Introduction
Acute promyelocytic leukemia (APL) is a form of
acute myeloid leukemia, which has been identified as the
M3 subtype. A unique chromosome translocation t(15;
17)(q22; q21) found in the majority of APL patients leads to the
formation of the promyelocytic leukemia retinoic acid receptor α
(PML-RARα) fusion gene (1). The fusion
protein encoded by the PML-RARα gene polymerizes and combines with
retinoid-X receptor. The resultant protein complexes enhance
histone deacetylase, thus repressing the transcription of the gene
and disrupting the retinoic acid signal pathway under physiological
concentrations of retinoic acid (1).
This change results in the excessive growth of malignant
promyelocytes and an inhibition of granulocyte differentiation.
All-trans retinoic acid (ATRA) as a successful model
of differentiation therapy has improved the curative effect and
extended the survival time of patients with APL. Clinical data has
shown that the application of ATRA combined with chemotherapy
increases the clinical complete response rate to ≤95% and the
2-year event-free survival rate was 86% (2). However, fatal retinoic acid syndrome and
ATRA resistance in the majority of patients ultimately leads to
treatment failure. Additionally, 31% of patients administered ATRA
combined with chemotherapy relapse within 4–5 years after complete
remission (3). Therefore, it is
essential to identify new drugs for APL patients.
Arsenic trioxide (As2O3) and
tetra-arsenic tetra-sulfide (As4S4), as
traditional medicines, have been used widely for the treatment of
newly diagnosed and relapse APL. The side effects, such as fluid
retention, skin rashes, leukocytosis, gastrointestinal discomfort,
pulmonary infiltrates, neuropathy, prolongation of the corrected QT
interval, liver function abnormality and sudden death, make it
difficult for APL patients to accept As2O3 as
a single agent for long-term treatment (4). Therefore, As2O3
should be incorporated into combination medications with ATRA or
used as a salvage therapy for relapse APL patients.
As4S4, which exerts similar
effectiveness and less toxicity, provides not only a better
quality-of-life, but is also advantageous in cytogenetic remission
and PML-RARα reversion for newly diagnosed and hematological
relapse patients. Following a single application of
As4S4, the leukemia-free survival rate (LFS)
for 1 and 3 years reached 86.1 and 76.6%, respectively, among newly
diagnosed APL patients, with a median follow-up time of 13.5
months. In addition, the LFS for 1 and 6 years was 96.7 and 87.4%
for the hematological complete remission group, with a median
follow-up of 23 months (5). A previous
study suggested that the LFS of APL patients at 2 years treated
with As4S4 is higher than for those treated
with As2O3 (6).
Therefore, oral As4S4 is not inferior to
intravenous As2O3 as an effective treatment
for APL and may be considered a routine treatment option for the
appropriate patients. The exact molecular mechanism of the drug's
action remains unclear and warrants further investigation. The aim
of the present study was to characterize the toxicity and apoptosis
induced by As4S4 in a specific human APL
NB4-R1 cell line that exhibits resistance to ATRA.
Materials and methods
Cell culture and reagents
The NB4-R1 APL-derived cells from an RA-resistant
promyelocytic cell line were generously supplied by Shanghai Second
Medical College (Shanghai, China). The NB4-R1 cells were cultured
in RPMI-1640 (Gibco-BRL, Carlsbad, CA, USA) medium supplemented
with 10% heat-inactivated (at 56°C for 30 min) fetal bovine serum,
100 U/ml penicillin and 100 µg/ml streptomycin, and maintained in a
5% CO2 humidified atmosphere at 37°C. The cells were
grown at an optimal cell density between 5×105 and
1×106/ml. Cell viability was evaluated via trypan blue
dye exclusion assays and the cell survival rate was >95%.
As4S4 (Xi'an Traditional Chinese Drug
Company, Xi'an, China) stock solution was obtained by dissolving in
the 1.0 M NaOH assistant agent. According to the IC50 of
NB4-R1 cells in our previous study (7), the exponentially growing cells were
treated with 25 µmol/l As4S4 for 0, 24 or 48
h and the cells were analyzed via flow cytometry, DNA ladder
electrophoresis and western blot analysis.
Measurement of apoptosis
Annexin V-FLUOS/propidium iodide (PI) binding
study using flow cytometry
Flow cytometric analysis using Annexin V-FLUOS and
PI (Roche Custom Biotech, Indianapolis, IN, USA) was performed to
differentiate between live, apoptotic and necrotic cells following
treatment with As4S4. Subsequent to the
treatment with 25 µmol/l As4S4 for 0, 24 or
48 h, 1×106 cultured cells were harvested and washed
twice with cold phosphate-buffered saline (PBS). The cells were
centrifuged at 200 × g for 5 min at 4°C and re-suspended in 100 µl
of Annexin V-FLUOS/PI labeling solution for 10–15 min in the dark
at room temperature. The stained cell suspension was immediately
analyzed using a flow cytometer (BD Biosciences FACSCalibur double
laser flow cytometer; BD Biosciences, Franklin Lakes, NJ, USA). The
data analysis was performed using the CellQuest software program
(BD Biosciences).
DNA ladder agarose gel
electrophoresis
DNA ladder fragmentation reflecting the endonuclease
activity is a characteristic feature of apoptosis. After incubation
for 0, 24 or 48 h with As4S4, the NB4-R1
cells were collected and washed twice with PBS. Subsequently,
1×106 cells were solubilized and the chromosomal DNA was
extracted and purified using an Apoptotic DNA Ladder kit (Beyotime
Institute of Biotechnology, Jiangsu, China) according to the
manufacturer's instructions. The DNA samples were electrophoresed
on a 1.5% agarose gel containing 1 mg/ml ethidium bromide at 60 V
for 2 h. The apoptotic DNA fragments were analyzed and photographed
using a Quantity One gel image analysis system (ChemiDOC XRS;
Bio-Rad, Richmond, CA. USA).
Cell cycle analysis
The cell cycle distribution was analyzed via flow
cytometry (BD Biosciences FACSCalibur double laser flow cytometer).
Following treatment with As4S4 for 0, 24 or
48 h, the NB4-R1 cells (1×106) were harvested and washed
twice with ice-cold PBS. The cells were suspended gently in 70%
chilled ethanol at −20°C overnight. After washing with PBS, the
cells were re-suspended in 500 µl PBS containing PI (50 µg/ml) and
RNase (50 µg/ml), and were incubated for 30 min at room temperature
in the dark. The cell cycle phase distribution of each experiment
was analyzed using 10,000 cells per sample. The proportion of cells
in the G0/G1, S and G2/M phases
were represented as DNA histograms.
Western blot analysis
After treatment with 25 µmol/l
As4S4 for 0, 24 or 48 h, the cultured cells
were harvested and washed three times with cold PBS. Subsequently,
the cells were solubilized in radioimmunoprecipitation assay buffer
containing a protease inhibitor cocktail (Sigma, St. Louis, MO,
USA). After incubation on ice for 10 min, the cell suspension was
centrifuged for protein at 15,500 × g for 15 min at 4°C. The
protein (30 µg) was separated on 10% SDS-PAGE and transferred to a
nitrocellulose membrane at 110 V for 2 h. The non-specific binding
sites on the membranes were blocked with 5% (w/v) skimmed milk in
Tris-buffered saline (TBS): [20 mmol/l Tris-HCl and 200 mmol/l NaCl
(pH 7.6)] for 2 h under gentle agitation at room temperature.
Subsequently, the membranes were incubated with the relevant
primary antibodies [poly ADP-ribose polymerase (PARP) rabbit
monoclonal, 1:10,000; Cell Signaling Technology, Inc., Danvers, MA,
USA; B-cell lymphoma 2 (Bcl-2) mouse monoclonal, 1:1,000; Bax
rabbit monoclonal, 1:1,000; caspase-3 rabbit monoclonal, 1:1,000;
GAPDH mouse monoclonal, 1:10,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA] directed against the protein or enzyme of interest
for 1 h at room temperature and subsequently at 4°C overnight. The
membranes were washed extensively with TBS containing 0.05%
Tween-20 (v/v) (TBST) and incubated with the appropriate
horseradish peroxidase-conjugated secondary antibody (Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Following washing
with TBST, the membranes were incubated under chemiluminescence and
wrapped in clear plastic wrap for film exposure. The bands on the
immunoblots were quantified using Quantity One version 4.6.2
software (Bio-Rad). The protein expression of each sample was
internally normalized to GAPDH and the quantity was compared with
the expression of the control groups.
Statistical analysis
Experiments were performed in duplicates or
triplicates of ≥3 independent experiments and the results are
presented as the mean ± standard deviation. Statistical analysis
between groups was carried out via a one-way analysis of variance
using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
As4S4 induces
NB4-R1 cell apoptosis in a time-dependent manner
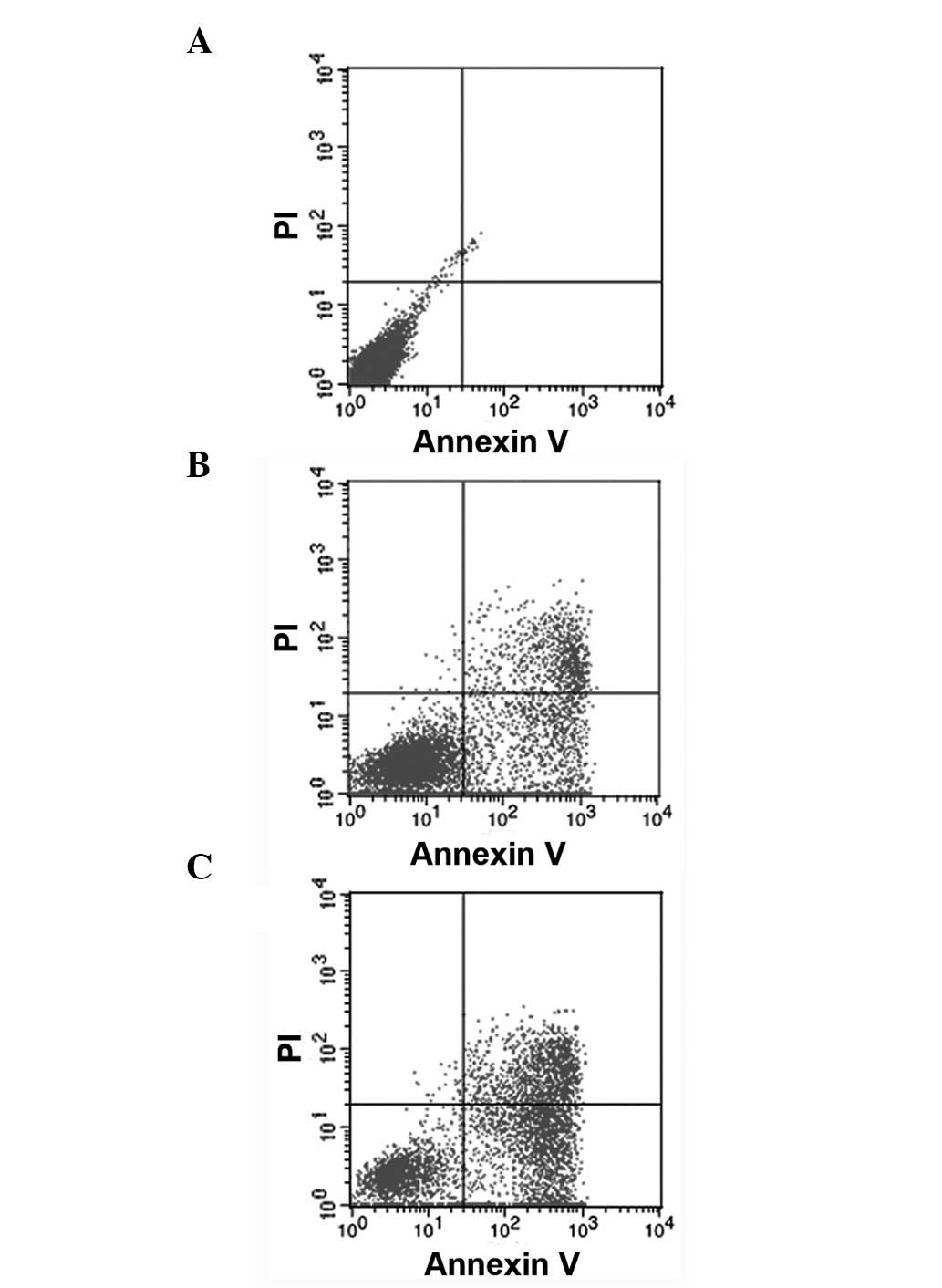
Apoptotic characterization was performed in the
As4S4-treated cells via Annexin V-FLUOS and
PI double staining and the samples were analyzed via flow
cytometry. The data revealed that the untreated cells showed normal
cell viability. In contrast to the control cells, the percentage of
early apoptotic cells treated with 25 µmol/l
As4S4 for 24 or 48 h (Annexin
V+/PI−, in the lower right quadrant)
significantly increased from 0.00 to 24.49 and 47.41% (P<0.05),
respectively. In addition, the percentage of late apoptotic cells
(Annexin V+/PI+, shown in the upper right
quadrant) significantly increased from 0.08 to 14.72 and 20.70%
(P<0.05), respectively. As shown, a progressive increase in the
number of apoptotic cells was observed, which suggests
time-dependent cytotoxicity (Fig. 1,
Table I).
 | Table I.Flow cytometric data showing the
effect of As4S4 (25 µmol/l) on Annexin V/PI
binding in the NB4-R1 cells. |
Table I.
Flow cytometric data showing the
effect of As4S4 (25 µmol/l) on Annexin V/PI
binding in the NB4-R1 cells.
| Action time, h | Viable cells | Early apoptotic
cells | Late apoptotic
cells |
|---|
| 0 |
99.53±0.39 |
0.00±0.00 |
0.08±0.13 |
| 24 |
60.27±5.12a |
24.49±4.05a |
14.72±1.82a |
| 48 |
31.60±1.48a |
47.41±4.78a |
20.70±3.89a |
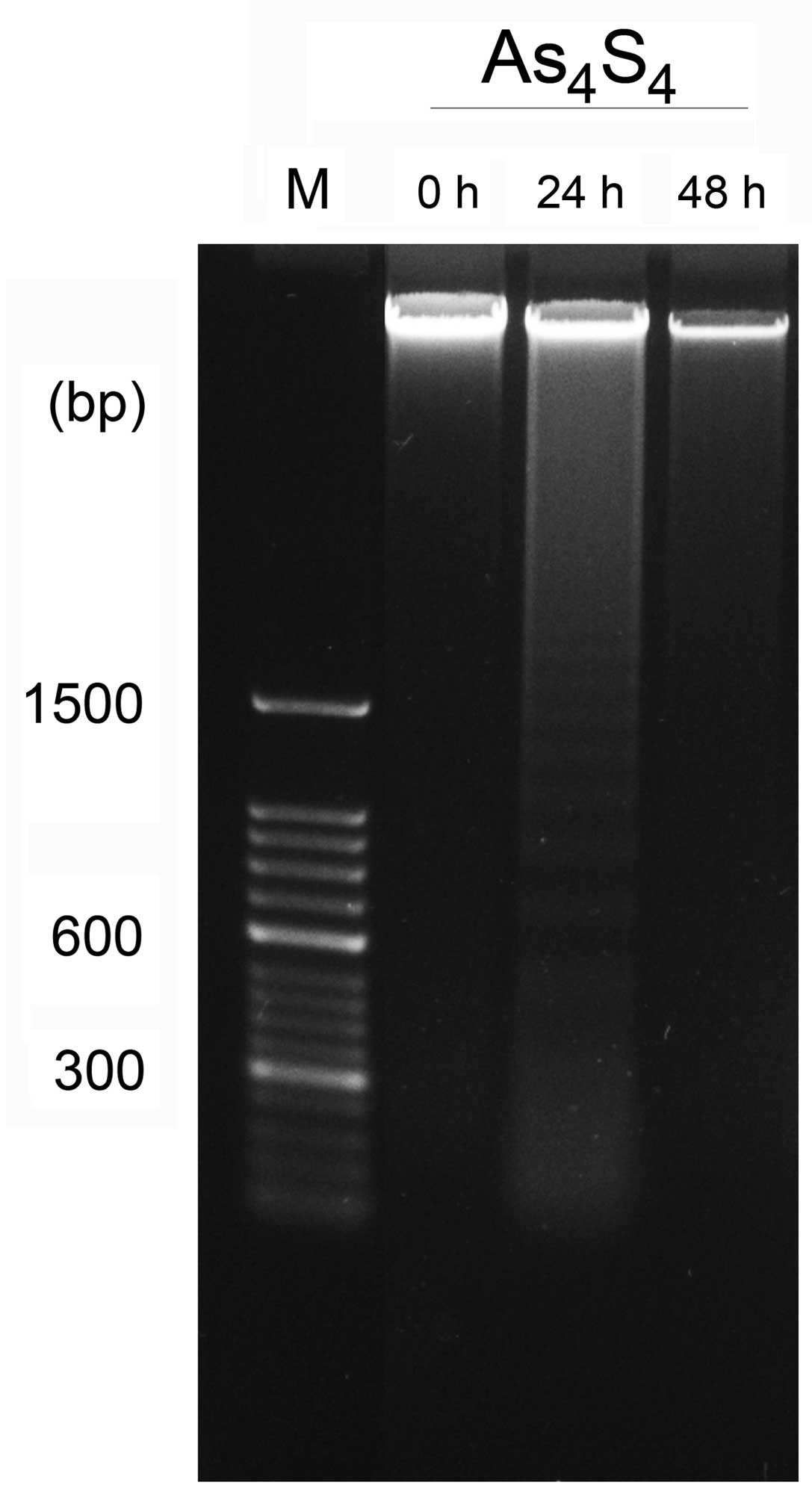
DNA ladder agarose gel electrophoresis was used to
distinguish apoptotic cells from necrosis. Apoptosis is
characterized by internucleosomal DNA ladder fragmentation through
agarose gel electrophoresis to show a ‘ladder’ pattern at 180-base
pair (bp) intervals due to the activation of endogenous
endonucleases, whereas random DNA fragmentation is a typical
manifestation of necrosis following electrophoretic separation. The
untreated cells contained only high-molecular-weight genomic DNA.
Compared with the control group, the NB4-R1 cells inoculated with
As4S4 exhibited the characteristic pattern of
nucleosomal laddering specific to apoptosis, which was visible as
faint bands on the gel. As4S4 produced DNA
fragments of low molecular weight consisting of multimers of
180–200 bp in the NB4-R1 cell line in the 24-h treatment groups. In
the 48-h treatment groups, DNA degradation failed to form the
typical bands but formed random DNA fragmentation, which indicates
necrosis of cells at this time point (Fig.
2).
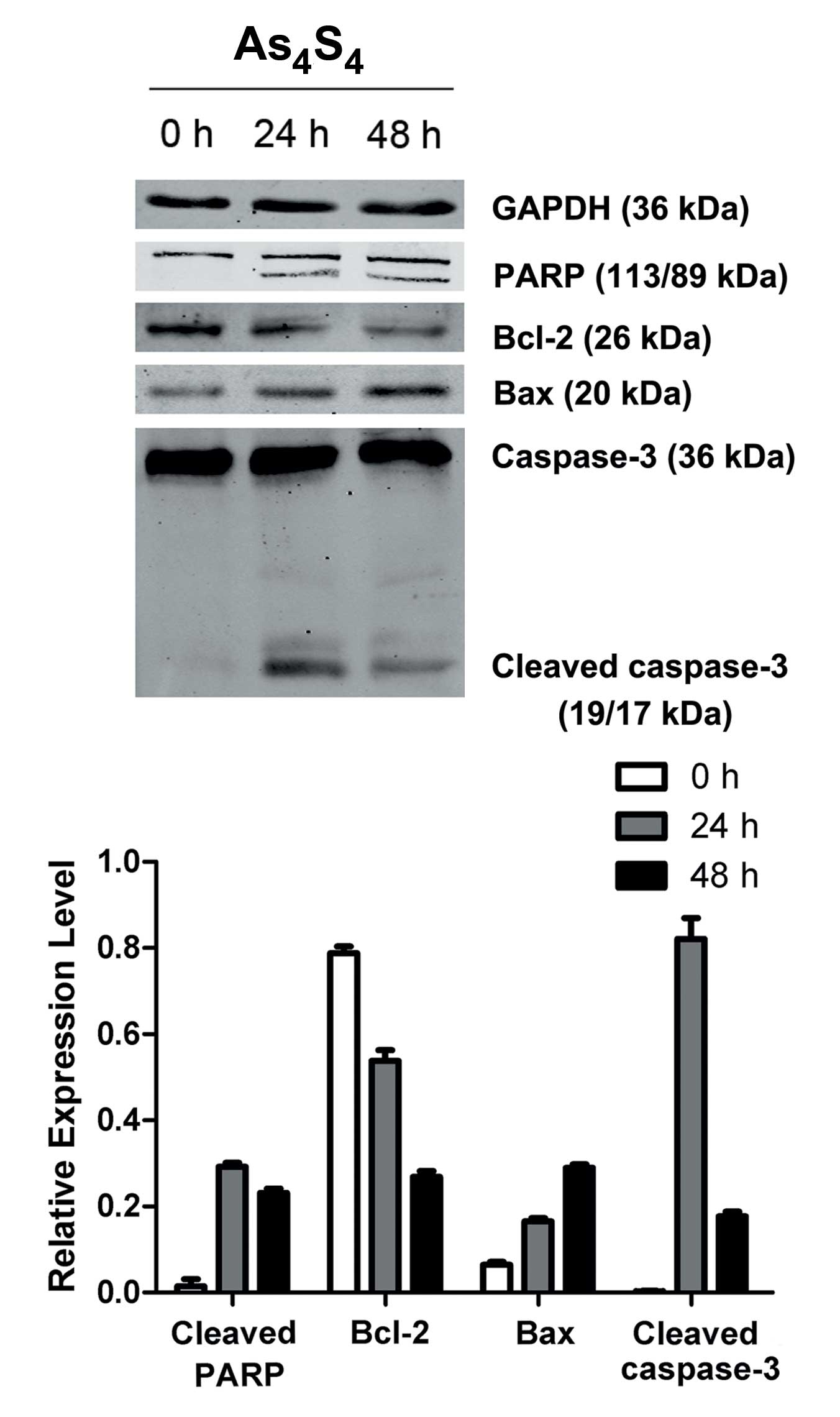
Effect of As4S4
on the proteins associated with NB4-R1 cell apoptosis
Based on the above result, the association between
apoptosis factor expression and apoptosis induction was
investigated in the NB4-R1 cells treated with
As4S4. The expression of several
apoptosis-related factors was studied. Bax levels increased
significantly over the control, whereas Bcl-2 showed a clear
reduction. All the factors exhibited variations in a time-dependent
manner.
As changes of Bax/Bcl-2 have been reported to play
significant roles in the activation of caspase signaling, the
activation of caspase-3 was detected in the following set of
experiments. Incubation of the NB4-R1 cells with
As4S4 for 24 h induced activation of
caspase-3. Pro-caspase-3 was cleaved into small active fragments of
19 or 17 kDa under apoptotic stimulation. The 113 kDa PARP, as the
specific substrate of caspase-3, was cleaved into 89 and 24 kDa
fragments after treatment for 24 h. No cleavage of PARP in the
control group was detected (Fig.
3).
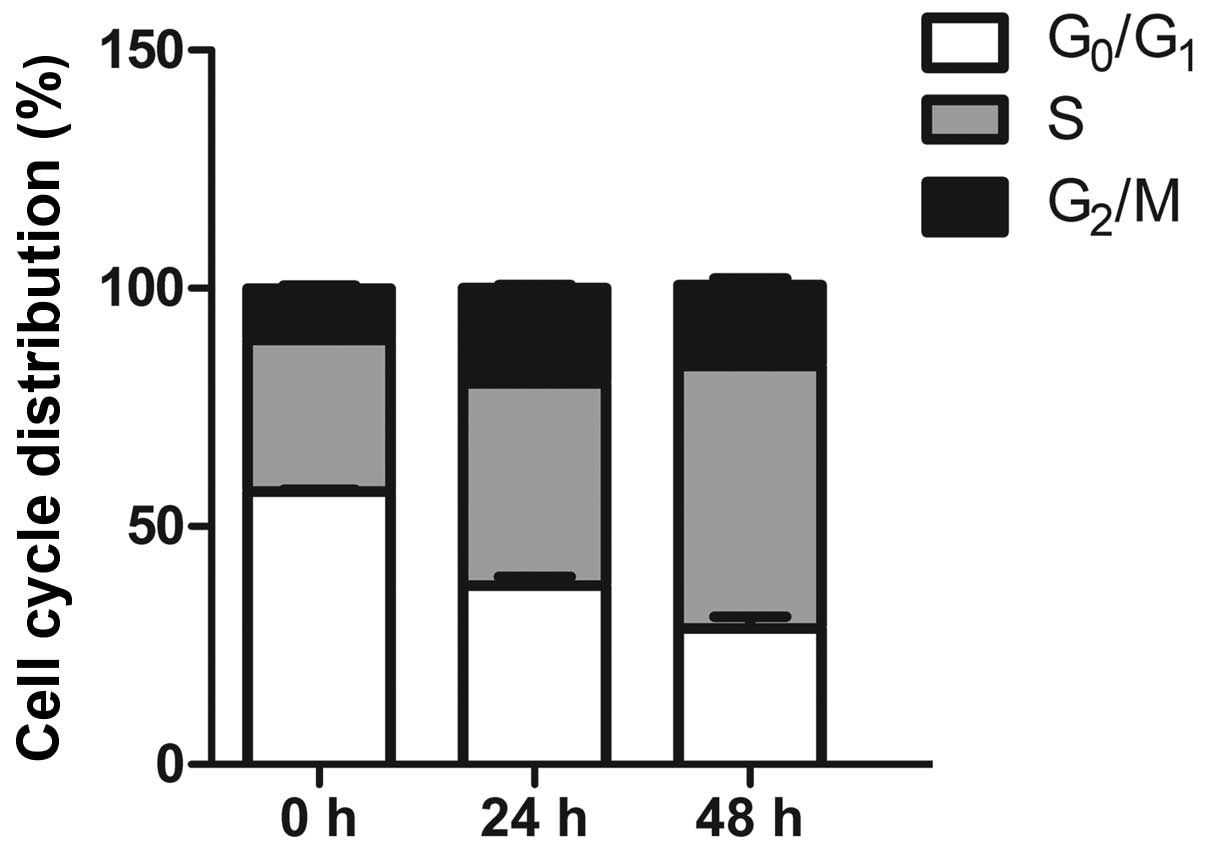
As4S4 induces
cell cycle arrest in NB4-R1 cells
Flow cytometry assays were performed to analyze the
effects of As4S4 on the cell cycle
distribution. According to the DNA histogram results, the drug
induced a significant increase in the cell population in the S
phase. Cells in the S phase increased from 31.85% of untreated
cells to 42.53 and 55.12% of cells in the experimental groups
(P<0.05), where the action of As4S4
induced nearly a 2-fold increase. Compared to the control group,
the distribution of NB4-R1 cells in the G0/G1
phases decreased from 57.29 to 37.57 and 28.51% (P<0.05),
respectively. The percentage of G2/M phase cells
detected at different time points increased from 10.79 to 19.91 and
17.01%, representing a slight increase compared to the controls
(P<0.05). These results show that the inhibitory effect on the
growth of NB4-R1 cells induced by As4S4 was
partially mediated by reducing the number of cells in the
G0/G1 phases and arresting the cell cycle in
the S phase and G2/M phases (Table II, Fig.
4).
 | Table II.Flow cytometric data showing the
effect of As4S4 (25 µmol/l) on the
progression of the cell cycle in NB4-R1 cells. |
Table II.
Flow cytometric data showing the
effect of As4S4 (25 µmol/l) on the
progression of the cell cycle in NB4-R1 cells.
| Action time, h |
G0/G1 | S |
G2/M |
|---|
| 0 |
57.30±0.35 |
31.85±0.91 |
10.79±0.67 |
| 24 |
37.56±1.85a |
42.53±2.71a |
19.91±1.83a |
| 48 |
28.51±2.53a |
55.12±0.13a |
17.01±1.44a |
Discussion
APL accounts for 10–15% of acute myeloid leukemia in
adults (1).
As4S4 has gained importance in the treatment
of the APL. Previous studies have shown that the therapeutic action
of As4S4 is also effective for other tumor
therapies (8,9). However, the molecular mechanism of the
action of As4S4 in RA-resistant APL therapy
remains unknown. The present results reveal a time-dependent toxic
action of As4S4 on RA-resistant NB4-R1 cells.
Flow cytometric analyses and DNA ladder agarose gel electrophoresis
confirmed that As4S4 inhibited tumor cell
growth via inducing apoptosis. To probe the cell signaling pathways
involved in this As4S4-induced apoptosis, the
protein expression levels of Bcl-2, Bax, caspase-3 and PARP were
detected via western blot analysis.
The Bcl-2 family of pro- and anti-apoptotic proteins
plays an important role in apoptosis that is induced by a variety
of stimuli. Bcl-2 proteins modulate the integrity of the
mitochondrial and endoplasmic reticulum membranes, cytochrome
c release, caspase activation and cell death (10). A reduction in Bcl-2 expression can lead
to a loss of signals that are required for survival. Bax is a major
pro-apoptotic member that is required for apoptotic cell death.
Previous evidence has indicated that Bcl-2 can constitute
homodimers and heterodimers with Bax, leading to an inhibition of
the formation of Bax/Bax pro-apoptotic homodimers (11,12). The
ratio between anti-apoptotic and proapoptotic members of the Bcl-2
family may determine the susceptibility of the cell to apoptosis.
The present study reported a decrease in Bcl-2 and an increase in
Bax following treatment of the NB4-R1 cells with
As4S4. The decrease in the Bcl-2/Bax ratio
leads to the translocation of Bax from the cytoplasm to
mitochondria, promoting the release of cytochrome c and the
activation of caspase. Variations in the levels of Bax and Bcl-2
can be deduced by apoptosis that is initiated via the intrinsic
pathway.
Caspase-3, as the most important executor of
apoptosis, participates in DNA degradation, nuclear condensation,
plasma membrane blebbing and proteolysis of certain caspase
substrates (13,14). Caspases are synthesized as relatively
inactive precursors (zymogens) that require proteolytic processing
for activation. As discovered in the NB4-R1 cells,
As4S4 cleaves the 36-kDa pro-caspase-3 into
small 17 or 19 kDa active fragments, leading to caspase-dependent
apoptosis. Subsequently, the cleaved caspase-3 activates
endonuclease caspase-activated DNase, leading to fragmentation of
the chromosomal DNA at internucleosomal sites (15). The present results show that cleaved
caspase-3 significantly increased after As4S4
incubation for 24 h while the DNA degradation revealed
characteristic DNA ‘ladder’ bands. The activity of this
endonuclease can be inhibited by PARP and the cleavage of PARP by
activated caspase-3 reverses the activity of the endonuclease
(16). In the present study, the
113-kDa PARP could be cleaved onto an 89-kDa C-terminal catalytic
fragment and an N-terminal 24-kDa fragment after 24 h of
As4S4 treatment, leading to a loss of DNA
repair function.
Various chemotherapy drugs inhibit the growth of
tumor cells by blocking the cell cycle. Numerous investigators have
reported that As4S4 blocks tumor cells at
different stages of the cell cycle (17,18).
Variations in experimental results may be associated with drug
concentration, action time and cell types. In the present study,
the accumulation of cells in the S and G2/M phases was
observed for NB4-R1 cells, suggesting that
As4S4 may exert its cytotoxic effects on
NB4-R1 cells through cell cycle arrest and cell apoptosis.
In conclusion, the present study revealed that
As4S4, a traditional medicine, inhibited the
growth of NB4-R1 cells in vitro. As4S4
induced cell apoptosis through changes in Bcl-2 and Bax, activation
of caspase-3 and cleavage of PARP. The results suggested the
apoptosis of NB4-R1 cells via a mitochondria-dependent pathway. In
addition, As4S4 may exert its cytotoxic
effects on NB4-R1 cells through blocking the cell cycle in the S
and G2/M phases. Thus, As4S4 may
be a potential anticancer drug candidate. The development of cell
apoptosis is a multi-factor, multi-step and multi-gene interactive
process. The signaling pathways and molecular mechanisms of
As4S4 in apoptotic regulation require further
investigations.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81000218). The authors would
like to express their gratitude to Dr Xinyang Wang for access to
the Oncology Research Laboratory, Key Laboratory of Environment and
Genes Related to Diseases (Xi'an, China) to complete the
experiments.
References
|
1
|
De Braekeleer E, Douet-Guilbert N and De
Braekeleer M: RARA fusion genes in acute promyelocytic leukemia: A
review. Expert Rev Hematol. 7:347–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo-Coco F, Avvisati G, Vignetti M, Thiede
C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona
E, et al: Gruppo Italiano Malattie Ematologiche dell'Adulto;
German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance
Leukemia: Retinoic acid and arsenic trioxide for acute
promyelocytic leukemia. N Engl J Med. 369:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fenaux P, Chevret S, Guerci A, Fegueux N,
Dombret H, Thomas X, Sanz M, Link H, Maloisel F, Gardin C, et al:
Long-term follow-up confirms the benefit of all-trans retinoic acid
in acute promyelocytic leukemia. European APL group. Leukemia.
14:1371–1377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Westervelt P, Brown RA, Adkins DR, Khoury
H, Curtin P, Hurd D, Luger SM, Ma MK, Ley TJ and DiPersio JF:
Sudden death among patients with acute promyelocytic leukemia
treated with arsenic trioxide. Blood. 98:266–271. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu DP, Qiu JY, Jiang B, Wang Q, Liu KY,
Liu YR and Chen SS: Tetra-arsenic tetra-sulfide for the treatment
of acute promyelocytic leukemia: A pilot report. Blood.
99:3136–3143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu HH, Wu DP, Jin J, Li JY, Ma J, Wang
JX, Jiang H, Chen SJ and Huang XJ: Oral tetra-arsenic tetra-sulfide
formula versus intravenous arsenic trioxide as first-line treatment
of acute promyelocytic leukemia: A multicenter randomized
controlled trial. J Clin Oncol. 31:4215–4221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi J, Zhang M, He P, et al: Establishment
of two-dimensional electrophoresis proteomic profiles of retinoid
acid resistant human acute promyelocytic leukemia NB4-R1 cells with
apoptosis induced by realgar. Chin J Integr Med. 31:391–396.
2011.
|
|
8
|
Wang XB, Gao HY, Hou BL, Huang J, Xi RG
and Wu LJ: Nanoparticle realgar powders induce apoptosis in U937
cells through caspase MAPK and mitochondrial pathways. Arch Pharm
Res. 30:653–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tse WP, Cheng CH, Che CT, Zhao M, Fan RQ
and Lin ZX: Realgar-mediated growth inhibition on HaCaT human
keratinocytes is associated with induction of apoptosis. Int J Mol
Med. 24:189–196. 2009.PubMed/NCBI
|
|
10
|
Rong Y and Distelhorst CW: Bcl-2 protein
family members: Versatile regulators of calcium signaling in cell
survival and apoptosis. Annu Rev Physiol. 70:73–91. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brajusković G, Orolicki SV, Cerović S,
Usaj SK, Marjanović S and Romac S: Bcl-2 and Bax protein
interaction in B-lymphocytes of peripheral blood in patients with
chronic lymphocytic leukemia. Vojnosanit Pregl. 62:357–363.
2005.(In Serbian). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sikdar S, Mukherjee A, Ghosh S and
Khuda-Bukhsh AR: Condurango glycoside-rich components stimulate DNA
damage-induced cell cycle arrest and ROS-mediated caspase-3
dependent apoptosis through inhibition of cell-proliferation in
lung cancer, in vitro and in vivo. Environ Toxicol Pharmacol.
37:300–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tong X, Han X, Yu B, Yu M, Jiang G, Ji J
and Dong S: Role of gap junction intercellular communication in
testicular leydig cell apoptosis induced by oxaliplatin via the
mitochondrial pathway. Oncol Rep. 33:207–214. 2015.PubMed/NCBI
|
|
15
|
Bayascas JR, Yuste VJ, Solé C,
Sánchez-López I, Segura MF, Perera R and Comella JX:
Characterization of splice variants of human caspase-activated
DNase with CIDE-N structure and function. FEBS Lett. 566:234–240.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boulares AH, Zoltoski AJ, Contreras FJ,
Yakovlev AG, Yoshihara K and Smulson ME: Regulation of DNAS1L3
endonuclease activity by poly(ADP-ribosyl)ation during
etoposide-induced apoptosis. Role of poly(ADP-ribose) polymerase-1
cleavage in endonuclease activation. J Biol Chem. 277:372–378.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen WX, Zhang F, Yang HC, et al: The
effect of realgar on apoptosis of transplanted ovarian SKOV3
carcinoma cells in nude mice. Tumor. 27:787–790. 2007.
|
|
18
|
Chen SY, Liu SX and Li XM: The dual
effects of realgar on acute promyelocytic leukemia cell: Inducing
apoptosis and promoting differentiation. J Xi'an Jiaotong Univ.
23:401–404. 2002.
|