Introduction
Cancer remains an extremely serious life-threatening
disease for all humans. Although continuous efforts have been made
to provide novel leads against cancers, and numerous cancer drugs
have been derived from plants or generated synthetically, the
current drugs used clinically have no significant effectiveness or
safety (1). Therefore, it is important
to undertake research into the discovery of new anticancer drugs of
plant origins. Numerous types of bioactive compounds have been
isolated from medicinal plants and several of these compounds are
currently undergoing further investigation (2).
As plants consumed by primates are assumed to be a
promising source of therapeutic agents for human disease
management, a series of studies have been conducted to search for
anticancer agents from plant sources with a focus on finding new
potential drugs or leads for breast cancer from primate-consumed
plants (3). Breast cancer is the most
malignant form of cancer among women, causing >1.2 million new
cases and 0.5 million mortalities annually (4). Our previous studies revealed that
kaempferol-3-O-rhamnoside, isolated from the leaves of
Schima walichii Korth, a plant commonly consumed by
primates, inhibited the proliferation of the MCF-7 breast cancer
cell line through activation of the caspase cascade pathway
(5). Furthermore, an evaluation of 42
species of Indonesian primate-consumed plants revealed that several
plant extracts, including the n-hexane fraction of the
Garcinia celebica (G. celebica) leaves extract, had
potent antiproliferative activity against MCF-7 cells (6). In the present study, a compound from the
n-hexane fraction of the G. celebica leaves extract
with antiproliferative activity against MCF-7 cell lines was
identified and the pro-apoptotic activity of the active compound
was evaluated.
Materials and methods
Collection of plant materials
Leaves of G. celebica were collected in the
Pangandaran Beach Conservation Area of West Java (Indonesia). The
plant species was identified by the Department of Biology, Faculty
of Mathematics and Natural Sciences, Universitas Padjadjaran
(Jatinangor, Indonesia). The leaves were dried in the open air away
from direct sunlight.
Isolation of an active compound
Dried G. celebica leaves were powdered and extracted
with 95% ethanol (three times every 24 h) at room temperature and
the solvent was evaporated under reduced pressure at 50°C to yield
concentrated extracts. The extract was partitioned with a mixture
of n-hexane-water (3:1) to generate hexane and water layers.
The water layer was further extracted with ethyl acetate to yield
ethyl acetate and water fractions. The n-hexane and ethyl acetate
fractions had inhibitory activities against MCF-7 cell
proliferation. Silica gel chromatography was used to elute the
n-hexane fraction (23.32 g) into 5 fractions of n-hexane-ethyl
acetate mixtures with increasing polarity (n-hexane:ethyl acetate,
9:1, 8:2, 7:3, 6:4, 5:5). Each fraction was tested for its toxicity
against A. salina larvae (7), and the
fraction with the highest toxicity was further chromatographed over
silica gel with the same elution system to generate 11 fractions,
of which fraction 6 contained the targeted compounds. Fraction 6
was subjected to preparative thin-layer chromatography using a
mixture of chloroform-methanol (9.5:05) as a developing solvent
system. The application of preparative chromatography resulted in a
compound (50.20 mg, white amorphous solid) that showed high
toxicity against A. salina larvae. The compound was identified by
an analysis of its spectroscopic data [ultraviolet (UV), infrared
(IR), mass spectrometry (MS) and nuclear magnetic resonance
(NMR)].
Cell culture and drug-sensitivity
assays
MCF-7 human breast cancer cell lines were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cell lines were cultured in RPMI-1640 medium (Sigma-Aldrich, St.
Louis, MO, USA) supplemented with 10% fetal bovine serum and
antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin). Cell
proliferation was analyzed using an
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT)
assay in cells treated with various concentrations of the active
compound isolated from leaves of G. celebica following the methods
of Abdulah et al (8). Briefly,
2×104 cells in 50 µl/well were plated in 96-well plates.
Following the initial cell seeding, different concentrations of the
active compound isolated from leaves of G. celebica were added and
incubated for 24 h. WST-8 assay cell-counting solution (Dojindo
Lab., Kumamoto, Japan) was added to each well (10 µl) and incubated
at 37°C for 3 h. After the addition of 100 µl/well of 1 N HCl, the
cell proliferation rate was subsequently determined by measuring
the absorbance at a wavelength of 450 nm. The absorbance was read
using a microtiter plate reader (Becton-Dickinson, Franklin Lakes,
NJ, USA).
Cell extraction and western blot
analysis
Protein concentrations were determined using a
bicinchoninic acid protein assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). In total, 40 µg protein was electrophoresed on
a Mini-PROTEAN TGX Precast gel (4–20%; Bio-Rad Laboratories,
Hercules, CA, USA) and electrotransferred to a 7×8 cm
Hybond-enhanced chemiluminescence membrane (GE Healthcare Life
Sciences, Little Chalfont, UK). Apoptosis-associated proteins were
analyzed by immunoblot analysis using poly(adenosine
diphosphate-ribose) polymerase (PARP; #9542) and Akt (#4685)
antibodies at a 1:1,000 dilution (both Cell Signaling Technology,
Danvers, MA, USA). β-actin (#A5441; Sigma-Aldrich) served as the
loading control.
Results
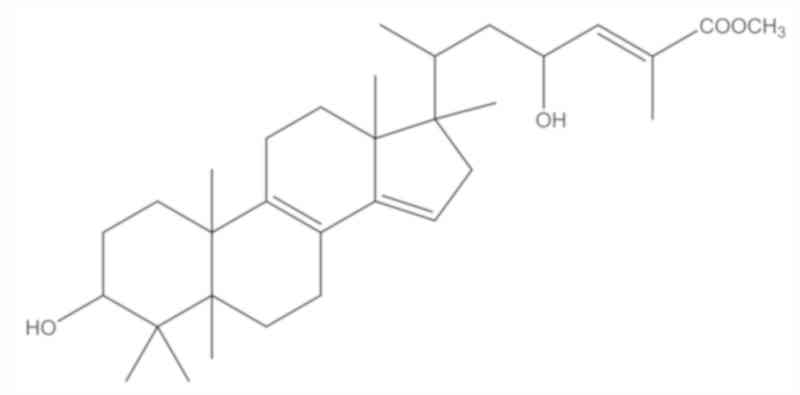
Structural determination of
methyl-3α,23-dihydroxy-17,14friedolanstan-8,14,24-trien-26-oat from
G. celebica leaves
An antiproliferative compound isolated from the
leaves of G. celebica was amorphous and was white in color. It
exhibited a molecular ion peak at m/z 484 in the electron
ionization mass spectrum indicating that this compound had a
molecular formula of C31H48O4.
The UV spectrum indicated absorption bands at 275
and 439 nm and the IR spectrum revealed the presence of a hydroxyl
group (3,500 cm−1) and a carbonyl group of an α,
β-unsaturated ester (1,700 cm−1). The presence of the
carbonyl functionality was further confirmed by a carbon signal
appearing at δ 168.7 in the 13C NMR spectrum.
The 1H and 13C NMR spectra of
the compound exhibited that this compound was assumed to be a
triterpenoid compound. The 1H NMR spectrum indicated the
presence of 5 tertiary methyls (δ 0.76, 0.89, 0.90, 0.98 and 1.01),
1 secondary methyl (δ 0.94, d, J=7.5 Hz), and 1 oxymethine
proton (δ 3.45, br s). These signals were assumed due to a
tetracyclic triterpene having a 3α-hydroxy group (9–11).
Furthermore, signals due to 1 olefinic proton (δ 6.72, qd,
J=7.5 and 1.5 Hz), 1 vinylic methyl (δ 1.87, d, J=1.5 Hz), 1
oxymethine proton (δ 4.57, ddd, J=10.7, 7.5 and 2.5 Hz), and
methoxy protons (δ 3.75, s) were observed. These signals
suggested that the compound had a side chain with a structure of
[-CH(Me)CH2CH(OH)CH=C(Me)COOCH3]. The
13C NMR spectrum indicated the presence of 7 methyl
carbons (δ 12.9, 15.5, 15.8, 17.3, 19.2, 22.4 and 28.2), 8
methylene carbons (δ 18.3, 22.9, 25.8, 26.9, 29.4, 30.3, 39.7 and
45.7), 6 methine carbons (δ 33.6, 44.6, 67.1, 76.0, 116.0 and
142.6), and 9 quartenary carbons (δ 37.8, 38.0, 48.2, 50.2, 123.1,
127.3, 144.6, 148.9 and 168.7). These NMR spectral data showed that
the compound may be friedolanostane triterpenoid with one
tetrasubstituted double bond and two trisubstituted double bonds.
One of the two trisubstituted double bonds was located in the side
chain and the two remaining double bonds in the tetracyclic system
were indicated to be conjugated. The conjugated double bonds were
assumed to be present at C-8/C-9 and C-14/C-15, therefore, the
vinylic proton appearing at 5.26 (s) was at C-15. Thus, the
structure of the compound was established as methyl-3α,
23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat (Fig. 1) which has been reported previously.
The 1H and 13C NMR data of the identified
compound is shown in Table I.
Confirmation of the structure was obtained by comparison of its
spectral data with those reported in previous studies (11,12).
 | Table I.1H and 13C NMR
data of
methyl-3α,23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat
isolated from G. celebica leaves. |
Table I.
1H and 13C NMR
data of
methyl-3α,23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat
isolated from G. celebica leaves.
|
| 13C
NMR | 1H
NMR |
|---|
|
|
|
|
|---|
| No. | Chemical shift | Position | Chemical shift | Position |
|---|
| 1 | 168.7 | C-26 |
|
|
| 2 | 148.9 | C-14 |
|
|
| 3 | 144.6 | C-9 |
|
|
| 4 | 142.6 | C-24 | 6.72 | H-24 (1H, qd,
J=7.5 & 1.5 Hz) |
| 5 | 127.3 | C-25 |
|
|
| 6 | 123.1 | C-8 |
|
|
| 7 | 116.0 | C-15 | 5.26 | H-15 (1H,
s) |
| 8 | 76.0 | C-3 | 3.45 | H-3 (1H, br
s) |
| 9 | 67.1 | C-23 | 4.57 | H-23 (1H, ddd,
J=10.7, 7.5 & 2.5 Hz) |
| 10 | 52.1 | OMe | 3.75 | OMe (3H,
s) |
| 11 | 50.2 | C-17 |
|
|
| 12 | 48.2 | C-13 |
|
|
| 13 | 45.7 | C-16 | 2.23-2.15 | H-16, H-20 (2H,
m) |
|
|
|
| 1.99–1.94 | H-16 (1H,
m) |
| 14 | 44.6 | C-5 |
|
|
| 15 | 39.7 | C-11 |
|
|
| 16 | 38.0 | C-10 |
|
|
| 17 | 37.8 | C-4 |
|
|
| 18 | 33.6 | C-20 |
|
|
| 19 | 30.3 | C-1 | 1.77–1.56 | 2× H-1, H2, H5, 2×
H-6, 2× H-12, H-11 (9H, m) |
| 20 | 29.4 | C-2 | 1.16–1.10 | H-2, H-11 (2H,
m) |
| 21 | 28.2 | C-28 | 0.98 | Me-28 (3H,
s) |
| 22 | 26.9 | C-7 | 2.37-2.31 | H-7, H-7 (2H,
m) |
| 23 | 25.8 | C-12 |
|
|
| 24 | 22.9 | C-22 | 2.08–2.05 | 2× H-22 (2H,
m) |
| 25 |
22.4 | C-29 | 0.89 | Me-29 (3H,
s) |
| 26 |
19.2 | C-30 | 1.01 | Me-30 (3H,
s) |
| 27 |
18.3 | C-6 |
|
|
| 28 |
17.3 | C-19 | 0.90 | Me-19 (3H,
s) |
| 29 |
15.8 | C-18 | 0.76 | Me-18 (3H,
s) |
| 30 | 15.46 | C-21 | 0.94 | Me-21 (3H,
d, J=7.5 Hz) |
| 31 | 12.93 | C-27 | 1.87 | Me-27 (3H,
d, J=1.5 Hz) |
Inhibitory activity of
methyl-3α,23-dihydroxy-17,14-friedo-lanstan-8,14,24-trien-26-oat
against MCF-7 cell line proliferation
Methyl-3α,23-dihydroxy-17,14-friedolanstan-8,
14,24-trien-26-oat was evaluated for its effect on the
proliferation of MCF-7 breast cancer cell lines by the MTT assay.
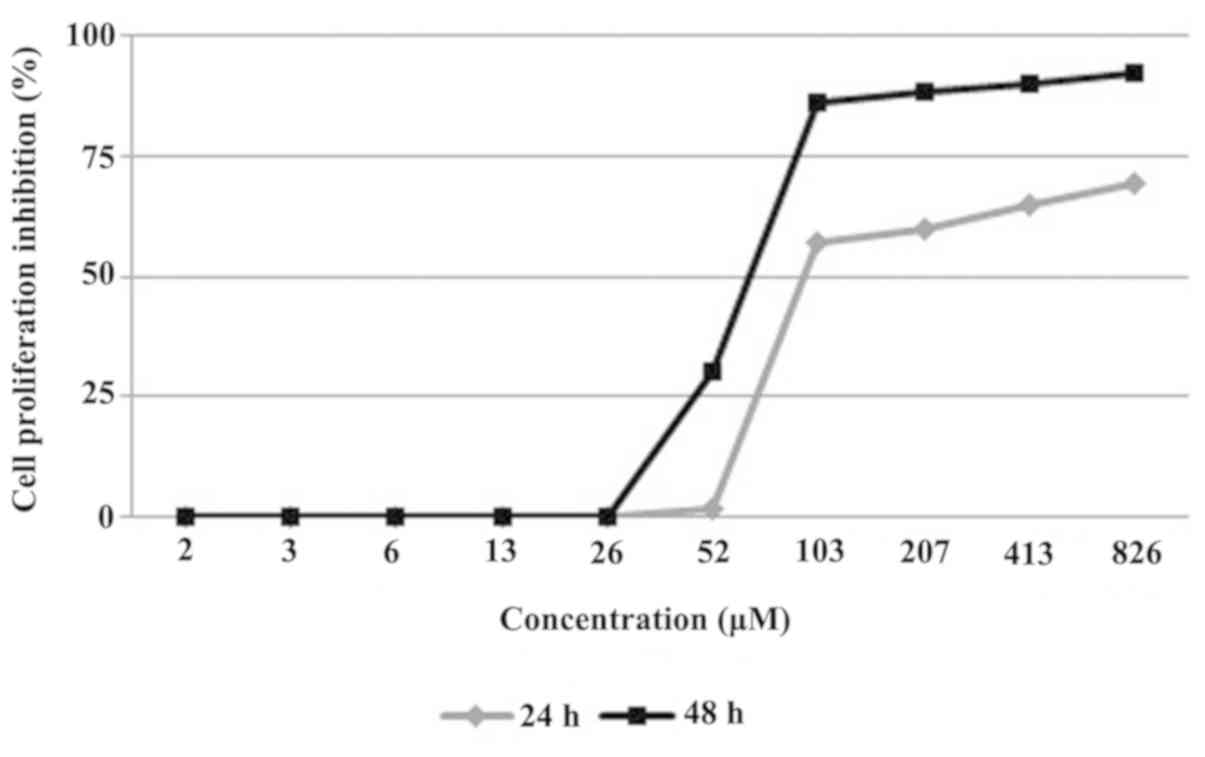
The evaluation resulted in a time- and dose-dependent manner
inhibition of the compound on the cell proliferation (Fig. 2). The compound strongly inhibited the
MCF-7 cell line proliferation in 24 and 48 h treatments, with the
IC50 value of 82 and 70 µM in the 24 and 48 h
measurements, respectively.
Proapoptotic activity of
methyl-3α,23-dihydroxy-17,14frie dolanstan-8,14,24-trien-26-oat
through PARP protein activation
The MTT assay showed strong inhibitory activity of
methyl-3α,23- dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat
against the MCF-7 cell line proliferation, thus, the compound was
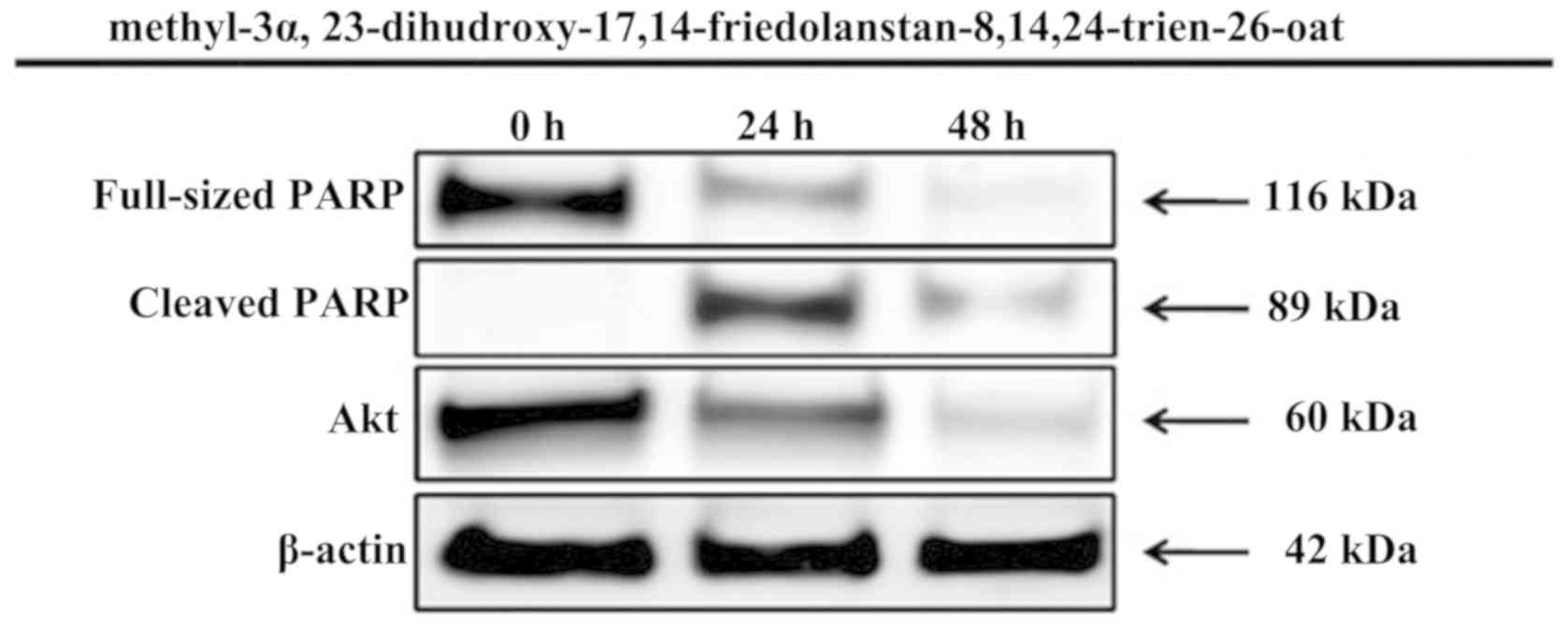
further examined for its proapoptotic activity through PARP protein
activation within 24 and 48 h of treatment. The inhibition of MCF-7
human breast cancer cells proliferation by the compound was
mediated by the induction of apoptosis, marked by PARP protein
activation, which is one of the most optimal biomarkers of
apoptosis. Furthermore, the compound also inhibited the expression
of Akt oncogene (Fig. 3).
Discussion
Traditional medicinal plants have long been regarded
as a source of potential therapeutic agents, and the search for new
drugs is usually based on this approach (13). In drug discovery, our previous studies
recently applied a new approach of selecting plants based on the
use of those plants by primates (3,5). In our
previous study, the extracts of the G. celebica leaves were
strongly cytotoxic to the MCF-7 breast cancer cell line (6). Thus, these extracts had the potential for
further investigation.
The present study was focused on identifying an
antiproliferative compound from the G. celebica leaves. This
study resulted in the isolation of a friedolanostane triterpenoid,
methyl-3α, 23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat,
which strongly inhibited the MCF-7 cell line proliferation in a
time- and dose-dependent manner, with IC50 values of 82
and 70 µM in the 24 and 48 h treatments, respectively.
This compound has not been reported previously in
connection with its cytotoxicity in these cancer cell lines.
Through investigating the anticancer activities of Garcinia
plants, numerous studies have focused on xanthone derivatives,
which are the main compounds of any Garcinia species
(14–16). These findings will thus be valuable
supporting information for revealing the efficacy of
Garcinia species as a potential anticancer source.
In the present study, this compound was also found
to induce the apoptosis of MCF-7 cells, which was indicated by the
changes in the expression levels of PARP. The expression levels of
PARP were analyzed after 24 and 48 h of treatment. The N-terminal
fragment of PARP, which is an 89-kDa peptide cleaved from
full-length PARP (116 kDa), was detected as early as 24 h after
treatment of the MCF-7 cells with methyl-3α,
23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat. Akt, as one
of the most important survival signaling pathways in malignancy,
has an important role in determining the chemosensitivity of cancer
cells. These survival signaling proteins were decreased by
treatment with methyl-3α,
23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat, as shown by
the reduced expression of the Akt protein.
However, the present study is not without
limitations. A detailed mechanism regarding the effect of
methyl-3α,23-dihydroxy- 17,14-friedolanstan-8,14,24-trien-26- oat
on the expression of several proteins that may directly be affected
were not performed, including pro- or anti-apoptotic, estrogen
receptor α and phosphorylated Akt (Ser473) proteins.
In conclusion, the present results suggest that
methyl-3α,23- dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat
inhibited the growth of MCF-7 cells through the induction of
apoptosis and downregulation of the oncogene Akt. Further
evaluation of its toxicity and detailed mechanisms of its
antiproliferative action is required to provide a scientific basis
for its chemopreventive and chemotherapeutic application in breast
cancer management.
Acknowledgements
The present study was financially supported by the
Directorate General of Higher Education of the Ministry of
Education and Culture of Indonesia (Grand-in-Aid for the
International Research Collaborations and Publications; grant no.
430/SP2H/PP/DP2 M/VII/2010) for AS.
References
|
1
|
Sakarkar DM and Deshmukh VN:
Ethnopharmacological review of traditional medicinal plants for
anticancer activity. Int J Pharm Tech Res. 3:298–308. 2011.
|
|
2
|
Kinghorn AD, Chai HB, Sung CK and Keller
WJ: The classical drug discovery approach to defining bioactive
constituents of botanicals. Fitoterapia. 82:71–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koshimizu K, Murakami A, Hayashi H, et al:
Biological activities of edible and medicinal plants from Indonesia
and Malaysia. Proceedings of The Tokyo International Forum on
Conservation and Sustainable Use of Tropical Bioresources (Tokyo).
203–208. 1998.
|
|
4
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diantini A, Subarnas A, Lestari K, Halimah
E, Susilawati Y, Supriyatna Julaeha E, Achmad TH, Suradji EW,
Yamazaki C, et al: Kaempferol-3-O-rhamnoside isolated from the
leaves of Schima wallichii Korth. Inhibits MCF-7 breast cancer cell
proliferation through activation of the caspase cascade pathway.
Oncol Lett. 3:1069–1072. 2012.PubMed/NCBI
|
|
6
|
Subarnas A, Diantini A, Abdulah R, et al:
Antiproliferative activity of primates-consumed plants against
MCF-7 human breast cancer cell lines. E3 J Med Res. 1:38–43.
2012.
|
|
7
|
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen
LB, Nichols DE and McLaughlin JL: Brine shrimp: A convenient
general bioassay for active plant constituents. Planta Med.
45:31–34. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdulah R, Faried A, Kobayashi K, Yamazaki
C, Suradji EW, Ito K, Suzuki K, Murakami M, Kuwano H and Koyama H:
Selenium enrichment of broccoli sprout extract increases
chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC
Cancer. 9:4142009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shiao MS, Lin LJ and Yeh SF: Triterpenes
in Ganoderma lucidum. Phytochemistry. 27:873–875. 1988. View Article : Google Scholar
|
|
10
|
Lin LJ, Shiao MS and Yeh SF: Triterpenes
from Ganoderma lucidum. Phytochemistry. 27:2269–2271. 1988.
View Article : Google Scholar
|
|
11
|
Rukachaisirikul V, Adair A, Dampawan P,
Taylor WC and Turner PC: Lanostanes and friedolanostanes from the
pericarp of Garcinia hombroniana. Phytochemistry. 55:183–188. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rukachaisirikul V, Saelim S, Karnsomchoke
P and Phongpaichit S: Friedolanostanes and lanostanes from the
leaves of Garcinia hombroniana. J Nat Prod. 68:1222–1225. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fabricant DS and Farnsworth NR: The value
of plants used in traditional medicine for drug discovery. Environ
Health Perspect. 109(Suppl 1): S69–S75. 2001. View Article : Google Scholar
|
|
14
|
Matsumoto K, Akao Y, Ohguchi K, Ito T,
Tanaka T, Iinuma M and Nozawa Y: Xanthones induce cell-cycle arrest
and apoptosis in human colon cancer DLD-1 cells. Bioorg Med Chem.
13:6064–6069. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suksamrarn S, Komutiban O, Ratananukul P,
Chimnoi N, Lartpornmatulee N and Suksamrarn A: Cytotoxic prenylated
xanthones from the young fruit of Garcinia mangostana. Chem Pharm
Bull (Tokyo). 54:301–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akao Y, Nakagawa Y, Iinuma M and Nozawa Y:
Anti-cancer effects of xanthones from pericarps of mangosteen. Int
J Mol Sci. 9:355–370. 2008. View Article : Google Scholar : PubMed/NCBI
|