Introduction
Proton pump inhibitors (PPIs) are commonly used to
treat acid reflux disease and ulcers of the stomach and the
duodenum. These compounds include omeprazole, rabeprazole,
esomeprazole, lansoprazole and pantoprazole, which are available
under a variety of brand names (1).
Multiple studies have examined the cells that line the stomach,
regarding the reduction of acid production, which is relatively
well-understood (2). Recently,
however, it was reported that treatment with PPIs could worsen the
symptoms of vitiligo in patients suffering from this skin
pigmentation disorder (3). In one
study, the investigators demonstrated that PPI treatment could lead
to increased destruction of melanocytes in vitiligo patients via
the induction of apoptosis (4).
Furthermore, one African-American patient receiving PPIs proceeded
to widespread vitiligo at the same time as when the daily use of a
PPI, 40 mg esomeprazole, began during the 3 years. Following the
termination of esomeprazole intake, these patients were able to
initiate an extremely slow repigmentation (5). The effects of PPIs on melanogenesis,
however, have rarely been recognized.
Melanogenensis is a complex process involving
numerous different metabolic signals and a series of enzymes. In
human melanocytes, the melanin pigment is derived from
hydroxylation of L-tyrosine to L-3,4-dihydroxyphenylalanine
(L-DOPA) by tyrosinase (TYR) (EC 1.14.18.1) with two copper ions at
the active center (6). Overexpression
of the genes involved in pigment formation was shown to result in
pigmentation disorders, including lentigo senilis and urticaria
pigmentosa, as well as in skin discoloration issues, such as
melasma, freckles and chloasma. Thus, TYR is a primary target of
screens designed to identify depigmentation agents. In addition to
its role in pigmentation disorders, TYR has been revealed to have a
role in Parkinson's disease (7) and
other neurodegenerative diseases (8).
Therefore, the development of anti-melanogenic agents is an
important goal for the cosmetic and medicinal industry (9). Melanin biosynthesis is modulated by the
expression of microphthalmia-associated transcription factor
(MITF), which regulates the transcriptional expression of the
TYR, TYR-related protein-1 (TRP-1) and TRP-2
genes in melanocytes (10).
A previous study demonstrated that omeprazole
treatment significantly reduced melanogenesis by inhibiting
ATP7A gene expression and by enhancing degradation of TYR
(11); however, the anti-melanogenic
effects of other PPIs are yet to be examined. The objective of the
present study was to assess the effects of PPIs on melanogenesis in
melanocytes.
Materials and methods
Materials
Arbutin, L-DOPA, L-tyrosine, N-phenylthiourea
(PTU), 12-O-tetradecanoylphorbol-13-acetate (TPA), TYR,
sodium hydroxide (NaOH), thiazolyl blue tetrazolium bromide (MTT),
dimethyl sulfoxide (DMSO), rabeprazole, esomeprazole, lansoprazole
and pantoprazole were obtained from Sigma-Aldrich (St. Louis, MO,
USA). All other reagents and chemicals were of high-grade and are
commercially available.
Measurement of mushroom TYR
activity
TYR activity was measured as described previously
(6). The mushroom TYR (EC 1.14.18.1)
reactions were performed in 96-well microplates (SPL Life Sciences
Co., Ltd., Pocheon, Gyeonggi, Korea) and consisted of 150 µl of 0.1
M phosphate buffer (pH 6.5), 3 µl of test sample, 36 µl of 1.5 mM
L-tyrosine and 7 µl of mushroom TYR [2,100 U/ml in 0.05 M phosphate
buffer, (pH 6.5)]. The initial absorbance of each mixture and the
absorbance after incubation at 37°C for 30 min, was measured at 490
nm using a microplate reader (PerkinElmer, Waltham, MA, USA). The
inhibitory activity was subsequently calculated using the following
formula: Inhibitory activity (%) = [(A - B) -
(C - D)]/(A - B) × 100; where A
refers to the absorbance of the control sample following the
reaction, B is the absorbance of the control sample prior to
the reaction, C is the absorbance of the treated sample
following the reaction and D is the absorbance of the
treated sample prior to the reaction.
Measurement of copper-chelating
activity
The copper-chelating activity was determined using
the pyrocatechol violet (PV) method as described previously, and
with slight modifications (12).
Samples were mixed with 1 ml of 50 mM sodium acetate buffer (pH
6.0) and 100 µl of 2 mM CuSO4. After 10 min of
incubation at room temperature, 100 µl of 2 mM PV was added.
Solutions were incubated for 20 min at room temperature and the
absorbance of each sample at 632 nm (A632) was measured.
DMSO and PTU were used as a negative control and as a standard
metal chelator, respectively. The copper-chelating activity was
calculated using the following formula: Copper-chelating activity
(%) = [1 - (A - B)/(C - D)] × 100; where A is the A632
of the sample + buffer + CuSO4 + PV mixture, B is the
A632 of the sample + buffer + buffer + PV mixture, C is
the A632 nm of the buffer + buffer + CuSO4 +
PV mixture and D is the A632 nm of the buffer + buffer +
buffer + PV mixture.
Cell cultures
The melan-a immortalized mouse melanocyte cell line,
which was derived from B57BL6 mice (13), was purchased from Bennett et al
(13) (St. George's University of
London, London, UK). The cells were cultured in RPMI-1640 medium
(HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(HyClone), streptomycin-penicillin (100 µg/ml each; HyClone) and
200 nM TPA (a potent tumor promoter) at 37°C in 5% CO2.
Cells were passaged every 3 days up to a maximal passage number of
40. Confluent monolayers of melanocytes were harvested using a
mixture of 0.05% trypsin and 0.53 mM EDTA (Lonza, Basel,
Switzerland).
Cell viability assay
Cell viability was determined using an MTT assay
(6). Several concentrations of test
samples (25, 50, 100 and 200 µM) were added to the cells and
incubated for 24 h. A total of 100 µl of MTT solution [5 mg/ml MTT
in phosphate-buffered saline (PBS)] was added to each well and
samples were incubated at 37°C for 1 h. After removal of the MTT
solution, 1 ml of DMSO was added to each well with vigorous mixing.
The absorbance of each well at 470 nm was determined using a
microplate reader (PerkinElmer).
Melanization inhibition assay using
melan-a cells
Cells were seeded in 24-well plates (BD Biosciences,
Franklin Lakes, NJ, USA) at a density of 1×105
cells/well and allowed to attach overnight. The supernatants were
subsequently removed and replaced with fresh media containing
various concentrations (25, 50, 100 and 200 µM) of the test
compounds. Cells were cultured for 72 h and further incubated for 1
day. Following washing with PBS, the cells were lysed with 250 µl
of 1 N NaOH and transferred to 96-well plates. The melanin content
was estimated by measuring the absorbance at 405 nm using a
microplate reader (PerkinElmer). Arbutin was used as a positive
control (10).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis of mRNA expression
Total RNA was extracted from melan-a cells using
TRIzol reagent (Invitrogen Life Technologies, Waltham, MA, USA),
according to the manufacturer's instructions, with slight
modification (14). Total RNA (2 µg)
was reverse transcribed with RT (MP Biomedicals, Santa Ana, CA,
USA) and oligo (dT) primers. cDNA was amplified using a PCR Thermal
Cycler Dice TP600 (Takara Bio, Inc., Shiga, Japan) and
oligonucleotide primers specific for mouse transcripts (6). The resulting PCR products were visualized
by ethidium bromide staining following agarose gel
electrophoresis.
Statistical analysis
Data are expressed as the means ± standard
deviation. Statistical significance was determined by the Student's
t-test for independent means using Microsoft Excel software
(Microsoft Corporation, Redmond, WA, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of PPIs on TYR and
copper-chelating activity
The effect of PPIs on the activity of TYR, a crucial
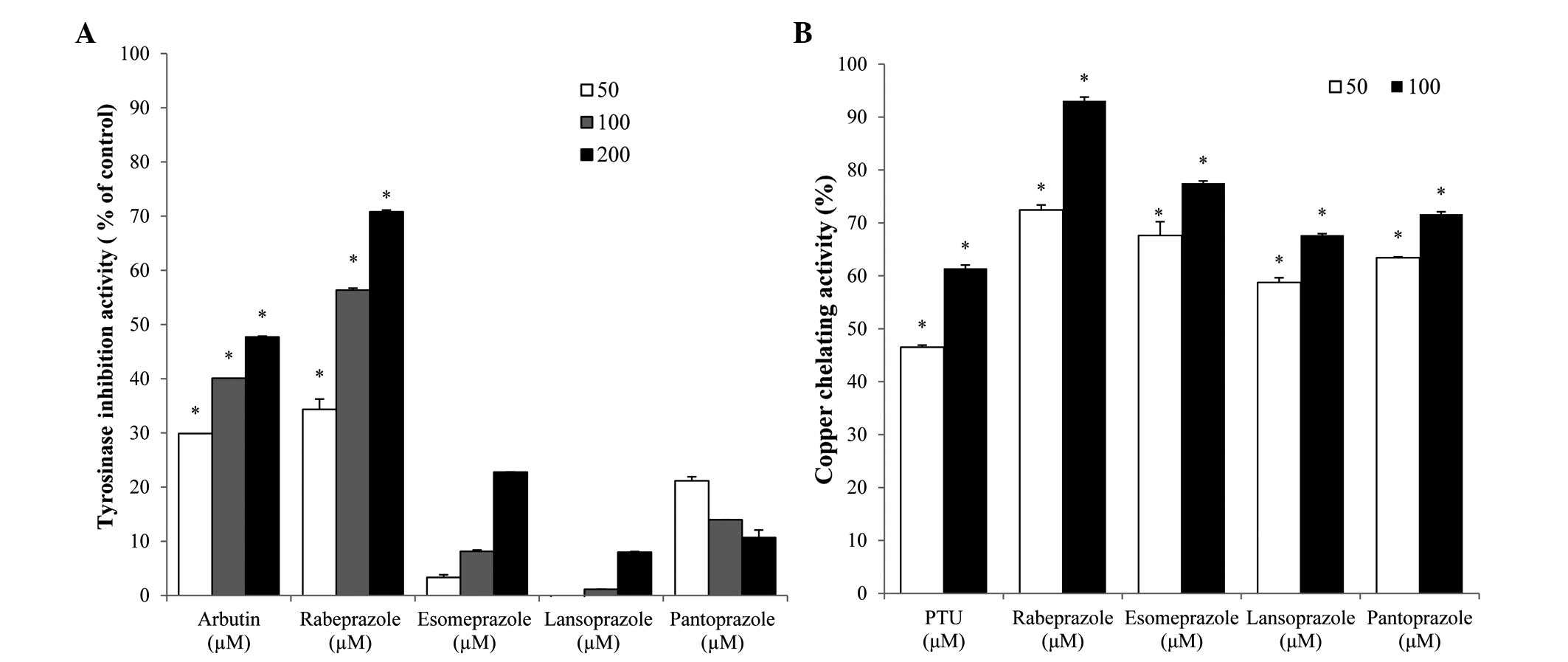
enzyme for melanin synthesis, was examined. As depicted in Fig. 1, rabeprazole treatment resulted in
significant, dose-dependent inhibition of TYR activity and the 50%
inhibitory concentration (IC50) of this compound was
110.1 µM. The IC50 of the arbutin positive control was
~212.3 µM (Fig. 1A). By contrast, the
PPIs esomeprazole, lansoprazole and pantoprazole exerted no
inhibitory effect on TYR activity.
To understand the role of metal chelation in PPI TYR
inhibition, the copper-chelating activities of each PPI were
examined. In general, each of the PPIs tested exhibited strong
copper-chelation activities. The copper chelation values of the
PPIs were higher compared with that of the PTU positive control
(Fig. 1B). While esomeprazole,
lansoprazole and pantoprazole did not affect TYR activity, these
compounds did exhibit copper-chelating activity. Conversely,
rabeprazole exhibited TYR inhibitory and copper-chelating activity,
indicating that this compound may inhibit TYR by chelating
copper.
Effects of PPIs on cell viability and
melanin content in melan-a cells
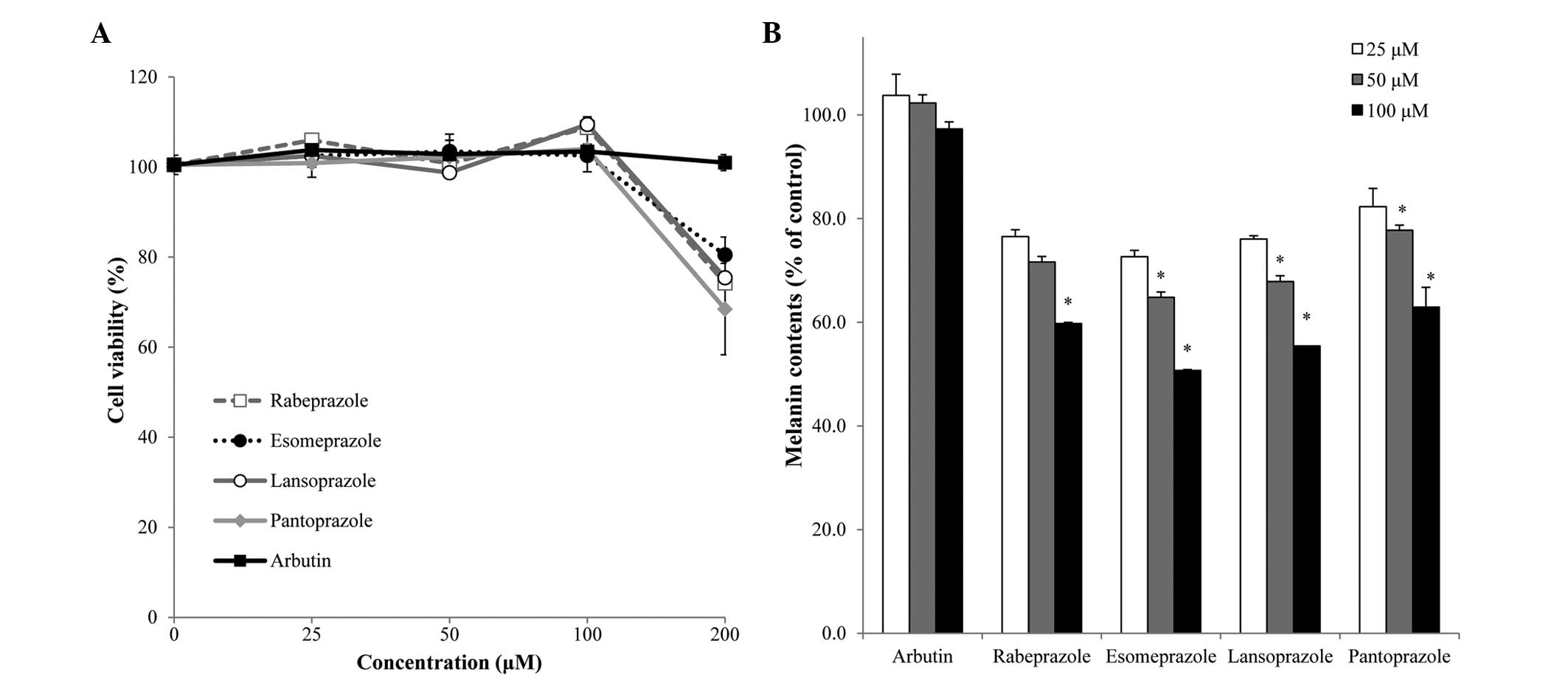
To examine the effect of PPIs on melanin
biosynthesis, melan-a cells were treated with various
concentrations (25, 50, 100 and 200 µM) of PPIs, and melanin
content and cell viability were subsequently assessed. No
cytotoxicity was observed in the melanocytes treated with PPIs or
arbutin at concentrations of ≤100 µM (Fig.
2A). Cytotoxicity in melan-a populations treated with 200 µM of
rabeprazole, esomeprazole, lansoprazole and pantoprazole was
observed as ~25.8±6.9, 19.5±3.9, 24.6±5.8 and 31.6±10.1%,
respectively.
At a concentration of 100 µM, PPI treatment resulted
in significant decreases in melanin content without inducing cell
death. Specifically, treatment with rabeprazole, esomeprazole,
lansoprazole and pantoprazole resulted in 40.2±0.2, 49.3±0.2,
44.6±0.0 and 37.1±3.8% decreases in melanin content, compared with
that of the control cell population, respectively (Fig. 2B). These results indicate that
treatment with PPIs decreased melanin synthesis without influencing
melanocyte viability in vitro.
Effects of PPIs on expression of
melanogenesis-associated genes in melan-a cells
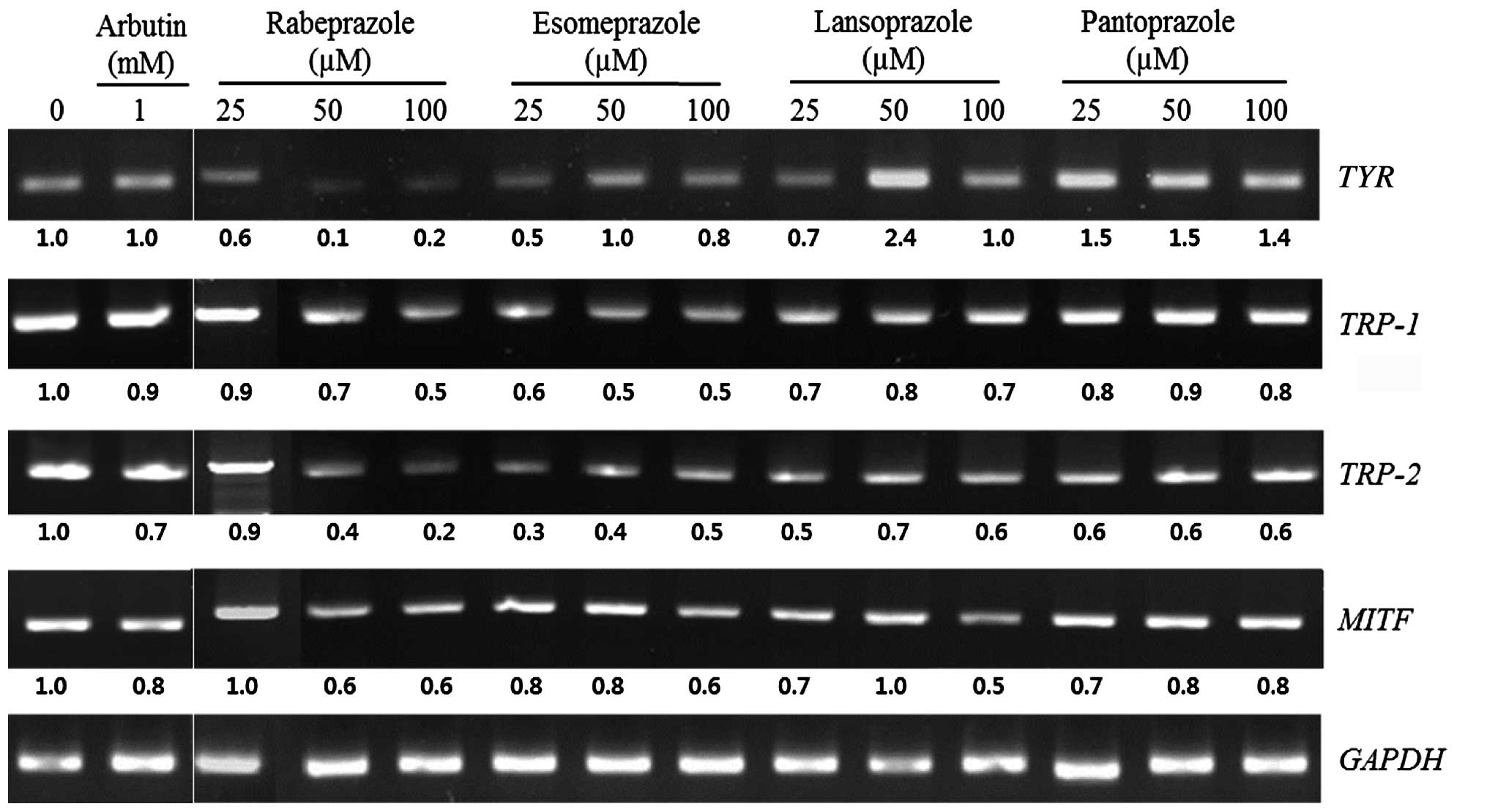
The influence of PPIs on the mRNA levels of the
TYR, TRP-1, TRP-2 and MITF genes was
examined using RT-PCR. Treatment with 100 µM rabeprazole suppressed
the expression of the TYR, TRP-1, TRP-2 and
MITF genes by 77.1, 48.3, 76.2 and 37.1%, respectively.
Esomeprazole (100 µM) decreased the expression of these genes by
23.1, 47.9, 53.9 and 30.7%, respectively, and 100 µM lansoprazole
inhibited the expression of the TRP-1, TRP-2 and
MITF by 25.9, 44.9 and 52.8%, respectively. Treatment with
100 µM pantoprazole suppressed the mRNA expression of the
TRP-1, TRP-2 and MITF genes by 22.3, 37.9 and
15.7%, respectively (Fig. 3). Taken
together, these results demonstrate that rabeprazole treatment
affected mRNA expression of each of the melanogenesis-associated
genes.
Discussion
The present study demonstrated that PPIs act as
inhibitors of melanogenesis in melanocytes in vitro. Melanin
biosynthesis begins with the hydroxylation of tyrosine to L-DOPA by
TYR. In the absence of thiols, the second enzyme, TRP-2, enables a
rapid conversion of dopaquinone to dopachrome, and subsequently to
5,6-dihydroxyindole (DHI) or indole 5,6-quinone 2-carboxylic acid
(DHICA). TRP-1 catalyzes the oxidation of DHICA to produce
eumelanins (15). The active site of
TYR contains a binuclear copper center, which catalyzes the
hydroxylation of phenols to catechols and the oxidation of
catechols to quinones (16).
Rabeprazole was the only PPI tested that exhibited mushroom TYR
inhibitory activity and copper-chelating activity (Fig. 1). Each of the PPIs tested decreased the
melanin content of melan-a cells without inducing cytotoxicity
(Fig. 2). Notably, while treatment
with esomeprazole, lansoprazole and pantoprazole had no effect on
TYR inhibitory activity, these compounds exhibited copper-chelating
activity and mediated decreases in melanin formation in
melanocytes. These data suggest that esomeprazole, lansoprazole and
pantoprazole inhibit melanogenesis via their copper-chelating
activity and not via effects on TYR activity.
In mammalian melanocytes, melanin biosynthesis is
directly modulated by 3 melanocyte-specific enzymes: TYR, TRP-1 and
TRP-2. These melanocyte-specific enzymes are directly regulated by
MITF, which is known to be the integral transcription factor for
the regulation of melanocyte differentiation and pigmentation
(17). Therefore, the mRNA levels of
TYR, TRP-1, TRP-2 and MITF were
measured following PPI treatment. Rabeprazole strongly suppressed
the expression of each of the melanogenesis-associated genes,
indicating that this compound transcriptionally regulates
TYR, TRP-1 and TRP-2 expression through
MITF during melanin biosynthesis. Treatment with
lansoprazole also resulted in decreased MITF mRNA levels;
however, these compounds had no effect on TYR expression
levels. Therefore, esomeprazole, lansoprazole and pantoprazole were
predicted to inhibit melanin formation through some other
mechanism. Melanogenesis is modulated by various stimuli, including
ultraviolet irradiation, hormone treatment and genetic mutations.
Stimulation of the melanocortin 1 receptor (also known as
melanocyte-stimulating hormone receptor) activates adenyl cyclase
through G protein signaling, thereby increasing cyclic adenosine
monophosphate production, which activates the expression of MITF
(6). It has also been reported that
stem cell factor (SCF)/c-kit signaling is associated with the
survival, proliferation and differentiation of melanocytes
(18). In the epidermis, SCF produced
by keratinocytes binds to the receptor on melanocytes and induces
the activation of the melanogenesis signaling cascade (19). Thus, further studies are necessary to
examine the effects of PPIs on other melanogenic processes.
Previous reports have indicated that PPIs induce
depigmentation in patients with vitiligo. In one study, patients
developed vitiligo following the use of oral PPIs for treatment of
occurred gastritis (20).
Additionally, Schallreuter and Rokos (5) identified that the progress of
repigmentation was much slower in patients who had received PPI
treatment compared with those who had previously not been exposed
to these compounds. PPIs are widely used to treat stomach and
intestinal diseases. PPIs inhibit gastric acid secretion by
blocking the gastric H+/K+-ATPase that is
necessary for the final step of this process (21). Melanocytes also express an
H+/K+-ATPase at the cytoplasmic membrane and
melanosomes contain a vacuolar type H+-ATPase (4). Matsui et al (11) demonstrated that omeprazole reduces
melanogenesis by inhibiting ATP7A expression and by
enhancing degradation of TYR. The present data are consistent with
recent findings that pantoprazole decreased melanin content and TYR
activity in B16 melanoma cells. However, the actions of
rabeprazole, esomeprazole and lansoprazole were not delineated.
Nevertheless, the study provides novel information regarding the
inhibitory effects of PPIs on melanogenesis.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(grant no. NRF-2014R1A2A2A01006882).
References
|
1
|
Jain KS, Shah AK, Bariwal J, Shelke SM,
Kale AP, Jagtap JR and Bhosale AV: Recent advances in proton pump
inhibitors and management of acid-peptic disorders. Bioorg Med
Chem. 15:1181–1205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rew JS: Clinical use of proton pump
inhibitors in gastrointestinal diseases. Korean J Gastroenterol.
47:181–190. 2006.PubMed/NCBI
|
|
3
|
Shin JM, Lee JY, Lee DY, Yoon TY, Lee JC,
Lim EH, Sohn KC, Lee YH, Im M, Seo YJ, et al: Proton pump
inhibitors as a possible cause of vitiligo: An in vivo and in vitro
study. J Eur Acad Dermatol Venereol. 28:1475–1479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Namazi MR: Proton pump inhibitors may
trigger vitiligo by rendering melanocytes prone to apoptosis. Br J
Dermatol. 158:844–845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schallreuter KU and Rokos H: From the
bench to the bedside: Proton pump inhibitors can worsen vitiligo.
Br J Dermatol. 156:1371–1373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baek SH, Ahn JW, Nam SH, Yoon CS, Shin JC
and Lee SH: S-(−)-10,11-dihydroxyfarnesoic acid methyl ester
inhibits melanin synthesis in murine melanocyte cells. Int J Mol
Sci. 15:12750–12763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan T, Li X and Jankovic J: The
association between Parkinson's disease and melanoma. Int J Cancer.
128:2251–2260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan T, Zhu J, Hwu WJ and Jankovic J: The
role of alpha-synuclein in melanin synthesis in melanoma and
dopaminergic neuronal cells. PLoS One. 7:e451832012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parvez S, Kang M, Chung HS and Bae H:
Naturally occurring tyrosinase inhibitors: Mechanism and
applications in skin health, cosmetics and agriculture industries.
Phytother Res. 21:805–816. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JH, Baek SH, Kim DH, Choi TY, Yoon TJ,
Hwang JS, Kim MR, Kwon HJ and Lee CH: Downregulation of melanin
synthesis by haginin A and its application to in vivo lightening
model. J Invest Dermatol. 128:1227–1235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsui MS, Petris MJ, Niki Y,
Karaman-Jurukovska N, Muizzuddin N, Ichihashi M and Yarosh DB:
Omeprazole, a gastric proton pump inhibitor, inhibits melanogenesis
by blocking ATP7A trafficking. J Invest Dermatol. 135:834–841.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rao F, Yuting Z, Yiran G and Fang C:
Antioxidant and tyrosinase inhibition activities of the
ethanol-insoluble fraction of water extract of Sapium
sebiferum (L.) Roxb. leaves. South Afr J Bot. 93:98–104. 2014.
View Article : Google Scholar
|
|
13
|
Bennett DC, Cooper PJ and Hart IR: A line
of non-tumorigenic mouse melanocytes, syngeneic with the B16
melanoma and requiring a tumour promoter for growth. Int J Cancer.
39:414–418. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heo JC and Lee SH: Alleviation of
asthma-related symptoms by a derivative of L-allo threonine. Int J
Mol Med. 31:881–887. 2013.PubMed/NCBI
|
|
15
|
Chao HC, Najjaa H, Villareal MO, Ksouri R,
Han J, Neffati M and Isoda H: Arthrophytum scoparium inhibits
melanogenesis through the down-regulation of tyrosinase and
melanogenic gene expressions in B16 melanoma cells. Exp Dermatol.
22:131–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beberok A, Wrześniok D, Otręba M and
Buszman E: Impact of sparfloxacin on melanogenesis and antioxidant
defense system in normal human melanocytes HEMa-LP - An in vitro
study. Pharmacol Rep. 67:38–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tuerxuntayi A, Liu YQ, Tulake A, Kabas M,
Eblimit A and Aisa HA: Kaliziri extract upregulates tyrosinase,
TRP-1, TRP-2 and MITF expression in murine B16 melanoma cells. BMC
Complement Altern Med. 14:166–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hachiya A, Kobayashi A, Ohuchi A, Takema Y
and Imokawa G: The paracrine role of stem cell factor/c-kit
signaling in the activation of human melanocytes in
ultraviolet-B-induced pigmentation. J Invest Dermatol. 116:578–586.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shin SH and Lee YM: Glyceollins, a novel
class of soybean phytoalexins, inhibit SCF-induced melanogenesis
through attenuation of SCF/c-kit downstream signaling pathways. Exp
Mol Med. 45:e172013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holla AP, Kumar R, Parsad D and Kanwar A:
Proton pump inhibitor induced depigmentation in vitiligo. J Cutan
Aesthet Surg. 4:46–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin JM and Sachs G: Pharmacology of
proton pump inhibitors. Curr Gastroenterol Rep. 10:528–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|