Introduction
Epidemiological investigation has shown the
association between the risk of breast cancer and total fat intake
(1). However, rather than total fat
intake, subtypes of fatty acids are considered to have a more
influential effect on breast cancer. Increased intake of n-3 fatty
acids derived from marine products and decreased intake of n-6
fatty acids found in vegetable oils and processed foods results in
a lower n-6/n-3 ratio and decreased breast cancer risk (2). Rat mammary cancer growth is suppressed by
eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)
(3,4) and
accelerated by n-6 fatty acids, such as linoleic acid (LA)
(5). An increased n-6/n-3 ratio is
associated with rat mammary carcinogenesis (6). In agreement with rat mammary
carcinogenesis studies, laboratory experiments have shown that n-3
fatty acids, such as EPA, suppress human breast cancer cell growth
(7), while n-6 fatty acids, such as LA
and arachidonic acid (AA), promote the growth of human breast
cancer cells (7,8). Experimental evidence shows critical roles
for n-3 and n-6 fatty acids in association with breast cancer
growth.
In contrast to n-3 and n-6 fatty acids, the role of
n-9 fatty acids in breast cancer has not been studied in detail.
One study showed that <15% of breast cancers could be prevented
if the populations of high-income countries shifted to the
traditional Mediterranean diet (9).
The Mediterranean diet contains high amounts of olive oil rich in
n-9 oleic acid (OA), and the possible effect of OA in suppressing
breast cancer has received attention. However, another study was
inconclusive in showing that olive oil consumption lowers the
breast cancer risk (10). In cell
culture, although OA causes growth inhibition at higher
concentrations, it produces growth acceleration of human breast
cancer cells at lower concentrations (11). Thus, the effects of OA on breast cancer
appear to be complex. Mead acid (MA) is an n-9 fatty acid produced
from OA when essential n-3 and n-6 fatty acids are deficient;
mammals elongate and desaturate OA to make the end product MA
(12,13). Epidemiologically, MA was inversely
associated with breast cancer risk as well as overall cancer risk
(14). Experimentally, MA suppressed
MCF-7 and KPL-1 human breast cancer cell growth in culture
(15,16). MCF-7 and KPL-1 are luminal A subtypes
according to intrinsic subtype classification (17). MA also suppressed the growth and
metastasis of KPL-1 cells transplanted in female athymic mice
(16).
Mammary carcinogenesis is a multistep process in
which normal breast epithelial cells experience DNA damage
(initiation phase), followed by enhanced cell proliferation
(promotion phase), and subsequently the acquisition of metastasis
(progression phase) to acquire a malignant potential. According to
in vivo and in vitro studies using breast cancer cell
lines, MA suppressed the promotion/progression phase of
carcinogenesis; however, the role of MA in the initiation phase
remains to be elucidated. In contrast to using breast cancer cell
lines, rodent mammary cancers induced by chemical carcinogens can
be preferentially used to evaluate the initiation/promotion stage
of mammary carcinogenesis. However, metastasis is hardly detectable
in rodent systems. Therefore, the present study was designed to
determine if MA can block the initiation/promotion stage of mammary
carcinogenesis to explore the possible use of MA as a
chemotherapeutic agent and a chemopreventive agent for mammary
cancer. The intrinsic classification of breast cancer can recommend
effective targeted therapy and predict responses to chemotherapy
(18). Therefore, the intrinsic
subtypes of chemically induced rat mammary cancers were determined
to see the effects of MA in association with the intrinsic subtypes
of induced mammary cancers.
Materials and methods
Diet
The experimental diets contained the same amount of
nutrients but with different fatty acid compositions (Table I). In brief, the MA diet and control
(CTR) diet were modifications of the AIN-76 diet. The MA diet
contained 5% SUNTGM33 (Suntory Wellness, Tokyo, Japan), which
contains 48.0% MA. SUNTGM33 is microbial oil obtained by fungal
fermentation (19). The CTR diet
contained 5% olive oil (Nacalai Tesque, Kyoto, Japan), which
contains 74.7% OA; OA is a precursor of MA. The detailed fatty acid
composition of SUNTGM33 and olive oil has been described
previously; the MA diet contained 2.4% MA while the CTR diet
contained 0% MA (16). Each
experimental diet was formulated by Oriental Yeast (Tokyo,
Japan).
 | Table I.Composition of experimental diets. |
Table I.
Composition of experimental diets.
| Components | MA diet | CTR diet |
|---|
| Casein | 20 | 20 |
| DL-Methionine | 0.3 | 0.3 |
| Cornstarch | 43 | 43 |
| α-Cornstarch | 12 | 12 |
| Sucrose | 10 | 10 |
| Cellulose |
5 |
5 |
| AIN-76 mineral
mix | 3.5 | 3.5 |
| AIN-76 vitamin
mix |
1 |
1 |
| Choline
bitartrate | 0.2 | 0.2 |
| SUNTGM33 |
5 |
0 |
| Olive oil |
0 |
5 |
Carcinogen
N-methyl-N-nitrosourea (MNU) in a
powder form was obtained from Sigma (St. Louis, MO, USA) and was
stored at 4°C in the dark. Immediately prior to use, MNU was
dissolved in physiological saline containing 0.1% acetic acid, and
a 5 mg/ml solution was prepared. A single dose of 50 mg/kg body
weight was administered intraperitoneally.
Animals and experimental
procedures
The study protocol and animal procedures were
approved by the Animal Care and Use Committee of Kansai Medical
University (Hirakata, Osaka, Japan; permit no. 14–111). In brief,
52 6-week-old virgin female Sprague-Dawley rats [Crl:CD(SD)] were
purchased from Charles River Japan (Hino, Japan). They were housed
in groups of 4 or 5 in plastic cages with paper bedding (Paper
Clean, SLC, Hamamatsu, Japan) in a specific pathogen-free
environment maintained at 22±2°C and 60±10% relative humidity with
a 12-h light/dark cycle (lights on at 8:00 a.m. and lights off at
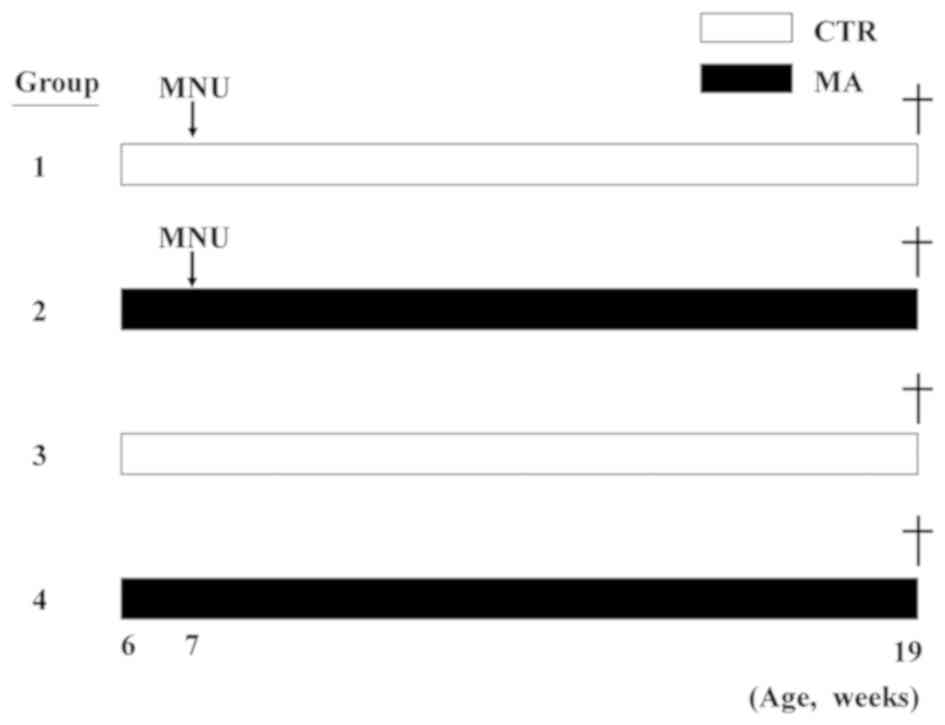
8:00 p.m.). The rats were randomly divided into four groups, which
were the CTR diet group and the MA diet group with or without MNU
(each n=13, Fig. 1). Fresh sterilized
stocks of the pellet diet were provided to the animals twice a week
starting at 6 weeks of age with any remaining diet being discarded
to minimize the ingestion of oxidized fatty acid. Half of the
animals received MNU at 7 weeks of age, and all animals remained on
the same diets for the remainder of the experiment (until 19 weeks
of age). Experimental diets and water were available freely. During
the experiment, the dose of diet ingested, body weight and tumor
volume were measured once a week. The tumor volume was calculated
using the standard formula: Width2 x length × 0.5. At
sacrifice, blood was sampled by inferior vena cava puncture and
subsequently the animals were sacrificed by exsanguination from
aortic transection. At necropsy, all the organs were examined
macroscopically, and macroscopically abnormal organs, mammary
glands and mammary tumors were examined histologically. Tissues
were fixed in 10% neutral buffered formalin, embedded in paraffin,
and stained with hematoxylin and eosin (HE); blood samples and
sections of the non-tumorous inguinal mammary tissues were used for
fatty acid analysis. Throughout the experiments, animals were cared
for in accordance with the Guidelines for Animal Experimentation of
Kansai Medical University.
Cell kinetics and microvessel
density
The cell kinetics (cell proliferation and cell
death) in the 6 largest MNU-induced tumors (Groups 1 and 2) were
evaluated. Cell proliferation was evaluated by anti-Ki-67 (Clone
SP6, ready to use; Nichirei Biosciences, Tokyo, Japan). Cell death
was evaluated by anti-phospho-histone H2A.X (γ-H2A.X) antibody
(Clone Ser139, 1:100; Cell Signaling, Danvers, MA, USA), an
immunomarker of the DNA damage response. Microvessel density was
evaluated by anti-CD34 antiserum (polyclonal, 1:50; Aviva Systemic
Biology, San Diego, CA, USA). Immunohistochemistry was performed
with the Histofine MAX-PO for rats kit (Nichirei Biosciences)
according to the manufacturer's protocol. Each slide was scanned
with a high-resolution digital scanner (NanoZoomer 2.0 Digital
Pathology; Hamamatsu Photonics, Hamamatsu, Japan) to prepare
digital images. The NDPI image files were opened in color mode with
NDP.view software (Hamamatsu Photonics). The images were changed to
JPEG files at magnification, ×40 in five randomly selected areas
within each tumor that was used to analyze immunohistochemical
staining (20,21). The Ki-67 and γ-H2A.X indexes were
assessed by positive cells/1,000 cells as an index of cell
kinetics, and CD34 was assessed by positive area/1 mm2
as a parameter of tumor angiogenesis.
Immunohistochemistry-based surrogate
intrinsic subtyping of MNU-induced mammary tumors
The intrinsic subtype of the MNU-induced tumors was
evaluated using the largest mammary tumor in each rat (Group 1,
n=13; Group 2, n=8). Estrogen receptor (ER) was visualized by
anti-ER antibody (Clone 6F11, 1:40; Leica Biosystems Newcastle,
Newcastle upon Tyne, UK), progesterone receptor (PgR) by anti-PgR
antibody (Clone PR6, 1:100; Abcam, Cambridge, UK), and HER2/neu by
anti-c-erbB2/Her2/neu antibody (Clone e2–4000+H3B5 antibody
cocktail, 1:300; Thermo Scientific, Waltham, MA, USA). Positive
status was evaluated according to previous studies (22–24). In
brief, ER and PgR were considered positive when there were ≥1%
positive tumor nuclei in the slide, and HER2 was considered
positive when there were ≥10% positive tumor cells with complete
and intense circumferential membrane staining.
Fatty acid analysis of serum and
mammary tissue
To determine fatty acid composition, blood samples
and mammary tissues were collected. Samples from Groups 1 and 2
were collected from the 5 and 6 rats that had the largest mammary
tumors, respectively. Samples from Group 3 and 4 were collected
from 5 and 6 randomly selected rats, respectively. Sera were
separated from whole blood that was cooled on ice by centrifugation
for 10 min at 1,640 × g. Non-tumorous mammary tissues stored at
−20°C were thawed and homogenized. The fatty acid composition of
the total phospholipid fraction of serum was determined. Total
lipids were extracted by the method of Bligh and Dyer (25). The total phospholipid fraction was
separated by thin-layer chromatography. For an internal standard,
1,2-diheptadecanoyl-sn-3-pfospfocholine (Avanti Polar Lipids, Inc.,
Alabaster, AL, USA) was added. Total phospholipid fractions were
transmethylated with HCl-methanol, and subsequently the fatty acid
composition was analyzed by gas chromatography (GC-2014, Shimadzu
Corporation, Kyoto, Japan) with a capillary column DB-225 (0.25 mm
×30 m ×0.25 µm; J&M Scientific, Folsom, CA, USA). The entire
system was controlled with gas chromatography software (GCsolution;
Shimadzu Corporation). The fatty acid composition of the total
lipid fraction of non-tumorous mammary tissues was determined. In
brief, frozen tissues were thawed, minced and homogenized three
times in 8 ml chloroform-methanol (2:1) by a polytron homogenizer
(Kinematica, Lucerne, Switzerland) for 10 sec. The fatty acid
analysis of total lipids in the mammary tissue was performed by the
same method as previously mentioned (16).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Body weight, tumor volume, tumor weight, cancer
multiplicity, number of Ki-67 and γ-H2A.X-positive cells/1,000
cells, CD34-positive area/1 mm2, ER and PgR-positive
cells/1 mm2, fatty acid composition, and the n-6/n-3
ratio among groups were analyzed by the t-test. The cancer
incidence was analyzed with the χ2 test.
Results
Host animals
During the experiment, the daily dose of food
ingestion was compatible among groups, and although MNU treatment
tends to decrease the body weight, the MA diet did not
significantly influence the weight as compared with the CTR diet
group. At necropsy, except for the development of mammary tumors,
no organs or tissues were macroscopically abnormal.
Mammary carcinogenesis
All mammary tumors examined were histologically
confirmed as mammary cancers. Therefore, the mammary tumors in the
present study are referred to as mammary cancers. In MNU-treated
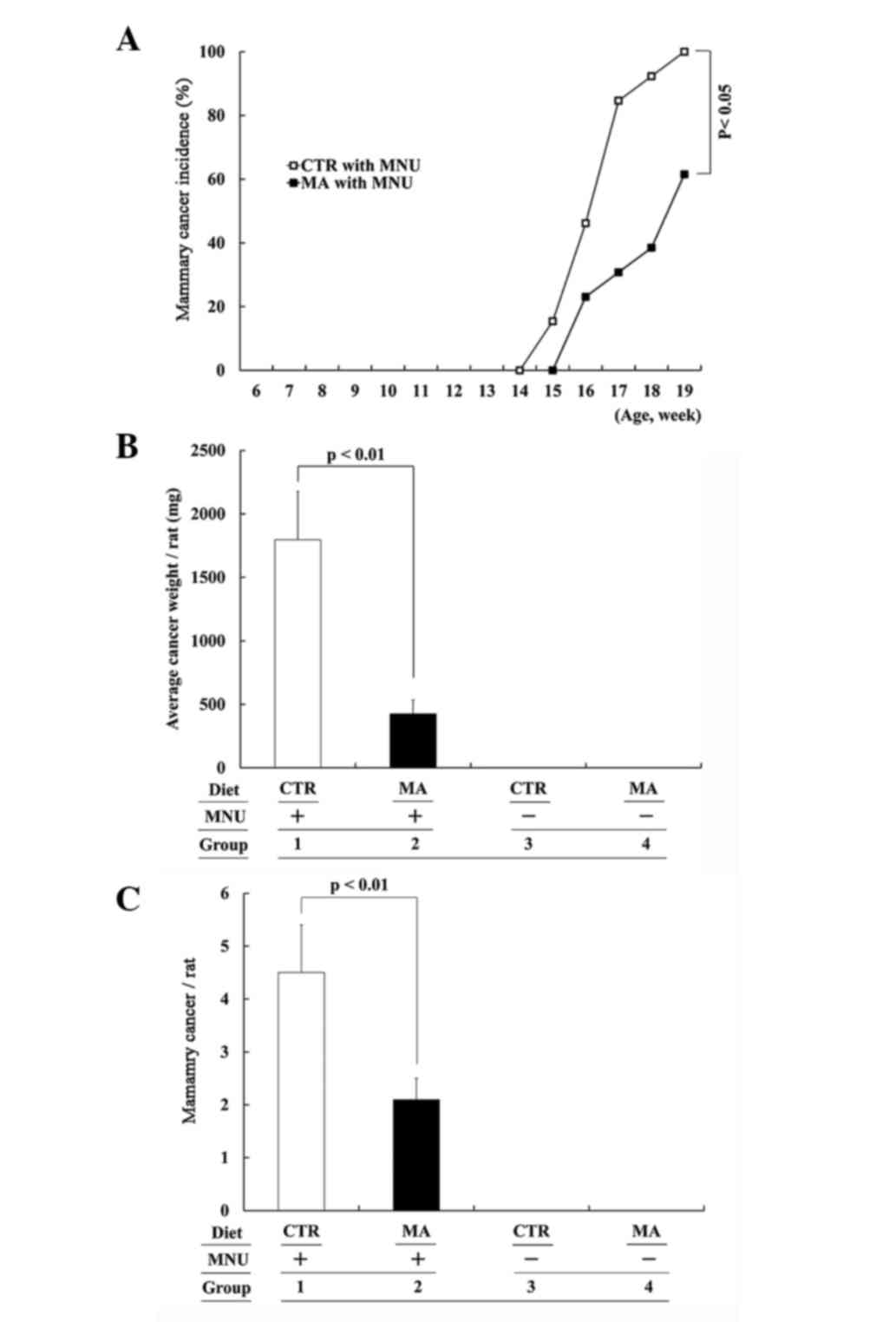
rats, the MA diet (Group 2) tended to have a delayed development of
palpable mammary cancer as compared to the CTR diet group (Group
1), and at the end of the experiment, the mammary cancer incidence
was significantly lower in MA dietfed rats (Group 2 vs. 1: 61.5 vs.
100%; P<0.05; Fig. 2A). At the end
of the study, the MA diet group had a significantly decreased
average cancer volume (data not shown) and final average cancer
weight (Group 2 vs. 1: 427.1±106.9 vs. 1,796.3±378.8 mg; P<0.01;
Fig. 2B) when MNU-induced macroscopic
mammary cancers were compared. Including histologically detectable
microscopic cancers, the MA diet significantly lowered cancer
multiplicity (Group 2 vs. 1: 2.1±0.4 vs. 4.5±0.9; P<0.05;
Fig. 2C. No mammary cancer was
detected in the MNU-unexposed rats on either the CTR or MA diet
(Groups 3 and 4, respectively).
Proliferation and apoptotic ratio of
MNU-induced mammary cancer
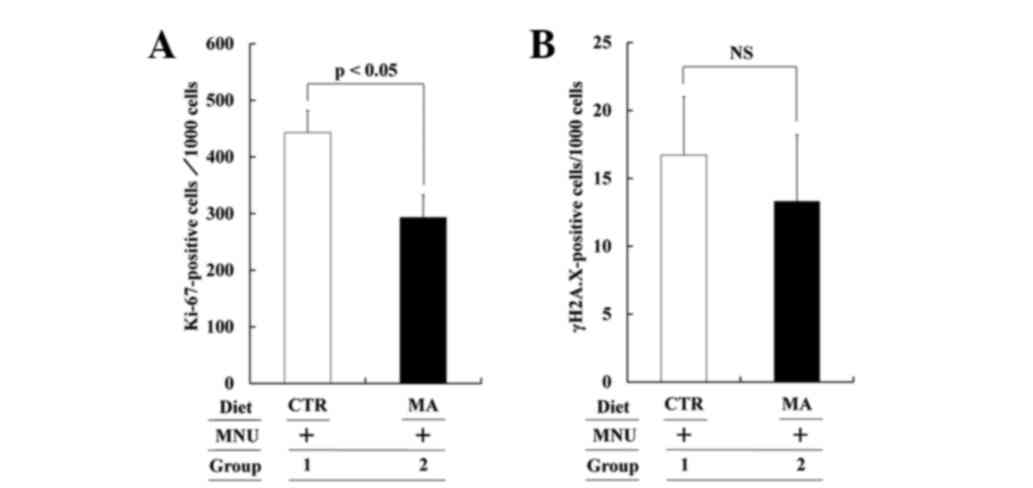
To compare the cell kinetics of MNU-induced mammary
cancers (cell proliferation and cell death), the number of
Ki-67-positive and γ-H2A.X-positive cells/1,000 cancer cells from
the CTR and MA diet groups was compared. The proliferation and
apoptotic cell number are shown in Fig. 3A
and B, respectively. The proliferation ratio in the CTR and MA
diet group was 44.3±3.9 and 29.3±3.9, respectively (Group 2 vs. 1:
P<0.05), while the apoptotic ratio in the CTR and MA diet group
was 1.7±0.4 and 1.3±0.5 (P>0.05). MA significantly suppressed
cancer cell proliferation but did not alter the cell death
ratio.
Microvessel density of MNU-induced
mammary cancer
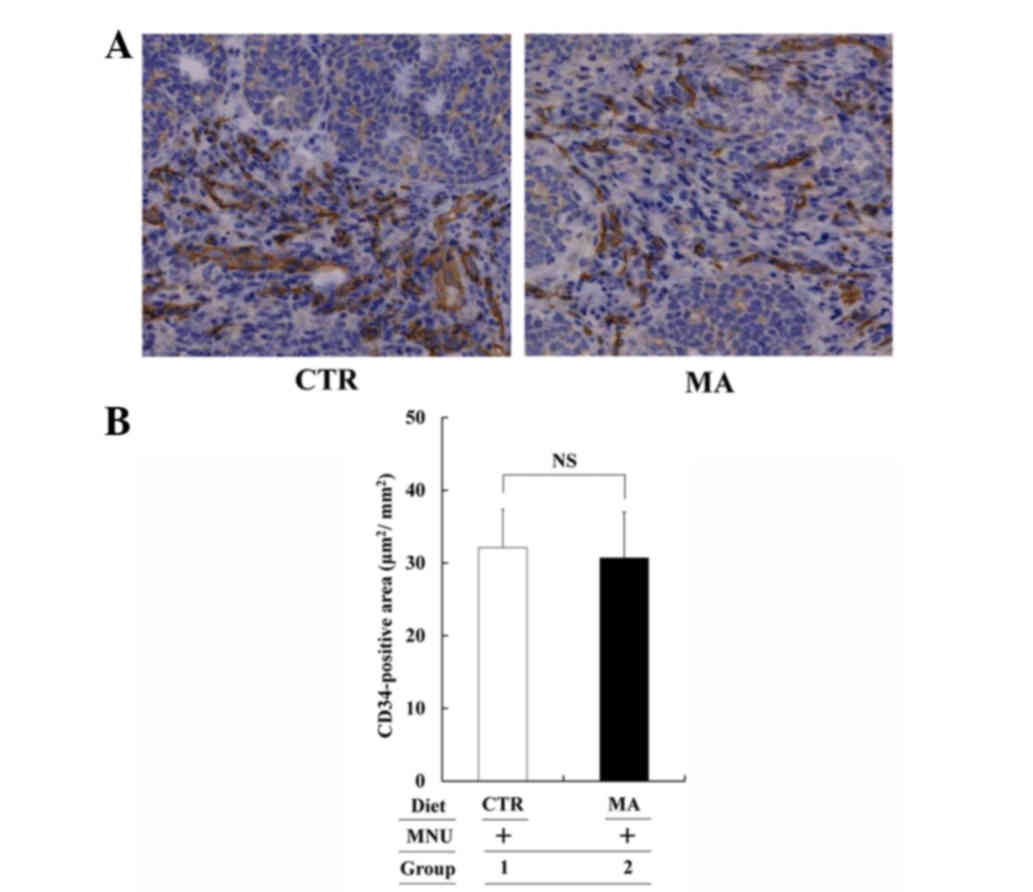
Although the cell growth was suppressed in the MA
diet group, microvessel density of MNU-induced tumors, as evaluated
by the CD34-positive area, was compatible between the CTR and MA
diet groups (Fig. 4). Therefore, the
MA diet did not affect tumor angiogenesis in the MNU-induced
mammary tumor system (Group 2 vs. 1: P>0.05).
Intrinsic subtype of MNU-induced
mammary cancer
In the CTR and MA diet group (13 and 8 mammary
cancers, respectively), all tumors were ER- and PgR-positive and
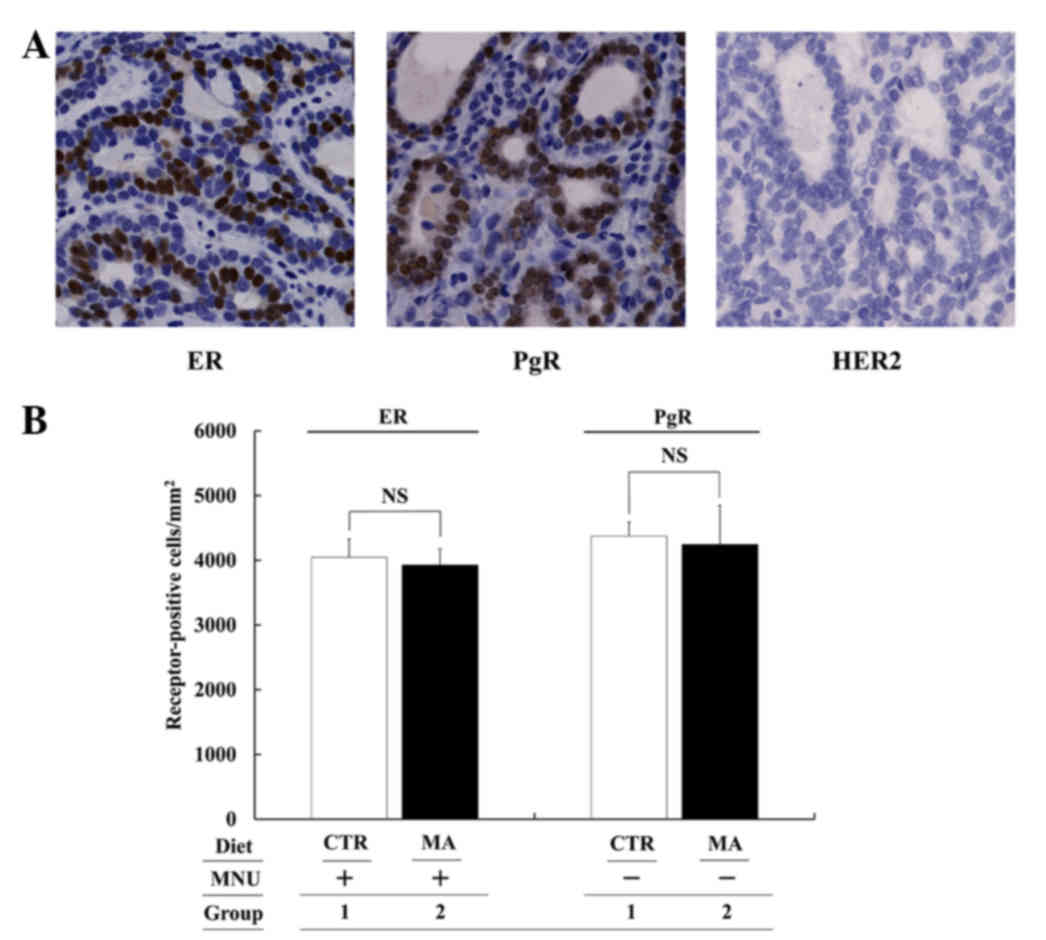
HER2-negative (all luminal A subtype). Representative staining is
shown in Fig. 5A. Therefore, MA
effectively suppressed the growth and development of luminal A
subtype tumors without affecting the number of ER- and PgR-positive
cells/1 mm2 (Group 2 vs. 1: not significant; Fig. 5B). However, the effect of MA against
other intrinsic subtype tumors could not be evaluated from the
MNU-induced mammary tumor system.
Fatty acid composition of serum and
mammary tissue
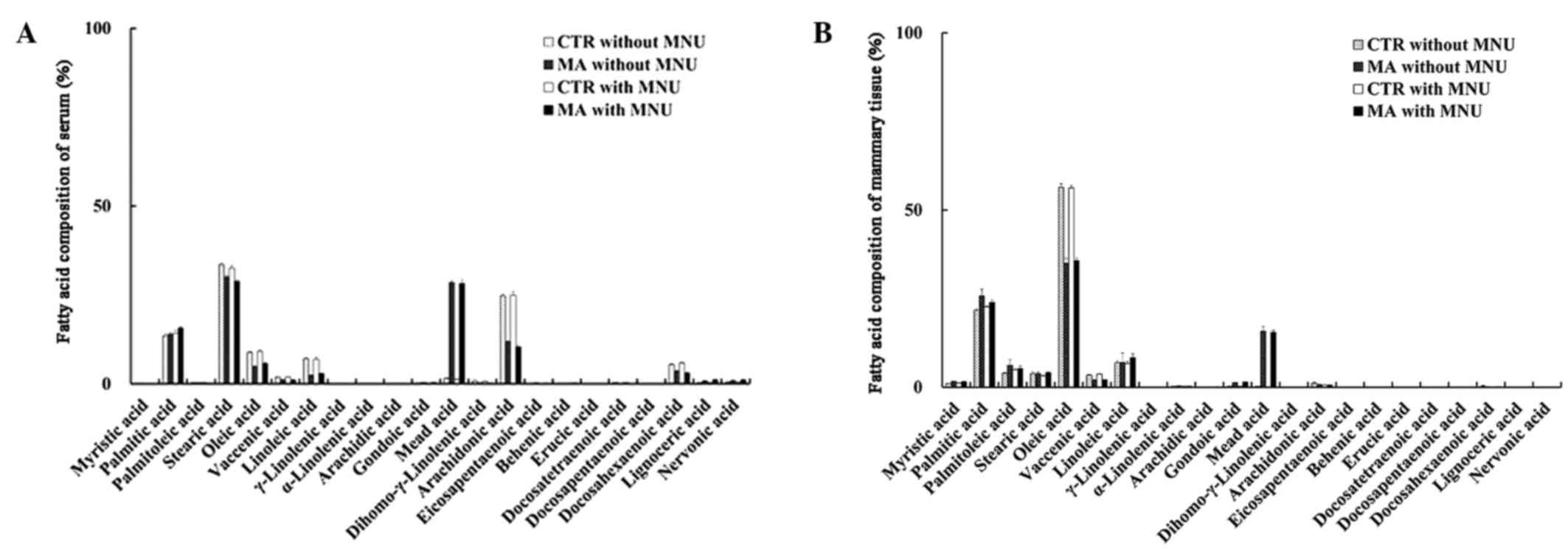
The different diet groups had different fatty acid
compositions of serum and mammary tissue that reflected the
differences in the fatty acid composition of the respective diets.
MNU treatment did not affect the fatty acid composition (Groups
1–4; Fig. 6A and B). Evident changes
in the major n-3, n-6 and n-9 fatty acid composition of serum in
the MA diet group were significant increase in MA concentration and
significant decreases in OA, LA, AA and DHA, as compared to the CTR
diet (Fig. 6A). Changes in the fatty
acid composition of non-tumorous mammary tissues were a significant
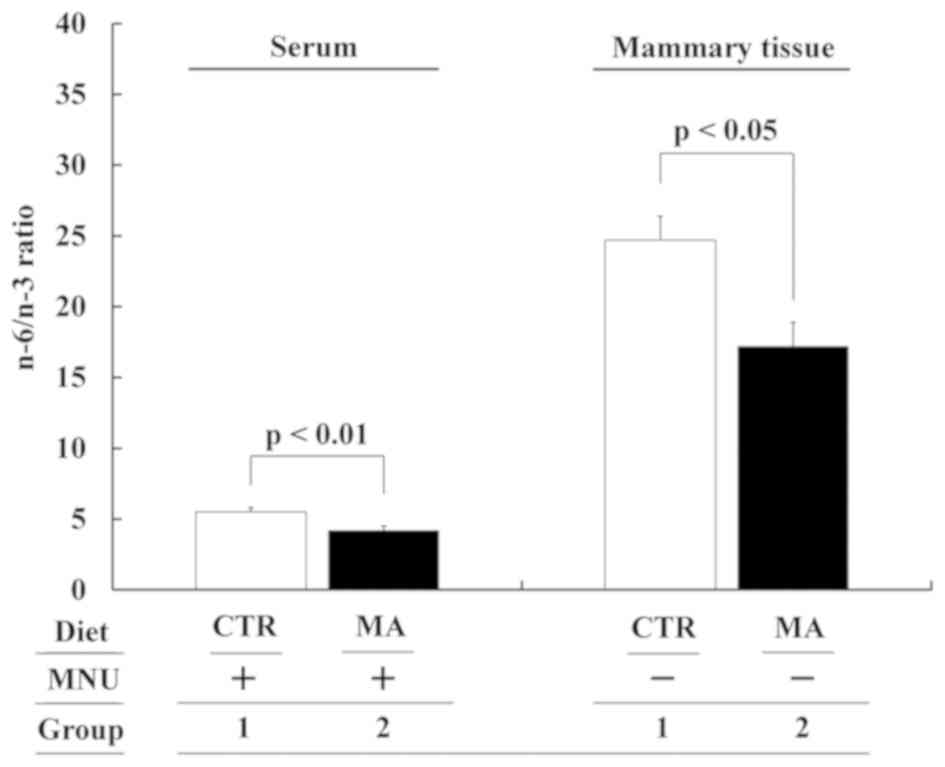
increase in MA and a significant decrease in OA (Fig. 6B). Changes in the fatty acid
composition of serum and mammary tissue resulted in significantly
decreased n-6/n-3 ratios in the sera and mammary tissues of the MA
group (Group 2 vs. 1: P<0.01 and P<0.05, respectively;
Fig. 7).
Discussion
The use of pharmacological or natural agents that
inhibit the development of invasive breast cancer either by
blocking the DNA damage that initiates cancer (blocking agent) or
by arresting or reversing the progression of premalignant cells in
which such damage has already occurred (suppressing agent) is
desired (26,27). In the present study, 2.4% MA in the
diet significantly suppressed MNU-induced mammary cancer incidence,
multiplicity and average cancer volume and final cancer weight.
These results indicate that MA suppressed the initiation and
promotion phases of carcinogenesis. Our previous study on KPL-1
human breast cancer cells showed that 2.4% MA in the diet
suppressed the promotion (growth) and progression phases
(metastasis) (16). Taken together,
2.4% MA in the diet acted as a blocking and suppressing agent that
suppressed all stages of mammary carcinogenesis; thus, MA is a
candidate molecule to be used as a chemotherapeutic and
chemopreventive agent.
The effects of n-9 OA on breast cancer remain
controversial (11). By contrast,
epidemiological results and experimental data show the beneficial
effects of n-9 MA against breast cancer (14–16); MA
suppressed the growth of MCF-7 and KPL-1 human breast cancer cells
in culture. In the present study, in agreement with a study of
KPL-1 cells transplanted into female athymic mice (16), MA suppressed the development and growth
of MNU-induced mammary cancer. In these two experiments, the
mechanism of growth suppression was decreased cell proliferation,
not accelerated apoptosis. Studies of the effects of MA on breast
cancer in humans and animals are limited (14–16). MA
dose-dependently inhibits vascular endothelial growth factor
(VEGF)-stimulated angiogenesis (28).
The VEGF pathway may act indirectly via endothelial cells and may
be involved in tumor angiogenesis; or the VEGF pathway may act
directly via the VEGF receptor in cancer cells and participate in
the growth modification of breast cancer cells (16). The VEGF-VEGFR interaction on cancer
cells may partially explain the actions of MA in modifying cancer
cell growth. However, the microvessel density in MNU-induced tumors
was not affected by MA, which is consistent with findings from
athymic mice implanted with KPL-1 cells (16). In contrast to the lack of detailed
studies on n-9 fatty acids, the growth inhibitory action of n-3
fatty acids and the growth stimulatory action of n-6 fatty acids
have been studied in detail (26).
Changes in the n-6/n-3 ratio appear to be noteworthy (6,29). The
present study showed that MA decreased the n-6/n-3 ratio in serum
and in mammary tissue, which may have been indirectly involved in
suppressing cell proliferation of MNU-induced mammary cancers.
However, more precise mechanisms of the MA-mediated decrease in
cell proliferation should be determined.
Detailed molecular characterization of individual
cancers will enable cancer patients to receive tailored targeted
therapies that improve outcomes and decrease therapy toxicity.
Immunohistochemistry-based surrogate intrinsic subtyping is
convenient when specimens for gene analysis are not available
(18). Immunohistochemical staining
for ER, PgR, and HER2 can differentiate breast cancer into luminal
A (ER+ and/or PgR+, HER2−),
luminal B (ER+ and/or PgR+,
HER2+), HER2 (ER− and PgR−,
HER2+), and triple negative (ER− and
PgR−, HER2−) subtypes (18,30).
Endocrine therapy for luminal A subtype, endocrine therapy and
trastuzumab plus chemotherapy for luminal B subtype, trastuzumab
plus chemotherapy for HER2 subtype, and chemotherapy for triple
negative cases are recommended (18,31). MCF-7,
KPL-1 (17), and all the MNU-induced
mammary cancers were luminal A, which means that MA supplementation
in addition to endocrine therapy may further improve the outcome of
luminal A breast cancer. However, assessments of the efficacy of MA
against other breast cancer subtypes are required.
Acknowledgements
The present study was supported by a grant from
MEXT-Supported Programs for the Strategic Research Foundations at
Private Universities (no. S1201038).
References
|
1
|
Freedman LS, Kipnis V, Schatzkin A and
Potischman N: Methods of epidemiology: Evaluating the fat-breast
cancer hypothesis-comparing dietary instruments and other
developments. Cancer J. 14:69–74. 2002. View Article : Google Scholar
|
|
2
|
Yang B, Ren XL, Fu YQ, Gao JL and Li D:
Ratio of n-3/n-6 PUFAs and risk of breast cancer: A meta-analysis
of 274135 adult females from 11 independent prospective studies.
BMC Cancer. 14:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takata T, Minoura T, Takada H, Sakaguchi
M, Yamamura M, Hioki K and Yamamoto M: Specific inhibitory effect
of dietary eicosapentaenoic acid on N-nitroso-N-methylurea-induced
mammary carcinogenesis in female Sprague-Dawley rats.
Carcinogenesis. 11:2015–2019. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuri T, Danbara N, Tsujita-Kyutoku M,
Fukunaga K, Takada H, Inoue Y, Hada T and Tsubura A: Dietary
docosahexaenoic acid suppresses N-methyl-N-nitrosourea-induced
mammary carcinogenesis in rats more effectively than
eicosapentaenoic acid. Nutr Cancer. 45:211–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen LA, Thompson DO, Maeura Y, Choi K,
Blank ME and Rose DP: Dietary fat and mammary cancer. I. Promoting
effects of different dietary fats on N-nitrosomethylurea-induced
rat mammary tumorigenesis. J Natl Cancer Inst. 77:33–42.
1986.PubMed/NCBI
|
|
6
|
Zhu Z, Jiang W, McGinley JN, Prokopczyk B,
Richie JP Jr, El Bayoumy K, Manni A and Thompson HJ: Mammary gland
density predicts the cancer inhibitory activity of the N-3 to N-6
ratio of dietary fat. Cancer Prev Res (Phila). 4:1675–1685. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Senzaki H, Iwamoto S, Ogura E, Kiyozuka Y,
Arita S, Kurebayashi J, Takada H, Hioki K and Tsubura A: Dietary
effects of fatty acids on growth and metastasis of KPL-1 human
breast cancer cells in vivo and in vitro. Anticancer Res.
18:1621–1627. 1998.PubMed/NCBI
|
|
8
|
Chang NW, Wu CT, Chen DR, Yeh CY and Lin
C: High levels of arachidonic acid and peroxisome
proliferator-activated receptor-alpha in breast cancer tissues are
associated with promoting cancer cell proliferation. J Nutr
Biochem. 24:274–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trichopoulou A, Lagiou P, Kuper H and
Trichopoulos D: Cancer and Mediterranean dietary traditions. Cancer
Epidemiol Biomarkers Prev. 9:869–873. 2000.PubMed/NCBI
|
|
10
|
Buckland G, Travier N, Agudo A,
Fonseca-Nunes A, Navarro C, Lagiou P, Demetriou C, Amiano P,
Dorronsoro M, Chirlaque MD, et al: Olive oil intake and breast
cancer risk in the Mediterranean countries of the European
prospective investigation into cancer and nutrition study. Int J
Cancer. 131:2465–2469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rose DP and Connolly JM: Effects of fatty
acids and inhibitors of eicosanoid synthesis on the growth of a
human breast cancer cell line in culture. Cancer Res. 50:7139–7144.
1990.PubMed/NCBI
|
|
12
|
Fulco AJ and Mead JF: Metabolism of
essential fatty acids. VIII. Origin of 5,8,11-eicosatrienoic acid
in the fat-deficient rat. J Biol Chem. 234:1411–1416.
1959.PubMed/NCBI
|
|
13
|
Ichi I, Kono N, Arita Y, Haga S, Arisawa
K, Yamano M, Nagase M, Fujiwara Y and Arai H: Identification of
genes and pathways involved in the synthesis of Mead acid (20:3
n-9), an indicator of essential fatty acid deficiency. Biochim
Biophys Acta. 1841:204–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pouchieu C, Chajès V, Laporte F,
Kesse-Guyot E, Galan P, Hercberg S, Latino-Martel P and Touvier M:
Prospective associations between plasma saturated, monounsaturated
and polyunsaturated fatty acids and overall and breast cancer
risk-modulation by antioxidants: A nested case-control study. PLoS
One. 9:e904422014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heyd VL and Eynard AR: Effects of
eicosatrienoic acid (20:3 n-9, Mead's acid) on some
promalignant-related properties of three human cancer cell lines.
Prostaglandins Other Lipid Mediat. 71:177–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinoshita Y, Yoshizawa K, Hamazaki K,
Emoto Y, Yuri T, Yuki M, Shikata N, Kawashima H and Tsubura A: Mead
acid inhibits the growth of KPL-1 human breast cancer cells in
vitro and in vivo. Oncol Rep. 32:1385–1394. 2014.PubMed/NCBI
|
|
17
|
Kurebayashi J, Kanomata N, Moriya T,
Kozuka Y, Watanabe M and Sonoo H: Preferential antitumor effect of
the Src inhibitor dasatinib associated with a decreased proportion
of aldehyde dehydrogenase 1-positive cells in breast cancer cells
of the basal B subtype. BMC Cancer. 10:5682010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sandhu R, Parker JS, Jones WD, Livas CA
and Coleman WB: Microarray-based profiling for molecular
classification of breast cancer and identification of new targets
for therapy. Lab Medicine. 41:364–372. 2010. View Article : Google Scholar
|
|
19
|
Sakuradani E, Kamada N, Hirano Y,
Nishihara M, Kawashima H, Akimoto K, Higashiyama K, Ogawa J and
Shimizu S: Production of 5,8,11-eicosatrienoic acid by a delta5 and
delta6 desaturation activity-enhanced mutant derived from a delta12
desaturation activity-defective mutant of Mortierella alpina 1S-4.
Appl Microbiol Biotechnol. 60:281–287. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanematsu S, Yoshizawa K, Uehara N, Miki
H, Sasaki T, Kuro M, Lai YC, Kimura A, Yuri T and Tsubura A:
Sulforaphane inhibits the growth of KPL-1 human breast cancer cells
in vitro and suppresses the growth and metastasis of orthotopically
transplanted KPL-1 cells in female athymic mice. Oncol Rep.
26:603–608. 2011.PubMed/NCBI
|
|
21
|
Redon CE, Weyemi U, Parekh PR, Huang D,
Burrell AS and Bonner WM: γ-H2AX and other histone
post-translational modifications in the clinic. Biochim Biophys
Acta. 1819:743–756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American society of clinical oncology/college of
American pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/college of American pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russo J: Significance of rat mammary
tumors for human risk assessment. Toxicol Pathol. 4:145–170. 2015.
View Article : Google Scholar
|
|
25
|
Bligh EG and Dyer WJ: A rapid method of
total lipid extraction and purification. Can J Biochem Physiol.
37:911–917. 1959. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsubura A, Uehara N, Kiyozuka Y and
Shikata N: Dietary factors modifying breast cancer risk and
relation to time of intake. J Mammary Gland Biol Neoplasia.
10:87–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cazzaniga M and Bonanni B: Breast cancer
chemoprevention: Old and new approaches. J Biomed Biotechnol.
2012:9856202012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hamazaki T, Nagasawa T, Hamazaki K and
Itomura M: Inhibitory effect of 5,8,11-eicosatrienoic acid on
angiogenesis. Prostaglandins Leukot Essent Fatty Acids. 86:221–224.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okuyama H, Kobayashi T and Watanabe S:
Dietary fatty acids-the N-6/N-3 balance and chronic elderly
diseases. Excess linoleic acid and relative N-3 deficiency syndrome
seen in Japan. Prog Lipid Res. 35:409–457. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lips EH, Mulder L, de Ronde JJ, Mandjes
IA, Koolen BB, Wessels LF, Rodenhuis S and Wesseling J: Breast
cancer subtyping by immunohistochemistry and histological grade
outperforms breast cancer intrinsic subtypes in predicting
neoadjuvant chemotherapy response. Breast Cancer Res Treat.
140:63–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members: Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St. Gallen international expert consensus on the primary
therapy of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|