Introduction
Osteoarthritis (OA), a common chronic joint disease,
is frequently observed in middle-aged and elderly patients, and is
also an important factor influencing the quality of life in these
age groups. Joints are load-bearing and their surfaces are covered
by articular cartilages. The layer of articular cartilages is a
significant component of the motor system, and can reduce the
friction coefficient of articular surfaces and disperse the
pressure load on the articular surface (1). With the progression of OA, the injury
of cartilage cells becomes aggravated. Investigating the changes of
cartilage cells is an important process to explore the
etiopathogenesis and therapy of OA. Currently, studies are focusing
on interpreting and clarifying the pathogenesis and biological
behaviors of OA in molecular and genetic perspectives, which is
also the premise and foundation of developing an effective and
precisely targeted treatment for OA in the future. Transient
receptor potential (TRP) channels are a group of important cation
channels located in the cell membrane, and can respond to
temperature, touch, pain, osmotic pressure, taste and other stimuli
(2,3).
TRP cation channel V5 (TRPV5) is one of the TRP
channel subtypes, and was cloned from rabbit proximal tubular
kidney epithelial cells. TRPV5 is a highly selective
receptor-activated ion channel; the current is highly selective for
Ca2+ via active TRPV5 and is feedback-regulated by
Ca2+ (4). TRPV5 is
expressed in the kidneys, duodenum, pancreas, prostate, placenta,
colon and rectum, and is highly expressed in distal convoluted
renal tubules and collecting tubules. In the human body, TRPV5 is
usually co-existent and co-expressed with active calcium-resorption
proteins, such as calcium binding proteins and the
Na+/Ca2+ exchanger. The expression level of
TRPV5 in channel mediating Ca2+ transmembrane transport
is regulated by vitamin D3, Ca2+ concentration, estrogen
and parathyroid hormone. TRPV5 can be opened or closed by
conformational change, thereby controlling the transmembrane flow
of Ca2+ and maintaining the balance of Ca2+
in vivo (5). Studies have
shown that TRPV5 may be associated with inflammation, endocrine and
other pathological processes (6),
and closely correlates with the occurrence of idiothic
hypercalciuria, osteoporosis and bone tumors (7,8). Thus
far, there are few studies regarding the association between TRPV5
and articular cartilage cells. The expression of TRPV5 in articular
cartilage cells and its mechanism of action in the development of
diseases have not been clarified.
A suitable exercise load can provide stress
stimulation for the proliferation, differentiation and functional
phenotype of articular cartilages. By contrast, an improper
mechanical load causes and even aggravates the degeneration of
articular cartilages, directly influencing and damaging joint
functions and eventually inducing OA. The aim of the present study
was to preliminarily investigate the protein expression
characteristics of TRPV5 in rat articular cartilage cells and its
biological significance under different exercise load modes, to
analyze the association between TRPV5 expression levels and
different exercise loads, and to preliminarily explore and analyze
the possible role of TRPV5 in chondrogenesis.
Materials and methods
Exercise-load models of normal rats
Sprague Dawley (SD) rats, aged 50 days, were forced
to exercise for 30 min, twice a day for 8 weeks on a self-made
animal treadmill (independently developed based on the Junxia
treadmill). During the exercise, the rats were stimulated with
sound or by prodding with a small wooden stick. No load was used in
the control group. There were 12 rats in each group. The rats were
maintained in a specific, pathogen-free environment. All the animal
procedures were performed according to the protocol approved by the
Experiment Animal Center of China Medical University (Shenyang,
Liaoning, China) in accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health.
Exercise-load models of OA rats
SD rats, aged 50 days, were orally administered
monosodium iodoacetate to induce OA, and following this they
underwent treadmill exercise. The method and intensity of exercise
and grouping were the same as with the normal rats.
Main reagents
Rabbit polyclonal antibody against human TRPV5
(Santa Cruz Biotechnology, Inc, Dallas, TX, USA) was used as the
primary antibody, and the immunohistochemistry Substance P
secondary antibody kit (Gene Technologies Ltd., Shanghai, China)
was used.
Macroscopic grading of experimental
samples
After 8 weeks of exercise, the experimental animals
were sacrificed by spine dislocation. The knee articular cartilages
were exposed and the cartilage lesions were graded according to the
Outerbridge Grading Standard (9) by
visual observation. The grading standard was as follows: Grade 0,
normal articular cartilage; grade 1, mild OA; grade 2, moderate OA;
grades 3 and 4, severe OA. Subsequently, the cartilage tissues were
sampled.
Histopathology
The resected surgical samples were fixed in 10%
neutral formalin-buffered solution, gradient-dehydrated with
alcohol, embedded in paraffin and cut into 4-μm tissue sections.
The sections were dried, dewaxed, gradient-hydrated with alcohol,
stained with hematoxylin and eosin (H&E) and observed for
pathological characteristics under a microscope.
Immunohistochemistry
Immunohistochemical staining was performed with the
Elivision™ tool kit (Maxim Corp., Fuzhou, China) according to the
manufacturer’s instructions. The anti-TRPV5 antibody was diluted by
1:50 with 0.01 mol/l phosphate-buffered solution (PBS), and added
onto the sections as a primary antibody. In the negative controls,
0.01 mol/l PBS was used instead of the primary antibody to exclude
false-positive results caused by non-specific antibody reactions.
All the samples were tested twice as described above to exclude
possible technical errors. The experimental steps are described as
follows: The sections were dewaxed, hydrated, blocked for 10 min in
3.0% H2O2, incubated with 0.01 mol/l sodium
citrate solution and placed into a microwave oven for
high-temperature antigen retrieval. Subsequently, these sections
were blocked in normal goat serum blocking buffer for 20 min at
37°C, incubated overnight at 4°C with primary antibody (1:50
anti-TRPV5 antibody), rewarmed for 45 min and washed with 0.01
mol/l PBS. The sections were incubated with biotinylated secondary
antibody for 20 min at 37°C, washed with 0.01 mol/l PBS, developed
with diaminobenzidine and counterstained with hematoxylin.
Following this, the above sections were dehydrated, vitrified and
mounted. Finally, the sections were photographed under a microscope
(×400 power) and five fields were randomly selected. The integrated
optical density of positive units was measured using the Olympus
BX52 microscopic image acquisition system (Olympus, Tokyo, Japan)
and the results were evaluated.
Statistical analysis
Data are expressed as (mean ± standard deviation).
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was used for
independent sample t-test. P<0.05 was considered to indicate a
statistically significant difference and P<0.01 were highly
statistically significant.
Results
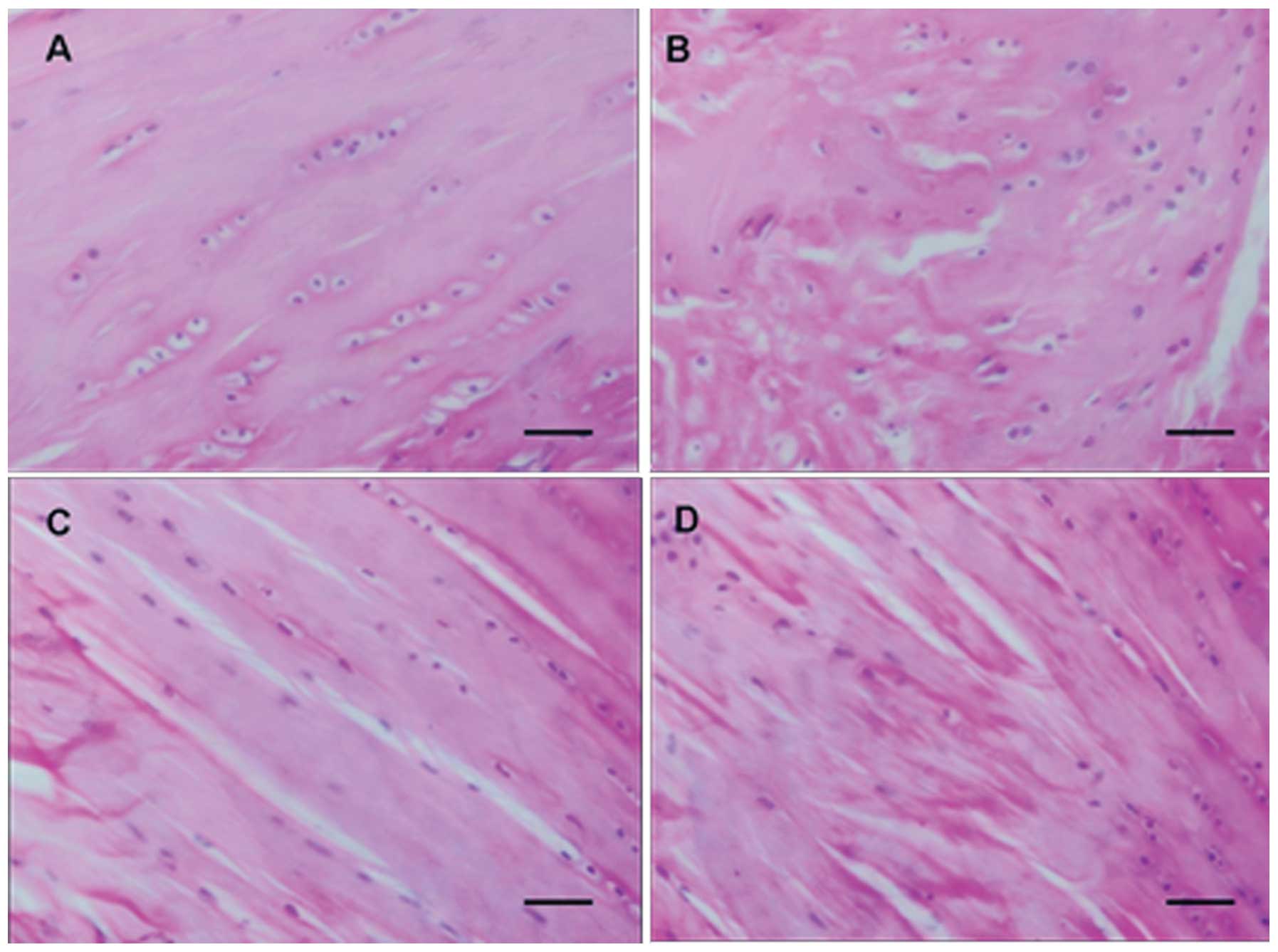
Histopathology
In the normal control group, the surface of rat
articular cartilages was smooth. The superficial cartilage cells
were fusiform and arranged intensely, and their long axis was
parallel to the cartilage surface. The intermediate cartilage cells
were round, arranged irregularly and distributed in a double-cell
back-to-back manner. The mast cells were gradually distributed from
double-cell to multi-cell mass, without a visible tidal line. There
were no significant pathological changes in the calcified cartilage
layer and subchondral bone. In the normal loading group, the ulcer
defects and minor fissures were observed on the surface of
articular cartilages, and the cartilage cells were reduced in
number, in a disorderly arrangement, and clustered. In the normal
control group, there were 1–2 layers of rat synovial tissues and
cells. The cells were distributed regularly and the synovial
tissues had no signs of chronic inflammation (Fig. 1A). In the normal loading group,
there was significant hyperplasia of rat synovial tissues, the
synovial cell layer changed into 3–5 layers due to hyperplasia, the
synovial cells were arranged irregularly and there was hyperplasia
of subsynovial fibrous tissues and blood capillaries, infiltration
of monocytes and lymphocytes, and synovial lipedema (Fig. 1B). In the OA group, the surface of
the cartilage tissues was coarse and uneven, the cartilage cells
were in a disorderly arrangement, decreased in number and
clustered, and there was degeneration and necrosis of cartilage
cells, decreased cartilage thickness, unevenly stained matrix and
metachromasia (Fig. 1C). In the OA
loading group, the cartilage cells were arranged extremely
irregularly and were significantly decreased in number. There was
also cell clustering and a larger number of cartilage cells became
degenerative and necrotic (Fig.
1D).
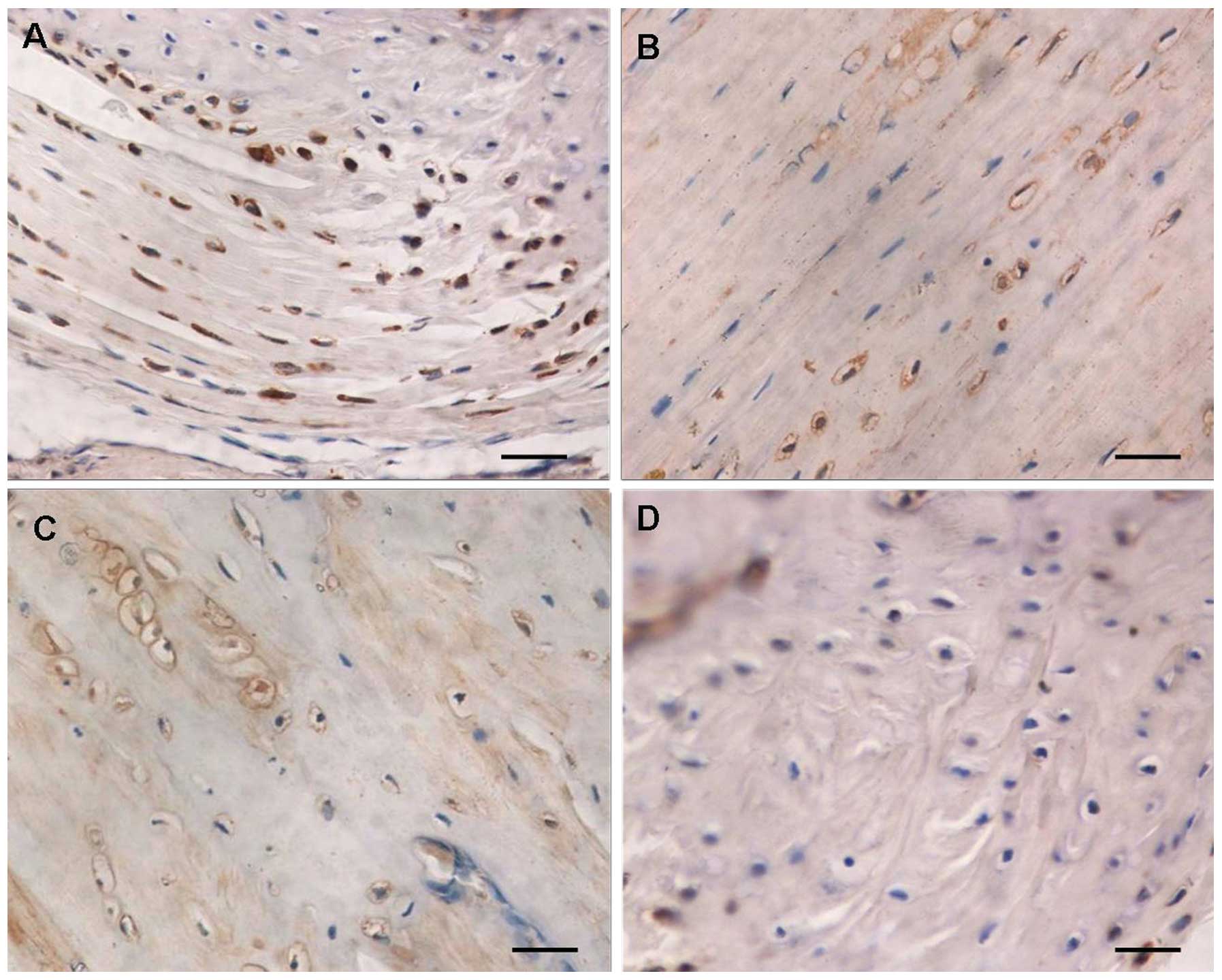
Immunohistochemistry of TRPV5
TRPV5 expression was mainly located at the membrane
of articular cartilage cells. The surface of clustered cartilage
cells demonstrated brown positive staining (Fig. 2). In the normal articular cartilage
group, there was a rich cartilage matrix, the cartilage cells
increased and were clearly distributed. The positive protein
expression of TRPV5 was observed in the superficial immature
cartilage cells and the deep isogenous cartilage cell clusters,
typically in the articular cartilage load-bearing region (Fig. 2A). In the normal loading group,
there was degeneration of articular cartilages, thinned cartilage
matrix, a decreased number of articular cartilage cells and
decreased brown TRPV5 protein-positive cells in the cartilage cell
clusters (Fig. 2B). The percentage
of positive expression areas in the normal articular cartilage and
normal loading articular cartilage groups was 34.3±5.8 and
18.1±4.9%, respectively (P<0.01). In the OA articular cartilage
group, there was a thin cartilage matrix, the number of cartilage
cells decreased significantly, the cartilage cells degenerated and
were irregular in morphology and distributed in disorderly fashion,
and the brown TRPV5 protein-positive cells in the cartilage cell
clusters were markedly reduced (Fig.
2C). In the OA loading articular cartilage group, the cartilage
matrix was injured and ulcerated, the subchondral bone was exposed
and sclerotized, the articular cartilage cells were significantly
decreased and were mainly present in the periarticular
non-load-bearing region, degenerated, irregular in morphology and
distributed in an extremely disorderly fashion. A small amount of
brown TRPV5 protein-positive expression was found in some of the
surviving articular cartilage cells (Fig. 2D). The percentage of positive
expression areas in the OA articular cartilage and OA loading
articular cartilage groups was 13.17±4.2 and 6.4±2.7%, respectively
(P<0.01).
Discussion
Based on the encoding amino acid sequences, the
TRP gene family is divided into seven subfamilies, which are
TRPC, TRPV, TRPM, TRPA, TRPP,
TRPML and TRPN (10).
TRPs are a group of voltage-independent cation channels that are
widely expressed on cell membranes of various organisms, and can
respond to several intra- and extra-cellular stimuli (4). The subtypes, such as TRPV, TRPM8,
TRPC3 and TRPC6, have been demonstrated to be correlated to the
occurrence of malignant tumors (11,12).
In the present study, TRPV5 was found to be expressed in the
articular cartilage cells to different extents, and its expression
level was associated with the severity of joint injury (including
the downregulation of TRPV5 expression level with an increase of
injury severity). TRPV5 expression has been indicated to possibly
play a role in the articular chondrogenesis process.
Thus far, TRP channels are a commonly used molecular
entity model used to study store-operated calcium channels (SOCs)
(13). The opening of SOCs is
triggered by calcium store depletion and SOCs are the major
channels for Ca2+ influx in non-excitable cells
(14). Ca2+, as the most
important second messenger in the body, is a necessary component of
several transmembrane signaling pathways (such as the
phosphatidylinositol-3-kinase messenger system). All the phases of
cell proliferation are associated with Ca2+ regulation
(15,16) and Ca2+ can extensively
participate in cell proliferation, differentiation and migration by
influencing cell volume, controlling cell membrane potential, and
contributing to cytoskeletal formation and intercellular responses.
Furthermore, the increased Ca2+ concentration in cells
can activate the endogenous endonuclease that segmentalize DNA, is
dependent on Ca2+/Mg2+, and plays an
important role in apoptosis (17).
In the TRP family, TRPV5 is a transient receptor potential channel
with high selectivity to Ca2+, and plays a critical role
in maintaining Ca2+ concentration in cells. The TRPV5
expression level influences intracellular Ca2+
homeostasis and thus, it may participate in the proliferation and
apoptosis of cartilage cells. In fact, studies indicate that TRPV5
is expressed in giant cell tumor tissues of the bone and may be
involved in the transmembrane transport of Ca2+ in tumor
cells (8). TRPV5 is co-expressed
with the majority of calcium-binding proteins in tissues, among
which Annexins (ANXs) are closely associated with cell growth,
differentiation and infiltration. Annexin A10 expression is a sign
of histocyte differentiation and growth arrest, which is closely
associated with the malignant phenotype of histocytes, vascular
invasion and tumor progression (18). Annexin A2 downregulation can block
TRPV5-mediated current. The aforementioned findings demonstrate
that ANXs may play a role by regulating TRPV5 (19). The present study confirms that TRPV5
is highly expressed in normal articular cartilage tissues and
injured articular cartilage tissues, but its expression level
varies among different pathological types. In addition, the
expression level of TRPV5 in cartilage tissues is associated with
the injury severity of cartilage histocytes. This suggests that
TRPV5 expression may play a role in the formation and development
of cartilage, and indicates that the TRPV5 expression level may be
helpful for prognosis prediction, although the specific mechanism
underlying its role in these processes requires further study.
Acknowledgements
The present study was supported by the National
Natural Scientific Foundation of China (grant no. 81272050) to
L.B.
Abbreviations:
|
OA
|
osteoarthritis
|
|
SD
|
Sprague Dawley
|
|
TRPV5
|
transient receptor potential cation
channel V5
|
|
H&E
|
hematoxylin and eosin
|
|
PBS
|
phosphate-buffered solution
|
References
|
1
|
Ruiz-Romero C, López-Armada MJ and Blanco
FJ: Proteomic characterizeation of human normal articular
chondrocytes: a novel tool for the study of osteoarthritis and
other rheumatic diseases. Proteomics. 5:3048–3059. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bang S, Yoo S, Oh U and Hwang SW:
Endogenous lipid-derived ligands for sensory TRP ion channels and
their pain modulation. Arch Pharm Res. 33:1509–1520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel A, Sharif-Naeini R, Folgering JR, et
al: Canonical TRP channels and mechanotransduction: from physiology
to disease states. Pflugers Arch. 460:571–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clapham DE: TRP channels as cellular
sensors. Nature. 426:517–524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Z, Qin DM, Gu GS, et al: The
correlations between TRPV5 and bone metabolism. J Jilin Univ Health
Sci. 31:645–648. 2005.
|
|
6
|
Topala CN, Bindels R and Hoenderop J:
Regulation of the epithelial calcium channel TRPV5 by extracellular
factors. Curr Opin Nephrol Hypertens. 16:319–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y and Zhang B: New progress in the
study of idiopathic hypercalciuria. Medical Recapitulate.
16:2969–2972. 2010.
|
|
8
|
Li FC, Gu GS, Jin CH, et al: Expression of
TRPV5 and TRPV6 in osteosarcoma and giant cell tumor of bone.
Shandong Med J. 47:73–74. 2007.
|
|
9
|
Outerbridge RE: The etiology of
chondromalacia patellae. J Bone Joint Surg Br. 43-B:752–775.
1961.PubMed/NCBI
|
|
10
|
Montell C, Birnbaumer L, Flockerzi V, et
al: A unified nomenclature for the superfamily of TRP cation
channels. Mol Cell. 9:229–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santoni G and Farfariello V: TRP channels
and cancer: new targets for diagnosis and chemotherapy. Endocr
Metab Immune Disord Drug Targets. 11:54–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun H, Shen F and Wu M: TRPC Mediates the
proliferation of hepatocellular carcinoma cell. Tumor. 26:396–401.
2006.
|
|
13
|
Putney JW Jr: New molecular players in
capacitative Ca2+ entry. J Cell Sci. 120:1959–1965.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rychkov G, Brereton HM, Harland ML and
Barritt GJ: Plasma membrane Ca2+ release-activated
Ca2+ channel with a high selectivity for Ca2+
identified by patch-clamp recording in rat liver cells. Hepatology.
33:938–947. 2001.
|
|
15
|
Xue Q and Wang Z: Ca2+
apoptosis and drug regulation. Chin Gen Pract. 12:1126–1127.
2009.
|
|
16
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signaling. Nat Rev Mol Cell
Biol. 1:11–21. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Sun Y, Song R, et al: Cadmium
induced apoptosis in rat hepatocytes by calcium overloading. Acta
Veterinaria et Zootechnica Sinica. 42:1168–1174. 2011.
|
|
18
|
Liu SH, Lin CY, Peng SY, et al:
Down-regulation of annexin A10 in hepatocellular carcinoma is
associated with vascular invasion, early recurrence, and poor
prognosis in synergy with p53 mutation. Am J Pathol. 160:1831–1837.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van de Graaf SF, Hoenderop JG, Gkika D, et
al: Functional expression of the epithelial Ca2+
channels (TRPV5 and TRPV6) requires association of the
S100A10-annexin 2 complex. EMBO J. 22:1478–1487. 2003.
|