Introduction
Sickness behavior is characterized as a reduction of
normal locomotor activities in response to circadian rhythms and
psychological changes that develop in inflammatory diseases,
including infections and cancers. Administration of the bacterial
endotoxin lipopolysaccharide (LPS) induces sickness behavior in
rodents. Recently, hypocretin (also known as orexin) signaling was
identified as one of the neural mediators against sickness behavior
(1). Grossberg et al (1), showed that LPS injection induced the
suppression of normal hypocretin signaling and inactivity. The
hypothalamic neuropeptide hypocretin regulates various
physiological processes such as wakefulness, sleep, food intake,
energy expenditure and reward (2,3). Expression
changes in the hypothalamic hypocretin have been reported regarding
food restriction (4) and other
physiological changes (5) to maintain
the vigilance state. However, there is no study of how the absence
of hypocretin is affected against sickness behavior.
In the present study, the significance of hypocretin
signaling against sickness behavior was examined using LPS-induced
inflammation in hypocretin-ataxin-3 transgenic mice, whose
hypocretin neurons were postnatally ablated (6). In addition, the present study is
important for the elucidation of the mechanism in narcolepsy
patients with inflammatory diseases, whose hypocretin neurons were
ablated (7).
Materials and methods
Ethical statement
All the experiments were conducted in accordance
with the ‘Guidelines for Care and Use of Laboratory Animals’ of the
National Institutes of Health and were approved by the Ethics
Committee on Animal Experiments of Tokyo Metropolitan Institute of
Medical Science (Tokyo, Japan).
Animals
The presence of the ataxin-3 transgene was
identified by polymerase chain reaction using tail DNA. Male mice
(12-week-old) were anesthetized and implanted with devices for
continuous monitoring of electroencephalography
(EEG)/electromyography (EMG), as described previously (8). Mice were housed under a 12-h light/dark
cycle with lights on from 8:00 a.m. to 8:00 p.m., corresponding to
Zeitgeber time (ZT) 0–12 h at 22–24°C, with ad libitum
access to food and water. Mice were allowed to habituate to the
recording conditions for 2 weeks.
LPS injections
On the second day at ZT8, mice received
intraperitoneal injections of either LPS (Sigma-Aldrich, St. Louis,
MO, USA) (250 µg/kg) dissolved in 0.9% saline or 0.9% saline alone.
Each mouse was recorded for 3 consecutive 24-h periods. EEG/EMG
were recorded without injection on the first day (intact day), and
mice received LPS or saline alone and underwent EEG/EMG on the
second day (LPS or saline day, respectively), and subsequently
EEG/EMG were recorded without injection on the third day again
(recovery day). A total of 4 male ataxin-3 transgenic mice and 4
matched wild-type littermates were recorded concurrently. EEG/EMG
signals were amplified using an EEG/EMG amplifier (MEG-6116; Nihon
Kohden Corp., Tokyo, Japan) through a 5-strand cable with a
slip-ring that allowed free movement of the mice. The amplifier was
connected to a personal computer with an analog-to-digital
converter and SleepSign software (Kissei Comtec, Nagano, Japan) for
acquiring and processing data. EEG/EMG records were visually scored
into 4-sec epochs of wakefulness (high EMG amplitude), rapid eye
movement (REM) sleep [silent low EMG amplitude, low EEG amplitude
with high values in the θ band (4.0–8.0 Hz)], and non-REM sleep
[low EMG amplitude, high EEG amplitude with high power density in
the δ band (0.5–4.0 Hz)] according to the standard criteria of
rodent sleep (9). Relative sleep and
wakefulness periods were standardized by the mean of each
proportion of the corresponding time on the intact day. The
standardized data are expressed as the mean of 4 independent
animals ± standard error.
Statistical analysis
Distributions of sleep/wakefulness periods in
ataxin-3 transgenic mice were compared to those in the matched
wild-type littermates using Student's t-test (two-tailed test).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Changes in sleep/wakefulness
periods
There was no significant difference regarding
non-REM sleep periods in the light phase following injection
(ZT9-ZT12) or wakefulness periods in the dark phase (ZT12-ZT18)
between the intact day and saline day in ataxin-3 transgenic mice
or matched wild-type littermates, respectively (data not
shown).
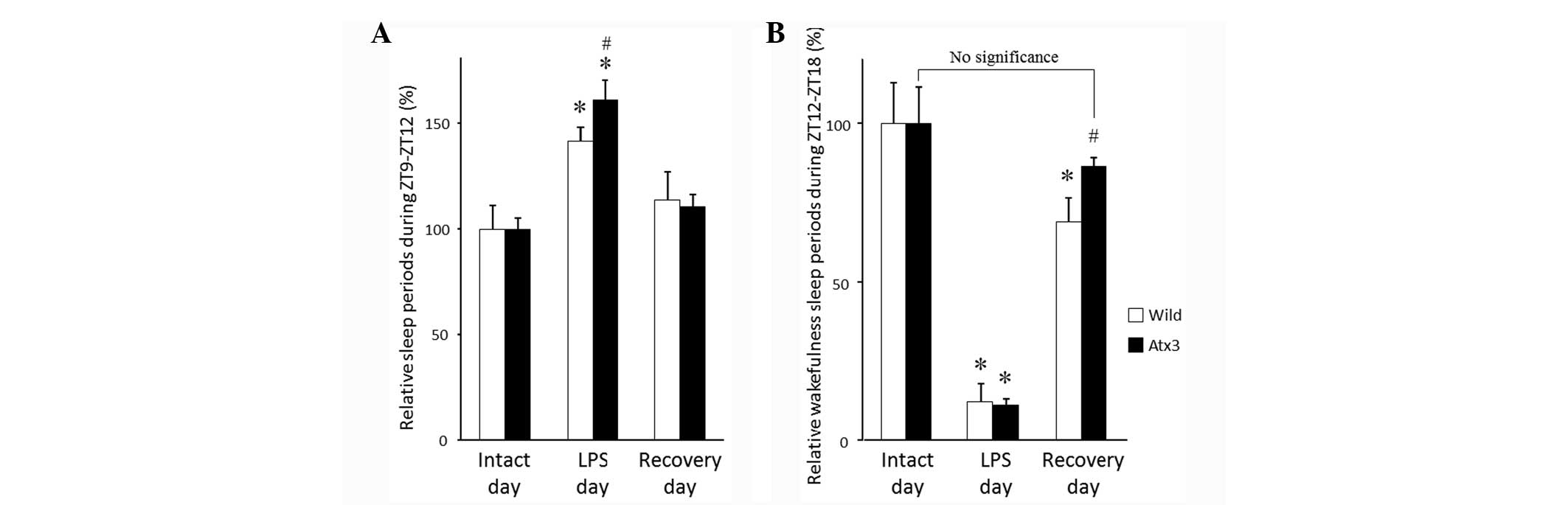
By contrast, non-REM sleep periods (ZT9-ZT12) were
significantly increased following LPS injection on the LPS day in
ataxin-3 transgenic mice (P<0.01) or matched wild-type
littermates (P<0.0005) compared with mice on the intact day
(Fig. 1A). Non-REM sleep periods
during ZT9-ZT12 of the ataxin-3 transgenic mice were significantly
increased following LPS injection on the LPS day compared with that
of the matched wild-type littermates (P<0.05) (Fig. 1A), suggesting that the ataxin-3
transgenic mice demonstrate more sleep during sickness behavior.
Wakefulness periods during ZT12-ZT18 were significantly decreased
following LPS injection on the LPS day in ataxin-3 transgenic mice
(P<0.0005) and matched wild-type littermates (P<0.0005)
compared with mice on the intact day (Fig.
1B). On the day following LPS injection (recovery day), the
significant reduction in the wakefulness period during ZT12-ZT18
was retained in the matched wild-type littermates (P<0.05);
however, there was no significant difference between wakefulness
periods on the intact day or recovery day in the ataxin-3
transgenic mice compared with mice on the intact day (Fig. 1B).
Discussion
Previously, LPS injection was reported to induce
increases in non-REM sleep (10). In
the present study, increases in the periods of non-REM sleep
induced by LPS injection were observed in the matched wild-type
littermates. In addition, further increases in sleep according to
hypocretin loss in ataxin-3 transgenic mice were demonstrated. The
marked reduction of activity was observed as expected following LPS
injection in the mouse lines. The marked increase in the quantity
of sleep was not observed on the recovery day in either line. The
complete recovery of the physical activity was not observed in the
matched wild-type littermates; however, it was confirmed that the
ataxin-3 transgenic mice recovered their physical activity to the
same as the intact day levels.
Hypocretin is known to regulate the sleep arousal
process. When there are no hypocretin neurons, it can be
hypothesized that the ataxin-3 transgenic mice will show an
increase in sleep. Hypocretin is suppressed in wild-type mice by
inflammation (1); however, it is
believed that hypocretin neurons are not completely inhibited in
LPS-induced sickness behavior of wild-type mice. Therefore,
differences in sickness behavior between ataxin-3 transgenic mice
and matched wild-type littermates on the LPS day in the present
study may be explained with/without hypocretin signaling.
Notably, it was suggested that the recovery from
sickness in the ataxin-3 transgenic mice was quicker when compared
with that in the matched wild-type littermates. As the ataxin-3
transgenic mice get more sleep, it is suggested that their faster
recovery is the result of longer rest on the LPS day.
Hypocretin is necessary for healthy people to
maintain wakefulness. Hypocretin, however, may be one of the
factors preventing recovery during sickness. The evolutionary merit
of this system is explained by wild animals, for whom an extended,
deep sleep may be dangerous. By contrast, humans are usually
completely protected when sick. Therefore, recovery during a cold
may be faster if combination therapies involving cold medicine and
a hypocretin antagonist (11,12) are administered.
Acknowledgements
The present study was supported by JSPS KAKENHI
grant no. 23700458 to S. Tanaka.
References
|
1
|
Grossberg AJ, Zhu X, Leinninger GM,
Levasseur PR, Braun TP, Myers MG Jr and Marks DL:
Inflammation-induced lethargy is mediated by suppression of orexin
neuron activity. J Neurosci. 31:11376–11386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Lecea L and Sutcliffe JG: The
hypocretins and sleep. FEBS J. 272:5675–5688. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakurai T: The neural circuit of orexin
(hypocretin): Maintaining sleep and wakefulness. Nat Rev Neurosci.
8:171–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mieda M, Willie JT, Hara J, Sinton CM,
Sakurai T and Yanagisawa M: Orexin peptides prevent cataplexy and
improve wakefulness in an orexin neuron-ablated model of narcolepsy
in mice. Proc Natl Acad Sci USA. 101:4649–4654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harris GC and Aston-Jones G: Arousal and
reward: A dichotomy in orexin function. Trends Neurosci.
29:571–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hara J, Beuckmann CT, Nambu T, et al:
Genetic ablation of orexin neurons in mice results in narcolepsy,
hypophagia and obesity. Neuron. 30:345–354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peyron C, Faraco J, Rogers W, et al: A
mutation in a case of early onset narcolepsy and a generalized
absence of hypocretin peptides in human narcoleptic brains. Nat
Med. 6:991–997. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chemelli RM, Willie JT, Sinton CM, et al:
Narcolepsy in orexin knockout mice: Molecular genetics of sleep
regulation. Cell. 98:437–451. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radulovacki M, Virus RM, Djuricic-Nedelson
M and Green RD: Adenosine analogs and sleep in rats. J Pharmacol
Exp Ther. 228:268–274. 1984.PubMed/NCBI
|
|
10
|
Morrow JD and Opp MR: Diurnal variation of
lipopolysaccharide-induced alterations in sleep and body
temperature of interleukin-6-deficient mice. Brain Behav Immun.
19:40–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao M and Guilleminault C: Hypocretin and
its emerging role as a target for treatment of sleep disorders.
Curr Neurol Neurosci Rep. 11:227–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roecker AJ and Coleman PJ: Orexin receptor
antagonists: Medicinal chemistry and therapeutic potential. Curr
Top Med Chem. 8:977–987. 2008. View Article : Google Scholar : PubMed/NCBI
|