Introduction
Clostridium difficile (C. difficile)
has been associated with a wide range of diseases, including toxic
megacolon, nosocomial diarrhea and pseudomembranous colitis
(1,2). Toxigenic and epidemic C.
difficile is a well-established health threat in the nosocomial
environment. Several studies have shown that C. difficile
causes community-acquired infections and it has been isolated from
various human, animal, food and environmental sources, often with
similar genetic profiles (3,4). The
pathogenicity of C. difficile is associated with its ability
to produce two toxins: Toxin A, an enterotoxin; and toxin B, a
potent cytotoxin (5), which are
responsible for the cellular damage linked to diseases. The
tcdA and tcdB genes that encode toxins A and B,
respectively, are located in the pathogenicity locus
(PaLoc), along with the positive and negative regulator
genes, tcdD and tcdC, respectively (6). Several polymerase chain reaction (PCR)
methods have been developed to detect the tcdA and
tcdB genes and there have been certain studies of the toxin
gene diversity, molecular epidemiology and antimicrobial resistance
of C. difficile isolated from hospitals. However, thus far,
limited data are available on the toxin gene diversity and
antimicrobial susceptibilities of the bacterium isolated from C.
difficile infection (CDI) patients in China. In the present
study, C. difficile isolates were analyzed from patients in
the Central Hospital of Taizhou City (Taizhou, China) for the
presence of the tcdD, tcdC, cdtA and
cdtB genes and the expression patterns were examined via
quantitative PCR (qPCR). Additionally, the susceptibility of the
C. difficile profiles to 12 antimicrobial agents, including
nemonoxacin and tigecycline, were investigated.
Materials and methods
Identification of C. difficile
isolates
The faecal samples were collected from various
departments of the Taizhou Central Hospital. Isolation of C.
difficile was performed on selective Columbia agar supplemented
(bioMerieux Co., Ltd., Shanghai, China) with cycloserine-cefoxitin
and amphotericin B (Bayer Co., Ltd., Shanghai, China) as described
previously (7). Briefly, the plates
were incubated in an anaerobic chamber at 37°C for 72 h. The C.
difficile isolates were identified by colony morphology, Gram
staining, odor and green-yellow fluorescence under UV light (365
nm).
PCR assays
All the PCR reactions were performed with a positive
and negative control using the primers (Table I) described by previous studies
(8,9). PCR was conducted with 2.5 pl cDNA and
15 pmol of each primer pair in a total volume of 50 p1 with 1 unit
of Taq DNA polymerase (Takara Bio, Inc., Shiga, Japan) in a
standard reaction mixture. Amplification was achieved by denaturing
at 95°C (1 min), primer annealing at 52°C (1 min) and extension at
72°C (1 min), which were repeated for 30 cycles.
 | Table IPrimers used in the present
study. |
Table I
Primers used in the present
study.
| Primers | Oligonucleotide
sequence (5′→3′) |
|---|
|
tcdA-PCR-F |
CCCAATAGAAGATTCAATATTAAGCTT |
|
tcdA-PCR-R |
GGAAGAAAAGAACT
TCTGGCTCACTCAGGT |
|
tcdB-PCR-F |
GGTGGAGCTGCTTCATTGGAGAG |
|
tcdB-PCR-R |
GTGTAACCTACTTTCATAACACCA |
|
tcdA-qPCR-F |
TCTACCACTGAAGCATTAC |
|
tcdA-qPCR-R |
TAGGTACTGTAGGTTTATTG |
|
tcdB-qPCR-F |
ATATCAGAGACTGATGAG |
|
tcdB-qPCR-R |
TAGCATATTCAGAGAATATTG |
|
tcdC-qPCR-F |
TCTCTACAGCTATCCCTGGT |
|
tcdC-qPCR-R |
AAAAATGAGGGTAACGAATTT |
|
tcdD-qPCR-F |
CTCAGTAGATGATTTGCAAGAA |
|
tcdD-qPCR-R |
TTTTAAATGCTCTATTTTTAGCC |
RNA extraction, cDNA synthesis and qPCR
assays
cDNA synthesis was performed as described previously
by Frias-Lopez et al (9).
RNA was extracted from cultured bacterial cells using AllPrep
DNA/RNA mini kit (Qiagen, Hilden, Germany) following the
manufacturer’s instructions and 2–3 μg RNA was expected to be
obtained. To eliminate the potential contamination by DNA, the
TURBO DNA-free™ kit was utilized (Applied Biosystems, Foster City,
CA, USA) following the manufacturer’s instructions. Subsequently,
RNA was reverse transcribed into first strand cDNA using the
SuperScript III First-Stand Synthesis SuperMix kit (Invitrogen Life
Technologies, Carlsbad, CA, USA) and random hexamer priming. qPCR
was performed using the KAPA SYBR FAST qPCR kit (Kapa Biosystems,
Woburn, MA, USA) following the manufacturer’s instructions:
Denaturing at 95°C (1 min), primer annealing at 52°C (1 min) and
extension at 72°C (1 min), repeated for 30 cycles. The increase in
fluorescence was measured in real-time during the extension step.
The primers used are listed in (Table
I). The ΔCt values for each sample between the toxin genes and
16S rRNA were calculated and are listed in Table II. The relative expression levels
of tcdC and tcdD were calculated based on the ΔCt
values between the two genes and 16S rRNA.
 | Table IIPCR and qPCR detection of the
tcdA and tcdB genes. |
Table II
PCR and qPCR detection of the
tcdA and tcdB genes.
| | | qPCR |
|---|
| | |
|
|---|
| | PCR | tcdA | tcdB |
|---|
| |
|
|
|
|---|
| Isolates | Unit | tcdA | tcdB | ΔCt1 | ΔCt2 | ΔCt3 | ΔCt1 | ΔCt2 | ΔCt3 |
|---|
| 1 | DN | + | + | 2.90 | 2.75 | 2.66 | 5.76 | 5.33 | 5.53 |
| 2 | DN | + | + | 2.11 | 2.23 | 2.83 | 4.32 | 4.36 | 4.57 |
| 3 | DN | + | + | 1.56 | 1.58 | 1.63 | 5.23 | 5.55 | 5.34 |
| 4 | DN | + | + | 2.52 | 2.67 | 2.54 | 2.98 | 2.75 | 2.54 |
| 5 | DN | + | + | 2.70 | 2.65 | 2.63 | 4.87 | 4.54 | 4.34 |
| 6 | DN | + | + | 2.55 | 2.44 | 2.48 | 3.21 | 3.45 | 3.65 |
| 7 | DN | + | + | 2.33 | 2.35 | 2.37 | 3.35 | 3.45 | 3.37 |
| 8 | DN | + | + | 3.64 | 3.70 | 3.72 | 3.34 | 3.75 | 3.44 |
| 9 | DN | + | + | 2.31 | 2.11 | 2.34 | 5.43 | 5.46 | 5.67 |
| 10 | DN | + | + | 1.06 | 1.05 | 0.91 | 4.83 | 4.92 | 4.44 |
| 11 | DN | + | + | 1.01 | 1.09 | 1.13 | 5.43 | 5.55 | 5.57 |
| 12 | ICU | + | + | 0.05 | 0.03 | 0.02 | 6.71 | 6.32 | 6.43 |
| 13 | ICU | + | + | 2.13 | 2.14 | 2.23 | 5.31 | 5.21 | 5.55 |
| 14 | DN | + | + | 2.37 | 2.39 | 2.30 | 5.73 | 5.77 | 5.78 |
| 15 | DN | + | + | 3.68 | 3.72 | 3.71 | 4.32 | 4.33 | 4.37 |
| 16 | DN | + | + | 2.97 | 2.95 | 2.92 | 3.21 | 3.22 | 3.34 |
| 17 | DN | + | + | 3.21 | 3.24 | 3.26 | 2.34 | 2.37 | 2.39 |
| 18 | DN | + | + | 1.09 | 1.11 | 1.13 | 1.29 | 1.27 | 1.25 |
| 19 | DN | + | + | 1.21 | 1.22 | 1.19 | 2.32 | 2.35 | 2.42 |
| 20 | DN | + | + | 1.24 | 1.29 | 1.27 | 2.55 | 2.57 | 2.60 |
| 21 | DD | + | + | 0.36 | 0.34 | 0.39 | 3.21 | 2.98 | 3.02 |
| 22 | DD | + | + | 1.02 | 1.03 | 1.05 | 4.56 | 4.57 | 4.58 |
| 23 | DD | + | + | 2.01 | 2.07 | 1.98 | 2.22 | 2.25 | 2.29 |
| 24 | DD | + | + | 3.03 | 3.05 | 3.07 | 4.59 | 4.54 | 4.56 |
| 25 | DD | + | + | 4.51 | 4.55 | 4.52 | 3.37 | 3.41 | 3.21 |
| 26 | DD | + | + | 2.72 | 2.71 | 2.69 | 2.21 | 2.29 | 2.31 |
| 27 | DD | + | + | 1.67 | 1.72 | 1.69 | 3.98 | 3.96 | 3.92 |
| 28 | DD | + | + | 1.79 | 1.78 | 1.78 | 6.53 | 6.52 | 6.32 |
| 29 | DD | + | + | 0.77 | 0.78 | 0.80 | 5.90 | 5.78 | 5.32 |
| 30 | DD | + | + | 0.34 | 0.35 | 0.36 | 4.90 | 4.93 | 4.53 |
| 31 | DH | + | + | 0.56 | 0.57 | 0.59 | 3.98 | 3.77 | 3.63 |
| 32 | DH | + | + | 2.31 | 2.34 | 2.35 | 3.29 | 3.27 | 3.25 |
| 33 | DH | + | + | 3.41 | 3.42 | 3.42 | 4.53 | 4.52 | 4.50 |
| 34 | DH | + | + | 2.79 | 2.78 | 2.77 | 2.98 | 2.97 | 2.93 |
| 35 | DH | + | + | 1.56 | 1.58 | 1.54 | 1.95 | 1.92 | 1.93 |
| 36 | DH | + | + | 1.96 | 1.94 | 1.93 | 4.78 | 4.79 | 4.70 |
| 37 | DH | + | + | NA | NA | NA | 4.56 | 4.67 | 4.98 |
| 38 | DH | + | + | NA | NA | NA | 3.32 | 3.45 | 3.56 |
| 39 | DPE | − | + | NA | NA | NA | 5.97 | 5.67 | 5.88 |
| 40 | DPE | − | + | NA | NA | NA | 2.60 | 2.70 | 2.66 |
| 41 | ICU | − | + | NA | NA | NA | 3.01 | 3.04 | 2.34 |
| 42 | ICU | − | + | NA | NA | NA | 3.77 | 3.58 | 3.60 |
| 43 | ICU | − | + | NA | NA | NA | 3.62 | 3.64 | 3.65 |
| 44 | DH | − | + | NA | NA | NA | 4.21 | 4.22 | 4.23 |
| 45 | DH | − | + | NA | NA | NA | 5.32 | 5.33 | 5.34 |
| 46 | DRO | − | + | NA | NA | NA | 5.29 | 5.29 | 5.27 |
| 47 | DRO | − | + | NA | NA | NA | 3.55 | 3.57 | 3.59 |
| 48 | DRO | − | + | NA | NA | NA | 3.11 | 3.12 | 3.17 |
| 49 | DRO | − | + | NA | NA | NA | 4.19 | 4.17 | 4.16 |
| 50 | DRO | − | + | NA | NA | NA | 2.48 | 2.47 | 2.39 |
| 51 | DRO | − | + | NA | NA | NA | NA | NA | NA |
| 52 | DRO | − | + | NA | NA | NA | NA | NA | NA |
| 53 | ICU | − | − | NA | NA | NA | NA | NA | NA |
| 54 | ICU | − | − | NA | NA | NA | NA | NA | NA |
| 55 | DRO | − | − | NA | NA | NA | NA | NA | NA |
| 56 | DRO | − | − | NA | NA | NA | NA | NA | NA |
| 57 | DRO | − | − | NA | NA | NA | NA | NA | NA |
Antimicrobial susceptibility testing
The antimicrobial susceptibility tests were
performed with 57 C. difficile isolates as described
previously (10). Briefly, an
inoculum of 105 CFU bacteria was applied to each plate
with a glass replicator on supplemented Brucella blood agar
(11). The plates were incubated in
an anaerobic chamber for 48 h at 37°C. The minimal inhibitory
concentration (MIC) was defined as the lowest concentration of each
antimicrobial agent that inhibited the growth of the tested
isolate. The antimicrobial agents (Sigma-Aldrich, St. Louis, MO,
USA) used are listed in Table
III.
 | Table IIIMinimal inhibitory concentrations
(MICs) of 12 antimicrobial agents for the 57 Clostridium
difficile isolates. |
Table III
Minimal inhibitory concentrations
(MICs) of 12 antimicrobial agents for the 57 Clostridium
difficile isolates.
| MIC (mg/l) |
|---|
|
|
|---|
| Antimicrobial
agent | MIC50 | MIC90 | Range | Resistant, % |
|---|
| Vancomycin | 0.40 | 1.50 | 0.28–2.00 | 0.00 |
| Piperacillin | 4.20 | 26.00 | 0.92–33.00 | 7.02 |
|
Ampicillin-sulbactam | 1.65 | 6 | 0.24–10.00 | 8.77 |
| Imipenem | 10.00 | 18.20 | 1.9–32.00 | 10.00 |
| Meropenem | 3.20 | 10.20 | 0.95–15.00 | 8.77 |
| Metronidazole | 0.80 | 2.56 | 0.125–5.50 | 17.54 |
| Tigecycline | 0.08 | 0.10 | 0.03–0.36 | 17.54 |
| Cefotetan | 35.00 | 168.00 | 1.8–185.00 | 21.05 |
| Moxifloxacin | 2.80 | 37.00 | 0.9–175.00 | 63.15 |
| Ertapenem | 4.00 | 39.00 | 0.03–67.00 | 87.7 |
| Cefoxitin | 101.00 | 145.00 | 45–156.00 | 100.00 |
| Clindamycin | 95.00 | 267.00 | 4–333.00 | 100.00 |
Results
Analysis of the toxin genes, tcdA and
tcdB
C. difficile were isolated from various
departments of the hospital: 20 from the Department of
Neurosurgery, 7 from the Intensive Care Unit, 10 from the
Department of Infectious Diseases, 10 from the Department of
Hematology and 10 from the Department of Radiation Oncology
(Table II). The PCR assay was used
to differentiate toxin A-negative and B-positive (toxin
A−, toxin B+) strains from the toxin-positive
(toxin A+, toxin B+) strains and the
toxin-negative (toxin A−, toxin B−) strains.
The primers used are listed in Table
I. As shown in Table II, 38
and 14 isolates of the A+B+ and
A-B+ strains were identified, respectively,
which were the toxigenic strains. The recovery rates of the
toxigenic strains were 85–100% according to the hospital studied.
By contrast, 5 isolates were A-B−. To
investigate the expression levels of tcdA and tcdB
genes, qPCR was performed. The Ct values were summarized in
Table II. Of the total 38
tcdA PCR-positive isolates, 36 could be detected for the
expression of this gene and the expression level varied slightly
between the 36 transcripts. Of the total 52 tcdB
PCR-positive isolates, the expression of this gene could be
identified in 50 qPCR transcripts and they showed slight variations
in the expression level as well. No transcription could be detected
in the A-B− isolates.
Detection of the tcdC and tcdD genes
Based on these results, the transcription of
tcdA could not be detected in isolates 37 and 38, and the
transcription of tcdB could not be detected in isolates 51
and 52. As mentioned above, TcdD and TcdC have been indicated as
the positive and negative regulators of toxin A and B expression,
respectively (6). In order to
investigate why the tcdA or tcdB genes are not
expressed in isolates 37, 38, 51 and 52, qPCR was performed to
detect the expression of tcdC and tcdD. Isolate 1,
which showed a high expression of the tcdA and tcdB
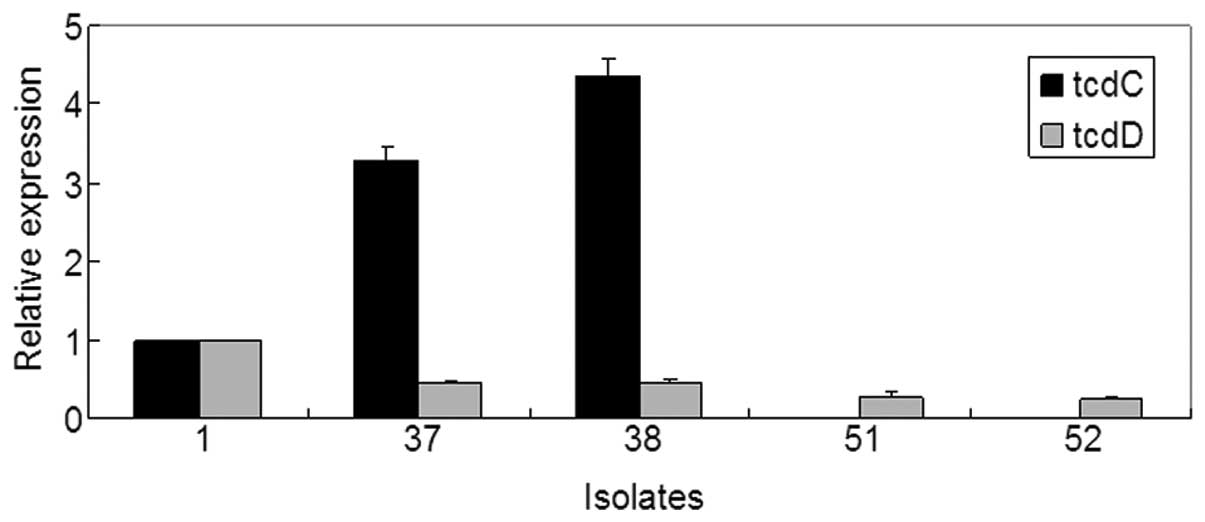
genes, was used as a positive control. The results (Fig. 1) showed that the mRNA level of
tcdD was lower in isolates 37 and 38 compared to isolate 1,
and by contrast, the tcdC mRNA level was relatively higher.
Furthermore, the mRNA level of tcdD was notably lower in
isolates 51 and 52, whereas no transcription of tcdC was
detected in these two isolates.
Antimicrobial susceptibilities
The MIC ranges, MIC50s, MIC90s and the percentages
of the susceptibility of 57 C. difficile isolates to 12
antimicrobial agents are summarized in Table III. All the isolates were
susceptible to vancomycin (MIC, ≤2 μg/ml). Susceptibility to
piperacillin, ampicillin-sulbactam, imipenem, meropenem,
metronidazole and tigecycline was shown in >80% isolates. The
resistance rates to cefotetan, moxifloxacin and ertapenem were
>70%. However, no isolates were susceptible to cefoxitin or
clindamycin. Tigecycline demonstrated the lowest MIC50 (0.08 mg/l)
and inhibited all the strains at 0.36 mg/l, whereas cefoxitin
showed the highest MIC50 (101 mg/l). Tigecycline was also the most
active with regards to MIC90 (0.1 mg/l), whereas clindamycin had
the highest MIC90 (267 mg/l).
Discussion
The toxin gene diversity has been investigated in
numerous studies by PCR (10). For
example, the multiplex PCR method by Persson et al (14) allowed the simultaneous
identification of the tcdA, tcdB, cdtA and
cdtB toxin genes. In addition, a multiplex qPCR method for
the detection of toxigenic C. difficile from stools and the
presumptive identification of the NAP-1 strain was developed by
Jayaratne et al (15). In
the present study, PCR and qPCR were carried out to identify the
tcdA and tcdB toxin genes in 57 isolated samples from
the Central Hospital of Taizhou City, and the study was a
systematic survey of the types of the C. difficile toxin
genes in China. The results showed that of the 57 isolates, 38
(66.67%) were A+B+, which plays a major role
in CDI. A total of 14 (24.56%) isolates were
A-B+ strains, which have been reported to be
significantly increased during recent years (16). In studies from various global
locations, different proportions of the A-B+
strains have been reported (17,18).
In the present study, based on the PCR results, the
A-B− strain accounted for only 5 (8.77%) of
the isolates. However, according to the qPCR results, not all the
A+ or B+ isolates showed detectable
expression of these genes. This can be explained by the inhibition
of tcdA or tcdB transcription by regulators in
certain strains. By contrast, it has also been reported that there
are certain activators, including σ factors and the positive
regulator TcdD, which are necessary for the expression of TcdA and
TcdB (19). Therefore, the absence
of these types of activators may be another reason why these two
genes are not expressed. However, this requires further
investigation.
Certain studies (20) have focused on the regulation of the
tcdA and tcdB toxin genes. Earlier studies (21) indicated that TcdC has a negative
influence on the transcription of the other genes in PaLoc,
consisting of the tcdA-E genes, and TcdD has a positive
regulatory function on the transcription of the tcdD,
tcdB, tcdE and tcdA genes. In the present
study, the mRNA levels of the tcdC and tcdD genes
were detected in isolates 1, 37, 38, 51 and 52 to identify why
tcdA or tcdB are not transcribed. Consistent with
previous studies (22), the mRNA
level of tcdC was significantly higher in isolates 37 and
38, in which the toxin tcdA was not detectable, indicating
that the transcription of tcdA could be inhibited by TcdC.
Notably, no transcription of tcdC could be detected in
isolates 51 and 52, possibly due to the absence of this gene in the
strains. Spigaglia et al (23) have previously reported the deletion
of tcdC in C. difficile clinical isolates. By
contrast, another reason why tcdA is not expressed in
isolates 37, 38, 51 and 52 could be due to the low expression of
tcdD.
The susceptibility of the 57 C. difficile
isolates to 12 antimicrobial agents was also investigated. All the
isolates of C. difficile showed susceptibility to
vancomycin, which is consistent with the fact that it is an
effective agent against C. difficile infection (24). In addition, the C. difficile
isolates in the present study were universally susceptible to
piperacillin, ampicillin-sulbactam, imipenem and meropenem. Based
on the study by Settle et al (25), it has been proved that piperacillin,
regarding its broad-spectrum activity particularly against
anaerobes, was relatively more likely to induce C. difficile
colonization or diarrhea. Thus, it is not widely used in hospitals.
Notably, 90% of the isolates in the present study were susceptible
to imipenem, which varies from the results of previous studies
(12,26). In an investigation of the inhibitory
activity of antimicrobial agents against clinical isolates of C.
difficile by Cheng et al (26), found resistance to imipenem in the
majority of tested strains. Ampicillin-sulbactam has been reported
to be active against C. difficile in numerous studies
(27,28). Similar to the results in the present
study, Lin et al (28) and
Hecht et al (29) also
demonstrated that meropenem had low MIC90 values (4 and 2 μg/ml,
respectively). Tigecycline had the lowest MIC90 value for C.
difficile isolates and was followed by vancomycin and
metronidazole (all, >3 mg/l), which is similar to previous
studies (30,31). Tigecycline has been proved to not
induce proliferation or cytotoxin production by epidemic C.
difficile strains, and patients with severe refractory CDI were
successfully treated with tigecycline (32,33).
Clindamycin showed the highest MIC90 of all the antimicrobial
agents tested, consistent with the study by Lin et al
(28) and as previously reported by
Critchley (34), the use of
clindamycin was independently associated with the infection of
C. difficile.
In conclusion, the present study identified the
presence of the tcdA and tcdB toxin genes in the
isolates of 57 C. difficile isolates from Chinese patients,
using PCR and qPCR. Similar to previous studies, the
A+B+ was the dominant ribotype. Certain
isolates were shown to be lacking tcdA or the tcdB
gene expression, possibly due to the absent or higher expression of
tcdC and lower expression of tcdD. The susceptibility
of the genes to 12 agents was also investigated. The isolates
showed similar susceptibility to specific agents, as reported
previously, such as ampicillin-sulbactam. However, certain agents
appeared to be more active against the isolates in the present
study compared to in previous studies, such as imipenem. These data
provide novel information on the characteristics of C.
difficile isolated from Chinese patients and would aid in the
future prevention of outbreaks.
Acknowledgements
The present study was supported by the Science and
Technology Plan of Medicine and Health of Zhejiang (grant no.
2010KYA186). The authors are grateful to Dr Lin Liu, Mrs. Beijia
Zheng, Mrs. Jinfeng Li and Mr. Xueyong Li for providing the
clinical samples in the study and to Caixia Zhu for critical
reading of the manuscript.
References
|
1
|
Bartlett JG, Chang TW, Gurwith M, et al:
Antibiotic-associated pseudomembranous colitis due to
toxin-producing clostridia. N Engl J Med. 298:531–534. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McFarland LV, Surawicz CM and Stamm WE:
Risk factors for Clostridium difficile carriage and C.
difficile-associated diarrhea in a cohort of hospitalized
patients. J Infect Dis. 162:678–684. 1990.
|
|
3
|
Freeman J, Bauer MP, Baines SD, et al: The
changing epidemiology of Clostridium difficile infections.
Clin Microbiol Rev. 23:529–549. 2010. View Article : Google Scholar
|
|
4
|
Arroyo LG1, Kruth SA, Willey BM, Staempfli
HR, Low DE and Weese JS: PCR ribotyping of Clostridium difficile
isolates originating from human and animal sources. J Med
Microbiol. 54:163–166. 2005.PubMed/NCBI
|
|
5
|
Hatheway CL: Toxigenic clostridia.
Clin Microbiol Rev. 3:66–98. 1990.
|
|
6
|
Spigaglia P and Mastrantonio P:
Comparative analysis of Clostridium difficile clinical
isolates belonging to different genetic lineages and time periods.
J Med Microbiol. 53:1129–1136. 2004.
|
|
7
|
Martirosian G: Recovery of Clostridium
difficile from hospital environments. J Clin Microbiol.
44:1202–1203. 2006.
|
|
8
|
Samie A, Obi CL, Franasiak J, et al: PCR
detection of Clostridium difficile triose phosphate
isomerase (tpi), toxin A (tcdA), toxin B (tcdB), binary toxin
(cdtA, cdtB), and tcdC genes in Vhembe District, South Africa. Am J
Trop Med Hyg. 78:577–585. 2008.
|
|
9
|
Frias-Lopez J, Shi Y, Tyson GW, et al:
Microbial community gene expression in ocean surface waters. Proc
Natl Acad Sci USA. 105:3805–3810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu S, Zhang L, Zhang C, et al: Comparison
of polymerase chain reaction ribotyping, toxinotyping and
nutritional aspects of toxin production of Clostridium
difficile strains. Biomed Rep. 2:477–480. 2014.PubMed/NCBI
|
|
11
|
Bélanger SD, Boissinot M, Clairoux N, et
al: Rapid detection of Clostridium difficile in feces by
real-time PCR. J Clin Microbiol. 41:730–734. 2003.
|
|
12
|
John R and Brazier JS: Antimicrobial
susceptibility of polymerase chain reaction ribotypes of
Clostridium difficile commonly isolated from symptomatic
hospital patients in the UK. J Hosp Infect. 61:11–14. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clinical and Laboratory Standards
Institute (CLSI). Methods for antimicrobial susceptibility testing
of anaerobic bacteria, approved standard M11-A7. 7th edition. CLSI;
Wayne, PA: pp. 309–315. 2007
|
|
14
|
Persson S, Jensen JN and Olsen KE:
Multiplex PCR method for detection of Clostridium difficile
tcdA, tcdB, cdtA, and cdtB and internal in-frame deletion of tcdC.
J Clin Microbiol. 49:4299–4300. 2011.PubMed/NCBI
|
|
15
|
Jayaratne PA, Monkman L, Broukhanski G, et
al: Real-time polymerase chain reaction method for detection of
toxigenic Clostridium difficile from stools and presumptive
identification of NAP1 clone. Diagn Microbiol Infect Dis.
75:121–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du P, Cao B, Wang J, et al: Sequence
variation in tcdA and tcdB of Clostridium difficile: ST37 with
truncated tcdA is a potential epidemic strain in China. J Clin
Microbiol. June.23–2014.(Epub ahead of print).
|
|
17
|
Geric B, Rupnik M, Gerding DN, et al:
Distribution of Clostridium difficile variant toxinotypes
and strains with binary toxin genes among clinical isolates in an
American hospital. J Med Microbiol. 53:887–894. 2004.
|
|
18
|
Kim H, Riley TV, Kim M, et al: Increasing
prevalence of toxin A-negative, toxin B-positive isolates of
Clostridium difficile in Korea: impact on laboratory
diagnosis. J Clin Microbiol. 46:1116–1117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saujet L, Monot M, Dupuy B, et al: The key
sigma factor of transition phase, SigH, controls sporulation,
metabolism, and virulence factor expression in Clostridium
difficile. J Bacteriol. 193:3186–3196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gerding DN, Johnson S, Rupnik M and
Aktories K: Clostridium difficile binary toxin CDT:
mechanism, epidemiology, and potential clinical importance. Gut
Microbes. 5:15–27. 2014. View Article : Google Scholar
|
|
21
|
Goldenberg SD and French GL: Lack of
association of tcdC type and binary toxin status with
disease severity and outcome in toxigenic Clostridium
difficile. J Infect. 62:355–362. 2011.
|
|
22
|
Antunes A and Dupuy B: Molecular methods
to study transcriptional regulation of Clostridium difficile
toxin genes. Methods Mol Biol. 646:93–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spigaglia P, Barbanti F, Dionisi AM and
Mastrantonio P: Clostridium difficile isolates resistant to
fluoroquinolones in Italy: emergence of PCR ribotype 018. J Clin
Microbiol. 48:2892–2896. 2010. View Article : Google Scholar
|
|
24
|
Gerding DN, Muto CA and Owens RC Jr:
Treatment of Clostridium difficile infection. Clin Infect
Dis. 46(Suppl 1): S32–S42. 2008.
|
|
25
|
Settle CD, Wilcox MH, Fawley WN, et al:
Prospective study of the risk of Clostridium difficile
diarrhoea in elderly patients following treatment with cefotaxime
or piperacillin-tazobactam. Aliment Pharmacol Ther. 12:1217–1223.
1998.PubMed/NCBI
|
|
26
|
Cheng SH, Chu FY, Lo SH and Lu JJ:
Antimicrobial susceptibility of Clostridium difficile by E
test. J Microbiol Immunol Infect. 32:116–120. 1999.
|
|
27
|
Ednie LM, Jacobs MR and Appelbaum PC:
Activities of gatifloxacin compared to those of seven other agents
against anaerobic organisms. Antimicrob Agents Chemother.
42:2459–2462. 1998.PubMed/NCBI
|
|
28
|
Lin YC, Huang YT, Tsai PJ, et al:
Antimicrobial susceptibilities and molecular epidemiology of
clinical isolates of Clostridium difficile in Taiwan.
Antimicrob Agents Chemother. 55:1701–1705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hecht DW, Galang MA, Sambol SP, et al: In
vitro activities of 15 antimicrobial agents against 110 toxigenic
Clostridium difficile clinical isolates collected from 1983
to 2004. Antimicrob Agents Chemother. 51:2716–2719. 2007.PubMed/NCBI
|
|
30
|
Larson KC, Belliveau PP and Spooner LM:
Tigecycline for the treatment of severe Clostridium
difficile infection. Ann Pharmacother. 45:1005–1010. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wilcox MH: Evidence for low risk of
Clostridium difficile infection associated with tigecycline.
Clin Microbiol Infect. 13:949–952. 2007.PubMed/NCBI
|
|
32
|
Baines SD, Saxton K, Freeman J, et al:
Tigecycline does not induce proliferation or cytotoxin production
by epidemic Clostridium difficile strains in a human gut
model. J Antimicrob Chemother. 58:1062–1065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herpers BL, Vlaminckx B, Burkhardt O, et
al: Intravenous tigecycline as adjunctive or alternative therapy
for severe refractory Clostridium difficile infection. Clin
Infect Dis. 48:1732–1735. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Critchley IA, Green LS, Young CL, et al:
Spectrum of activity and mode of action of REP3123, a new
antibiotic to treat Clostridium difficile infections. J
Antimicrob Chemother. 63:954–963. 2009. View Article : Google Scholar : PubMed/NCBI
|