis also revealed a strong
association between the MTHFR C677T gene polymorphism and EH
in the Chinese population (allelic: OR, 1.39; 95% CI, 1.26–1.55;
P=0.000; dominant: OR, 1.52; 95% CI, 1.34–1.71; P=0.000; recessive:
OR, 1.45; 95% CI, 1.24–1.70; P=0.000; and homozygote: OR, 1.71; 95%
CI, 1.44–2.03; P=0.000). None of these genetic models suggested any
association between MTHFR 677T and EH among the Japanese
population (Figs. 6–7 and Table
III).
In addition, to explore other potential sources of
heterogeneity, a random-effects meta-regression analysis including
all 27 studies was conducted under the allelic, dominant and
homozygote models. The publication year, population origin (Chinese
as 1; Japanese as 2; other Asian, including Indian and Turk, as 3;
and Caucasian as 4), case sample size, control sample size, total
sample size, the ratio of case and control sample, the percentage
of males, age of cases and controls, study design [hospital-based
(HB) as 1 and population-based (PB) as 2] and genotyping method
[polymerase chain reaction (PCR)-restriction fragment length
polymorphism as 1; others, including Sequenom and real-time
fluorescence quantitative-PCR, as 2] were considered as independent
variables. In contrast to the subgroup analysis, no association of
population origin was observed under any genetic model. However, a
significant and marginal association of study design was revealed
under the dominant (P=0.035) and allelic genetic models (P=0.068),
respectively. Therefore, another stratified analysis was conducted
by organizing studies into HB or PB studies under the dominant and
allelic genetic models. There was no significant heterogeneity in
the PB subgroup under the genetic models (Table IV), whereas the HB subgroup yielded
a significance under the allelic genetic model indicating that
study design may play a role in heterogeneity. Additionally, an
association between the MTHFR C677T gene polymorphism and EH
was found to be stronger in the HB studies compared to the PB
studies.
 | Table IVSummary of the subgroup analysis
results of all included studies stratified by study design. |
Table IV
Summary of the subgroup analysis
results of all included studies stratified by study design.
| Subgroup | Allelic genetic
model | Dominant genetic
model | Studies number | Case size, n | Control size,
n |
|---|
|
|
|---|
| OR (95% CI),
P-value | Heterogeneity | OR (95% CI),
P-value | Heterogeneity |
|---|
| HB | 1.45 (1.26–1.66),
P=0.000a |
I2=57.9%, P=0.002a | 1.55 (1.34–1.79),
P=0.000a |
I2=31.6%, P= 0.104 | 17 | 3198 | 2587 |
| PB | 1.17 (1.05–1.31),
P=0.006a |
I2=31.6%, P=0.156 | 1.22 (1.06–1.42),
P=0.007a |
I2=24.0%, P=0.222 | 10 | 2220 | 2410 |
| Overall | 1.32 (1.20–1.45),
P=0.000a |
I2=54.7%, P=0.000a | 1.39 (1.25–1.55),
P= 0.000a |
I2=35.6%, P=0.036a | 27 | 5418 | 4997 |
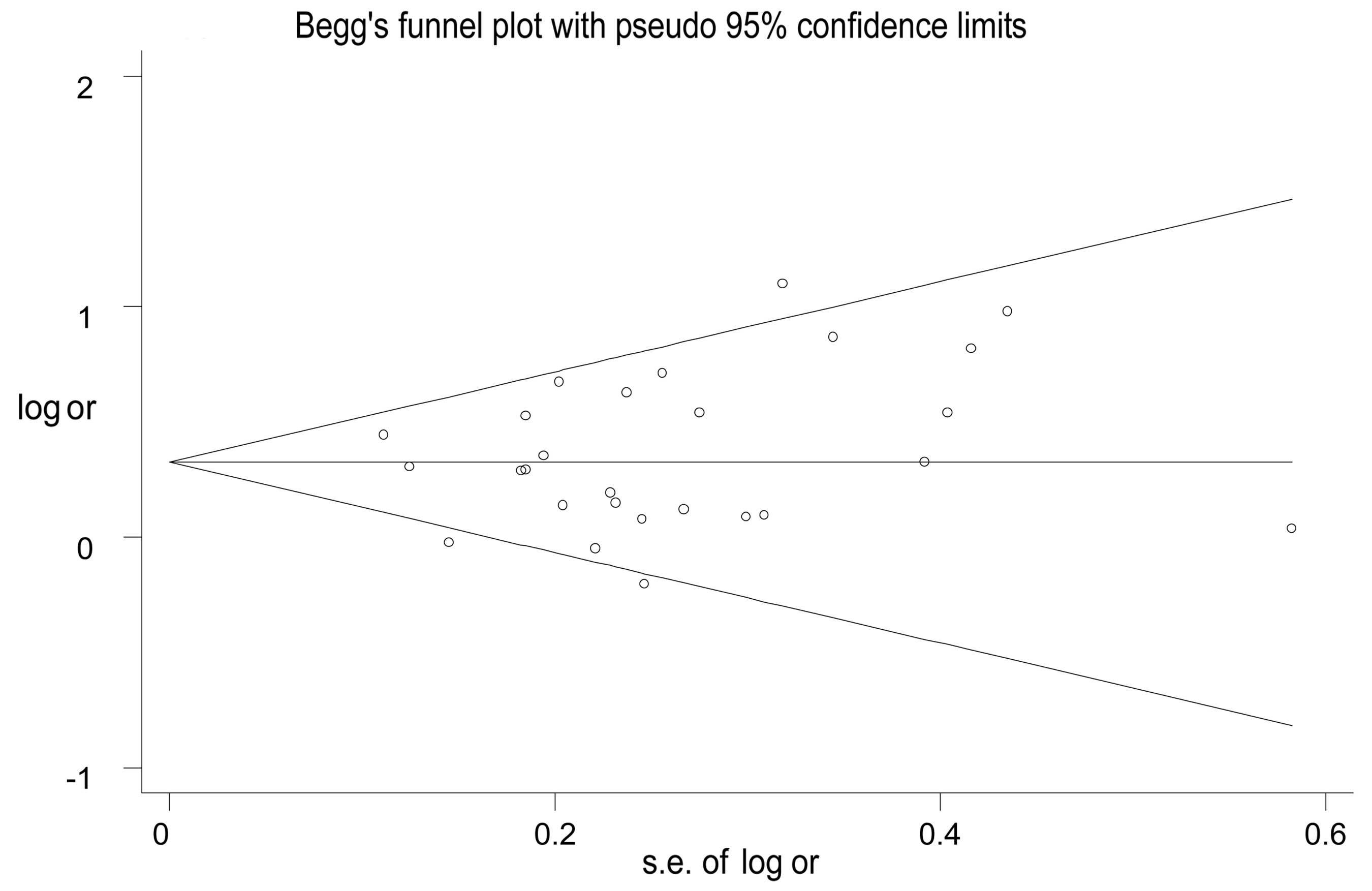
Publication bias
Publication bias was assessed by Begg’s funnel plot
and Egger’s test. The visible symmetrical funnel shape indicated no
apparent evidence of publication bias in the meta-analysis, which
is in accordance with the results of Egger’s test (allelic:
P=0.122; dominant: P=0.378, recessive: P=0.078, homozygote:
P=0.104; and heterozygote genetic model: P=0.550). There was no
significant difference revealed, which suggests a low probability
of publication bias (Fig. 8).
Discussion
Meta-analysis is an efficient method to obtain a
pooled effect of various studies with relatively small sample sizes
and to achieve more comprehensive conclusions. Several
meta-analyses that have been conducted in our Department of
Geriatrics (First Affiliated Hospital of Nanjing Medical
University, Nanjing, China) recently provided reliable results for
the association between different gene polymorphisms and
cardiovascular diseases (38,39).
The present meta-analysis, which involved 5,418 cases and 4,997
controls from 27 studies carried out worldwide, indicated a strong
association between the MTHFR C677T gene polymorphism and EH
in the whole population under the allelic (OR, 1.32; 95% CI,
1.20–1.45; P=0.000), dominant (OR, 1.39; 95% CI, 1.25–1.55;
P=0.000), recessive (OR, 1.38; 95% CI, 1.18–1.62; P=0.000),
homozygote (OR, 1.59; 95% CI, 1.32–1.92, P=0.000), and heterozygote
(OR, 1.32; 95% CI, 1.20–1.45; P=0.000) genetic models.
A significant association was detected in the Asian
and Caucasian groups (P<0.05) suggesting that carriers of 677T
or 677TT in these populations may suffer a significantly increased
risk of developing EH. In the further subgroup analysis of the
Asian population, stratified as Chinese, Japanese and others
(Indian, Turk), the significant heterogeneity no longer existed in
any subgroup suggesting that the population origin may contribute
to heterogeneity. Future studies should pay increasing attention to
the possible differences in genetic background of various
populations. In addition, the pooled analysis of 6,188 Chinese
subjects from 15 studies showed a strong association between the
MTHFR C677T gene polymorphism and EH, revealing that it was
a possible risk factor for EH in the Chinese population. There was
no apparent association between the MTHFR C677T gene
polymorphism and EH among the Japanese population. Considering that
only three studies were included in the subgroup, a meta-analysis
including more Japanese studies and future studies with larger
samples could be conducted to confirm this result. Additionally,
the study design was identified as an explanation for between-study
heterogeneity in the meta-regression analysis, as well as the
subgroup analysis. A stronger association together with greater
heterogeneity was observed in HB studies compared to PB studies.
This difference may be explained by the fact that control subjects
from hospitals tend to have more complicated health states and
confounding factors, whereas the controls selected from the general
population would be more representative. The poor comparability
between patients and controls in HB studies may contribute to the
heterogeneity and exaggerate the association of the MTHFR
677T gene polymorphism and EH to a certain degree (40).
Two meta-analyses, by Qian et al (41) and Niu et al (42), were conducted previously in the
whole population and in the Chinese population, respectively.
Compared to these studies, the present meta-analysis had
substantial advantages in the following aspects: First, only nine
studies with 1,520 patients and 1,334 controls in the study by Niu
et al (42) and 12 out of 25
studies in the study by Qian et al (41) were studies regarding the association
between the MTHFR C677T gene polymorphism and EH. The
remaining studies in the two meta-analyses assessed hypertension in
pregnancy (HIP). Considering the multiple pathogenesis and
complicated physiopathological changes in HIP cases, it is not
suitable to analyze the pooled effect of the association between
the MTHFR C677T gene polymorphism and hypertension with HIP
patients. Therefore, with an increase of 18 studies from the latest
meta-analysis regarding the association between MTHFR C677T
and EH (42), the present study
assessed 27 eligible studies with a total of 5,418 EH cases and
4,997 controls. This provides more reliable results with less
clinical heterogeneity. One overlapping study and particular
studies of secondary hypertension, which were included previously,
were excluded. Second, compared to the unexplained heterogeneity of
the Asian subgroup in the Qian et al (41) study, the heterogeneity of the Asian
population no longer existed in the Chinese, Japanese and other
(Indian and Turk) subgroups following stratified analysis in the
present study. This suggested that the population origin may be one
reason for heterogeneity. Third, in terms of meta-regression, while
only a marginal association (P=0.082) of study design was observed
in the Niu et al (42)
study, the association in the present study was significant
(P=0.035). This may be due to the relatively few included studies
in the study by Niu et al, and studies involved in
meta-regression should usually be ≥10 (43). Therefore, with stronger evidence,
the present meta-analysis demonstrated the effect of study design
in heterogeneity. Finally, the present meta-analysis involved a
wide range of various populations and the pooled effect of 15
Chinese studies with 3,420 cases and 2,768 controls was also
revealed in the subgroup analysis. For this reason, the study
renewed the two previous meta-analyses with additional supplements
and appropriate adjustments, and thus, to the best of our
knowledge, becoming the most comprehensive meta-analysis regarding
the association between MTHFR C677T and EH.
The present meta-analysis, although notably having
advantages, such as large sample sizes, reliable statistical
results and sound heterogeneity management, has certain
limitations. First, large-scale studies on the association between
the MTHFR gene polymorphism and EH are inadequate. Second,
as discussed previously, the MTHFR C677T gene mutation is
postulated to play a role in developing EH through generating
hyperhomocysteinemia. However, the level of plasma Hcy is also
influenced by certain environmental factors, including lack of
folate and vitamin B6 in the diet, and is associated with plasma
folate levels (8,26). The interaction between gene and
environment was not taken into account. Therefore, future
prospective cohort studies with large sample sizes should be
performed to confirm these results and studies should include these
considerations.
In conclusion, the present meta-analysis, including
27 studies with 10,415 subjects, indicates that the MTHFR
C677T gene polymorphism is associated with increased EH risks in
different populations as a whole, as well as in separate subgroups,
such as Asian, Caucasian and Chinese. Carriers of the 677T allele
are susceptible to EH. MTHFR C677T should therefore be
tested for as a predictive screening for the identification of the
individuals that may develop EH in the future. As an improvement on
the previous studies with noteworthy reinforcement and development,
the present results may potentially provide a stronger foundation
for the identification of the mechanism through which the
MTHFR gene may play a role in developing EH. More studies
should be conducted to confirm the conclusion, and polymorphisms
within the same linkage disequilibrium area should be focused on as
well.
Acknowledgements
The authors would like to thank all the subjects
participating in the present study. The study was supported by the
‘six elite peaks’ project, funded by the Jiangsu Province
Government, China (grant no. 2008-329).
References
|
1
|
Mein CA, Caulfield MJ, Dobson RJ and
Munroe PB: Genetics of essential hypertension. Hum Mol Genet.
13:R169–R175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dominiczak AF, Negrin DC, Clark JS,
Brosnan MJ, McBride MW and Alexander MY: Genes and hypertension:
from gene mapping in experimental models to vascular gene transfer
strategies. Hypertension. 35:164–172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lupton SJ, Chiu CL and Lind JM: A
hypertension gene: are we there yet? Twin Res Hum Genet.
14:295–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Men C, Tang K, Lin G, Li J and Zhan Y:
ENOS-G894T polymorphism is a risk factor for essential hypertension
in China. Indian J Biochem Bio. 48:154–157. 2011.PubMed/NCBI
|
|
5
|
Goyette P, Pai A, Milos R, et al: Gene
structure of human and mouse methylenetetrahydrofolate reductase
(MTHFR). Mamm Genome. 9:652–656. 1998. View Article : Google Scholar
|
|
6
|
Frosst P, Blom HJ, Milos R, et al: A
candidate genetic risk factor for vascular disease: a common
mutation in methylenetetrahydrofolate reductase. Nat Genet.
10:111–113. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tawakol A, Omland T, Gerhard M, Wu JT and
Creager MA: Hyperhomocyst(e)inemia is associated with impaired
endothelium-dependent vasodilation in humans. Circulation.
95:1119–1121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heux S, Morin F, Lea RA, Ovcaric M,
Tajouri L and Griffiths LR: The methylentetrahydrofolate reductase
gene variant (C677T) as a risk factor for essential hypertension in
Caucasians. Hypertens Res. 27:663–667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li YY: Methylenetetrahydrofolate reductase
C677T gene polymorphism and coronary artery disease in a Chinese
Han population:a meta-analysis. Metabolism. 61:846–852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alam MA, Husain SA, Narang R, Chauhan SS,
Kabra M and Vasisht S: Association of polymorphism in the
thermolabile 5, 10-methylene tetrahydrofolate reductase gene and
hyperhomocysteinemia with coronary artery disease. Mol Cell
Biochem. 310:111–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Homocysteine Studies Collaboration.
Homocysteine and risk of ischemic heart disease and stroke: a
meta-analysis. JAMA. 288:2015–2022. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodríguez-Esparragón F, Hernández-Perera
O, Rodríguez- Pérez J, et al: The effect of
methylenetetrahydrofolate reductase C677T common variant on
hypertensive risk is not solely explained by increased plasma
homocysteine values. Clin Exp Hypertens. 25:209–220.
2003.PubMed/NCBI
|
|
13
|
Lin PT, Cheng CH, Wei JC and Huang YC: Low
plasma pyridoxal 5′-phosphate concentration and MTHFR 677C-->T
genotypes are associated with increased risk of hypertension. Int J
Vitam Nutr Res. 78:33–40. 2008.
|
|
14
|
Yin RX, Wu JZ, Liu WY, et al: Association
of several lipid-related gene polymorphisms and blood pressure
variation in the Bai Ku Yao population. Am J Hypertens. 25:927–936.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fowdar JY, Lason MV, Szvetko AL, Lea RA
and Griffiths LR: Investigation of homocysteine-pathway-related
variants in essential hypertension. Int J Hypertens.
2012:1909.232012.PubMed/NCBI
|
|
16
|
Nakata Y, Katsuya T, Takami S, et al:
Methylenetetrahydrofolate reductase gene polymorphism relation to
blood pressure and cerebrovascular disease. Am J Hypertens.
11:1019–1023. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu HY, Ma P and Xu QB: The correlation
between polymorphisms of N5,10 methylenetetrahydrofolate reductase
and essential hypertension in Han population in Ningxia. Guangdong
Med J. 32:1977–1980. 2011.
|
|
18
|
Liu H, Chen S, Ma P and Xu Q: The
association between gene polymorphisms of
N5,10-methylenetetrahydrofolate reductase and essential
hypertension in patients of Ningxia Hui nationality. Tianjin Med J.
39:1095–1098. 2011.
|
|
19
|
Cai YM and Gong WX: Linkage study on
methylenetetrahydrofolate reductase single nucleotide polymorphisms
and hypertension in the elderly with rheumatoid arthritis. Chin J
Birth Health and Heredity. 17:14–17. 2009.
|
|
20
|
Luo JW, Tang Y, Chen H, Wu XY, Wu YN and
Deng YL: Study on MTHFR C677T polymorphism in hypertensive subjects
with blood stasis syndrome. J Beijing Univ Tradit Chin Med.
31:351–354. 2008.
|
|
21
|
Xing X and Hua Q: Relationships between
the polymorphism of methylenetetrahydrofolate reductase gene C677T
and hypertension, cardiac structure and function. Med J Chin PLA.
32:741–744. 2007.
|
|
22
|
Tang Y, Chen H, Wu XY and Luo JW: The
C677T point mutation of N5,10-methylenetetrahydrofolate reductase
(MTHFR) and essential hypertension. Mol Cardiol China. 7:205–207.
2007.
|
|
23
|
Wang L, Guo H and Li Y: MTHFR gene C 677 T
polymorphisms and variation of plasma homocysteine level in primary
hypertension. Tianjin Med J. 30:579–582. 2002.
|
|
24
|
Ilhan N, Kucuksu M, Kaman D, Ilhan N and
Ozbay Y: The 677 C/T MTHFR polymorphism is associated with
essential hypertension, coronary artery disease, and higher
homocysteine levels. Arch Med Res. 39:125–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Markan S, Sachdeva M, Sehrawat BS, Kumari
S, Jain S and Khullar M: MTHFR 677 CT/MTHFR 1298 CC genotypes are
associated with increased risk of hypertension in Indians. Mol Cell
Biochem. 302:125–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ng X, Boyd L, Dufficy L, et al: Folate
nutritional genetics and risk for hypertension in an elderly
population sample. J Nutrigenet Nutrigenomics. 2:1–8. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fridman O, Porcile R, Vanasco V, Junco MN,
Gariglio L, et al: Study on homocysteine levels and
methylenetetrahydrofolate reductase gene variant (C677T) in a
population of Buenos Aires City. Clin Exp Hypertens. 30:574–584.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhan S, Gao YY, Yin X, et al: Elevated
serum homocysteine, MTHFR gene mutataion and essential hypertension
in Chinese. Chin J Hypertens. 8:21–25. 2000.
|
|
29
|
Lwin H, Yokoyama T, Yoshiike N, et al:
Polymorphism of methylenetetrahydrofolate reductase gene (C677T
MTHFR) is not a confounding factor of the relationship between
serum uric acid level and the prevalence of hypertension in
Japanese men. Circ J. 70:83–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Wang H, Zhang X, Wang L and Wu G:
Relationship between homocysteine, methylene tetrahydrofolate
reductase C677T polymorphisms and essential hypertension in Kazak
nationality in Xinjiang. J Clin Cardiol (China). 28:570–573.
2012.
|
|
31
|
Cao Z: The Association between
methylenetetrahydrofolate reductase gene polymorphism and
hypertensive elderly patient with acute myocardial infarction. Chin
J Gerontol. 32:5118–5120. 2012.
|
|
32
|
Li X and Huang W: The analysis of MTHFR
gene polymorphism in patients with renal damage caused by
hypertension and patients with renal parenchymal hypertension. J
Capital Univ Med Sci. 27:497–500. 2006.
|
|
33
|
Hu R, Niu GM, Zhao SG, Zhang CY, Hu RL,
Wang ZG and Jiang MF: The association between gene polymorphisms of
N5, 10-methylenetetrahydrofolate reductase and Mongolian patients
with drimary hypertension. Chin J Hypertension. 14:274–276.
2006.
|
|
34
|
Liu JW, Ye L, Liu J and Li XY: Study on
homocysteine metabolism-related enzymes gene polymorphisms in
elderly essential hypertension patients with peripheral arterial
occlusive disease. Chin J Geriatr. 24:332–335. 2005.
|
|
35
|
Hui P, Nakayama T, Morita A, et al: Common
single nucleotide polymorphisms in Japanese patients with essential
hypertension: aldehyde dehydrogenase 2 gene as a risk factor
independent of alcohol consumption. Hypertens Res. 30:585–591.
2007. View Article : Google Scholar
|
|
36
|
Tylicki L, Födinger M, Puttinger H, et al:
Methylenetetrahydrofolate reductase gene polymorphisms in essential
hypertension. Am J Hypertens. 18:1442–1448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Benes P, Kanková K, Muzík J, Groch L and
Benedik J: Methylenetetrahydrofolate reductase polymorphism, type
II diabetes mellitus, coronary artery disease, and essential
hypertension in the Czech population. Mol Genet Metab. 73:188–195.
2001. View Article : Google Scholar
|
|
38
|
Li YY: α-Adducin Gly460Trp gene mutation
and essential hypertension in a Chinese population: a meta-analysis
including 10,960 subjects. PLoS One. 7:e302142012.
|
|
39
|
Li Y: Plasminogen activator inhibitor-1
4G/5G gene polymorphism and coronary artery disease in the Chinese
Han population: a meta-analysis. PLoS One. 7:e335112012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ruano-Ravina A, Pérez-Ríos M and
Barros-Dios JM: Population-based versus hospital-based controls:
are they comparable? Gac Sanit. 22:609–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qian X, Lu Z, Tan M, Liu H and Lu D: A
meta-analysis of association between C677T polymorphism in the
methylenetetrahydrofolate reductase gene and hypertension. Eur J
Hum Genet. 15:1239–1245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Niu WQ, You YG and Qi Y: Strong
association of methylenetetrahydrofolate reductase gene C677T
polymorphism with hypertension and hypertension-in-pregnancy in
Chinese: a meta-analysis. J Hum Hypertens. 26:259–267. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Higgins J, Thompson S, Deeks J and Altman
D: Statistical heterogeneity in systematic reviews of clinical
trials: a critical appraisal of guidelines and practice. J Health
Serv Res Policy. 7:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|