Introduction
Prostate cancer (PCa) is one of the most commonly
diagnosed malignant tumors and the second cause of cancer in males
(1,2).
Due to the changes of population ages and diet structure, the
global incidence of PCa has increased annually. The pathogenesis of
PCa remains to be fully elucidated. Therefore, identifying a marker
with a high correlation with occurrence and development of PCa is
important for early diagnosis and treatment of PCa.
Matrix metalloproteinases (MMPs) are a series of
protein hydrolases, which are closely associated with tumor growth,
invasion and metastasis (3,4). MMPs can degrade the extracellular matrix
(ECM), and can control the formation of tumor blood vessels
(5). They have numerous subtypes, and
MMP-2 is one of the most researched. There are a number of studies
regarding the association between MMP-2 and PCa, which showed that
the serum MMP-2 level was significantly higher compared to the
control subjects (6–9). However, the impact of MMP-2 expression on
the progress of PCa patients remains disputed. Certain studies have
shown that MMP-2 has a high expression level in PCa; however, the
sample sizes of these studies were small, or they were not
contrasted further to the case group. Thus, the present
meta-analysis was performed to explore the association of the level
of MMP-2 expression and PCa.
Materials and methods
Search strategy
The following electronic databases were
comprehensively searched: PubMed, Cochrane Library and China
National Knowledge Infrastructure performed until July 2015. The
following search terms were used: ‘MMP-2’ or ‘matrix
metalloproteinase-2’, ‘prostate cancer’ or ‘prostate tumor’ or
‘prostate’. Subsequently, the literature was retrieved for further
screening.
Study selection
The following criteria was used to evaluate the
retrieval literature, which is consistent with the analysis
included in the request: It should be the original and independent
research; it must be the malignant tumor originating in the
prostate; the association between MMP-2 expression and PCa should
be shown; it must be a case-control study, with benign prostatic
hyperplasia (BPH) as the control; the MMP-2 expression should be
detected in formalin-fixed and paraffin-embedded (FFPE) tumor
tissues. Simultaneously, the following exclusion criteria was used:
Cell lines or animals were used; review articles; and the data was
incomplete.
Data extraction
Data were extracted from the included studies as
follows: Surname of the first author, the year of publishing,
country, median age of patients, study sample size, the percentage
of MMP-2 positive, survival outcomes, method of hazard ratio (HR)
estimation, method of survival analysis, HR and 95% confidence
interval (CI), and odds ratio (OR). Two investigators (Tiancheng
Xie and Binbin Dong) extracted the data independently. Any
disagreement regarding data was resolved by another investigator
(Yangye Yan) to adjudicate the result.
Study quality
Two independent authors (Tiancheng Xie and Binbin
Dong) evaluated the quality of the included studies in the
meta-analysis, according to the Newcastle-Ottawa Scale (NOS) for
case-control studies. The NOS is from 0 to 9 stars. Any controversy
was solved by discussion with the third investigator (Yangye Yan)
to adjudicate any disagreement.
Statistical analysis
Heterogeneity was analyzed by calculating the Q test
statistics. When the data suggest P>0.10 with no significant
heterogeneity, the fixed effects model was used, otherwise, the
random effects model was employed. Due to the rare incidence rate
of PCa, HR could be approximately equal to OR. Therefore, OR was
used instead of HR. Egger's funnel plot was explored to identify if
there was any evidence of publication bias (10).
Results
Information from the literature
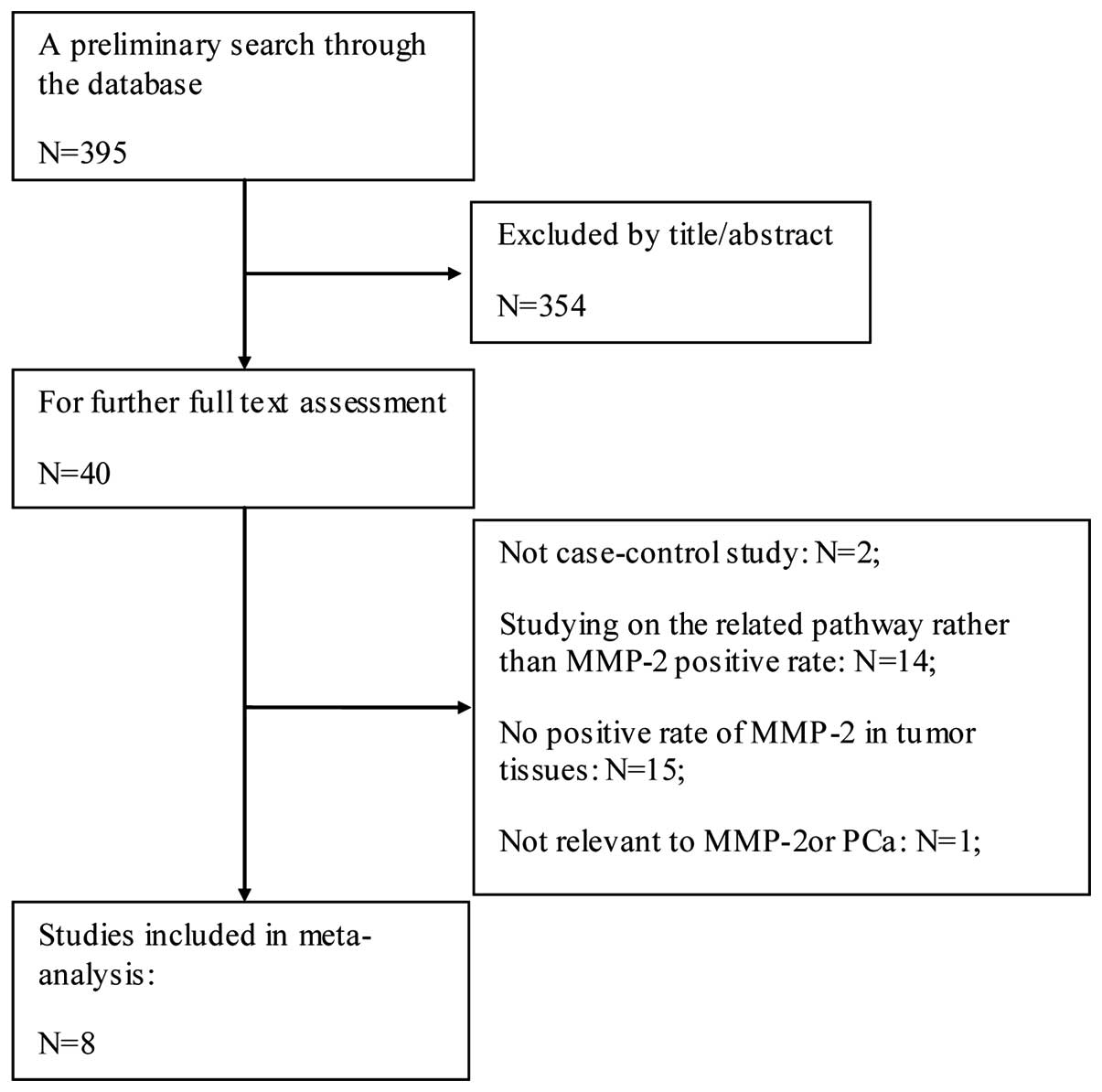
Based on the above search strategy, 395 relevant
studies were identified. Subsequently, the initial screening
occurred by reading the title and abstract of these retrieved
studies. A total of 355 studies were excluded as they did not focus
on the association of MMP-2 expression and PCa. Following this, 1
study was excluded as it was not associated to MMP-2 or PCa.
Following reading of the remaining studies, 29 were excluded as
they were continuous variables or others form of data, or
non-comprehensive data. In addition, 2 studies were excluded that
were not case-control studies. Finally, 8 case-control studies (2
in English and 6 in Chinese) were included in the meta-analysis
(9,11–17). The
selection process is shown in Fig.
1.
Study characteristics
All the studies were published between 2005 and
2014. There were 7 studies of the Asian population (9,11–13,15–17) and 1 of the Caucasian population
(14). A total of 7 studies compared
the positive rate of MMP-2 between the PCa and BPH groups, and 5 of
these compared the MMP-2 positive rate subdivided by the Gleason
score in the cancer group (11,12,15–17).
Furthermore, in the PCa group, 2 studies compared
the positive rate of MMP-2 between low and high serum
prostate-specific antigen (PSA) groups (12,15), and 2
studies compared the positive rate of MMP-2 between clinical stages
(Jewett stages) AB and CD groups (11,16). An
average NOS score of 6 indicated a reliable quality. The
characteristics of the included studies are shown in Table I.
 | Table I.Characteristics of the eligible
studies in the meta-analysis. |
Table I.
Characteristics of the eligible
studies in the meta-analysis.
|
|
|
|
|
|
|
|
|
| Subgroups, n |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
|
|
|
| Sample size, n | Age, years | Gleason score (in
case) | PSA | Clinical stages |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| First author,
year | Ethnicity | Source of
controls | Case | Control | Case | Control | Low (≤7) | High (>7) | Low | High | AB | CD | (Refs.) |
|---|
| Ma, 2014 | Asian | HB | 30 | 60 | 67 | 65 | 24 | 36 | 25 | 35 | N | N | (12) |
| Li, 2013 | Asian | HB | 20 | 78 | N | N | N | N | N | N | N | N | (13) |
| Escaff, 2011 | Caucasian | HB | 50 | 133 | 54–70 | 44–79 | N | N | N | N | N | N | (14) |
| Jia, 2010 | Asian | HB | 20 | 40 | 59 | 81 | 26 | 14 | N | N | 15 | 25 | (11) |
| Wu, 2009 | Asian | HB | 20 | 48 | N | N | 33 | 15 | N | N | N | N | (17) |
| Zhong, 2008 | Asian | HB | 62 | 15 | 73.9±12.1 | N | N | N | N | N | N | N |
(9) |
| Wu, 2005 | Asian | HB | 12 | 46 | <40 | 53–70 | 39 | 7 | N | N | 26 | 20 | (16) |
| Zhang, 2005 | Asian | HB | 10 | 51 | 34 (17) | 31 (20) | 34 | 17 | 31 | 20 | N | N | (15) |
Meta-analysis
BPH and cancer
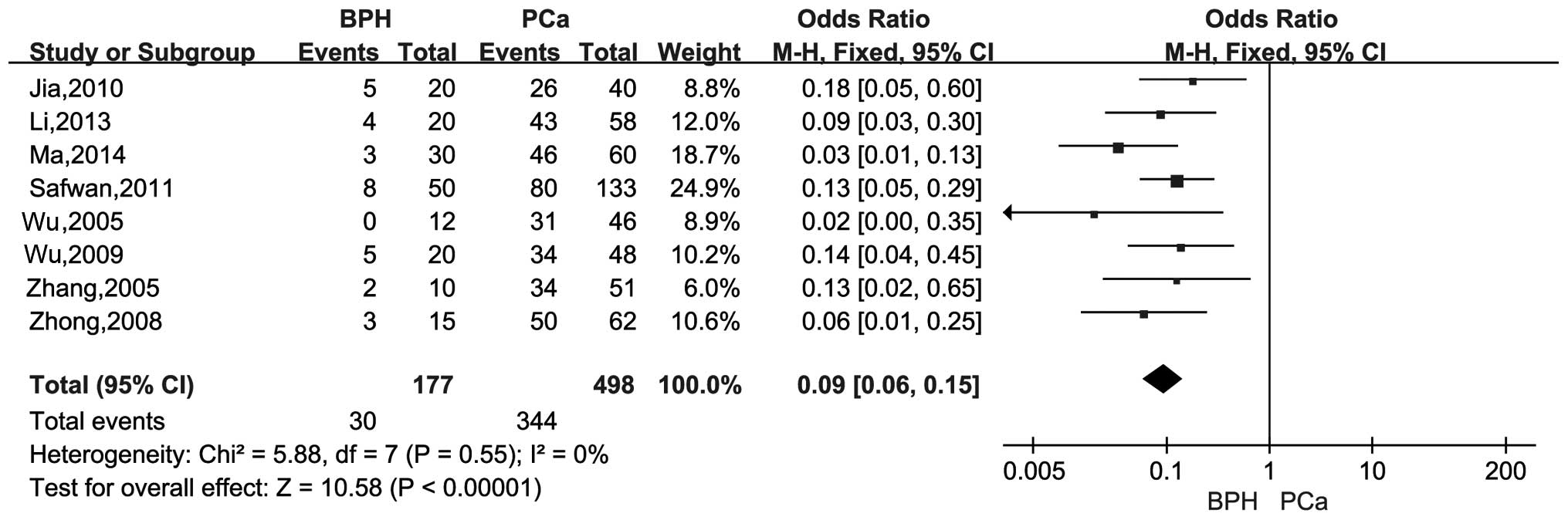
There were 8 studies with a total of 498 cases and
177 controls that compared the differences of the MMP-2 positive
rate between case-control groups. There was no significant
heterogeneity (I2=0%, P=0.55), and the pooled OR was
0.09 (95% CI, 0.06–0.15; Z=10.58; P<0.00001). The results showed
that the MMP-2 expression was significantly associated with PCa
(Fig. 2).
Gleason score
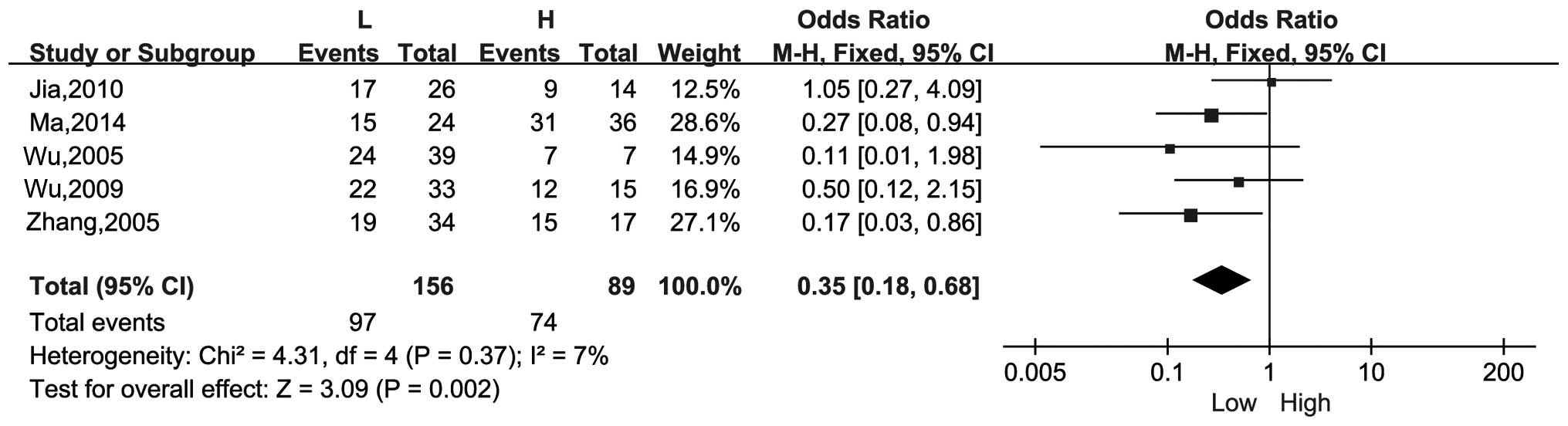
There were 5 studies that compared the differences
in the positive rate of MMP-2 in the Gleason score high and low
groups. The combined OR was 0.35 (95% CI, 0.18–0.68; Z =3.09;
P=0.002) with no significant heterogeneity (I2=7%,
P=0.37). Therefore, the expression level of MMP-2 has a positive
association with the Gleason score of patients (Fig. 3).
Subgroup analysis
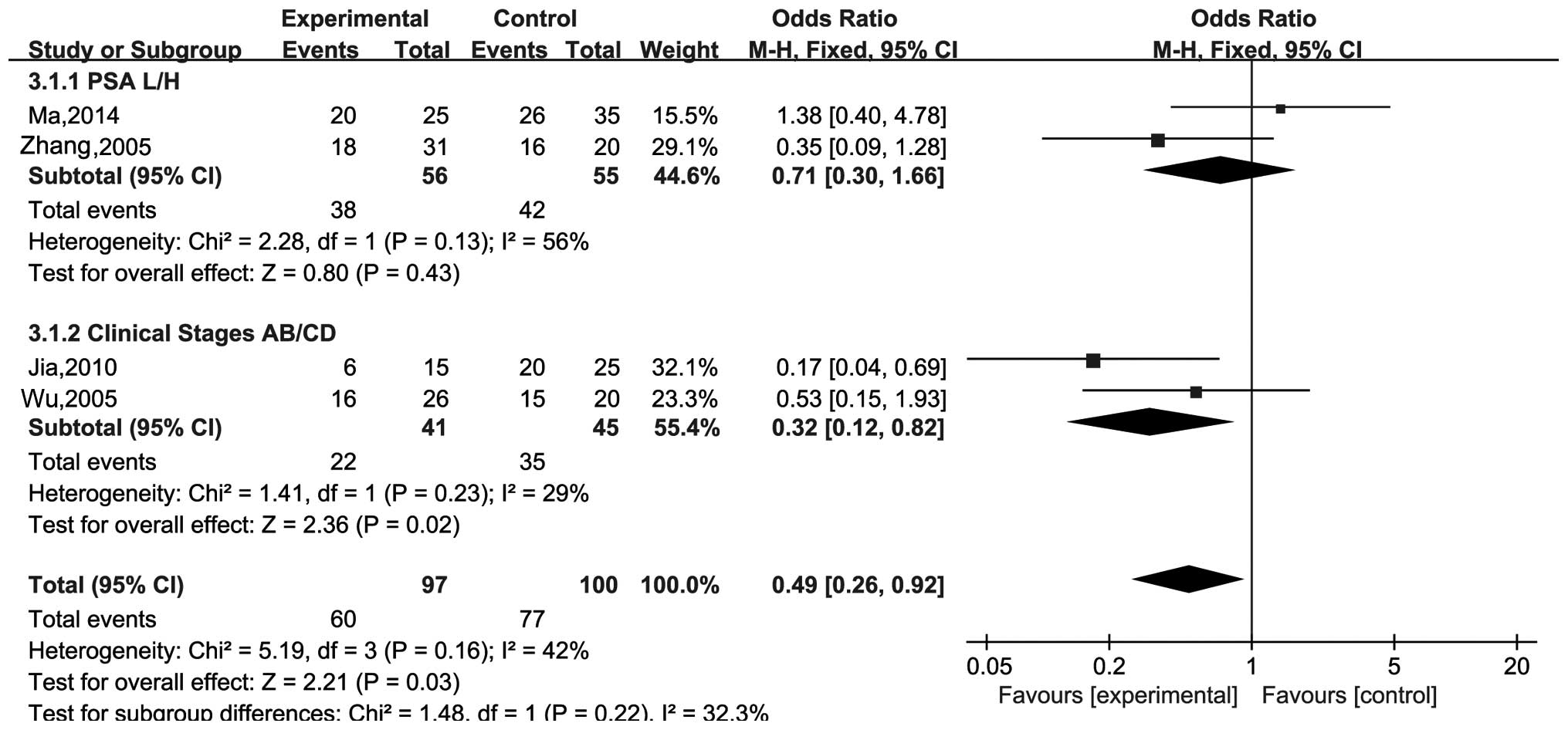
In addition, there were 2 subgroup analyses. One was
regarding serum PSA, and it explored whether MMP-2 has a
correlation between high and low serum PSA. The heterogeneity was
not significant (I2=56%, P=0.13), and the pooled OR was
0.71 (95% CI, 0.30–1.66; Z=0.80; P=0.43). Another compared the
positive rate of MMP-2 between clinical stages AB and CD. The
pooled OR was 0.32 (95% CI, 0.12–0.82; Z=2.36; P=0.02) without
significant heterogeneity (I2=29%, P=0.23). These
results suggested that MMP-2 was significantly correlated with
clinical stages, but not significantly correlated with serum PSA
(Fig. 4).
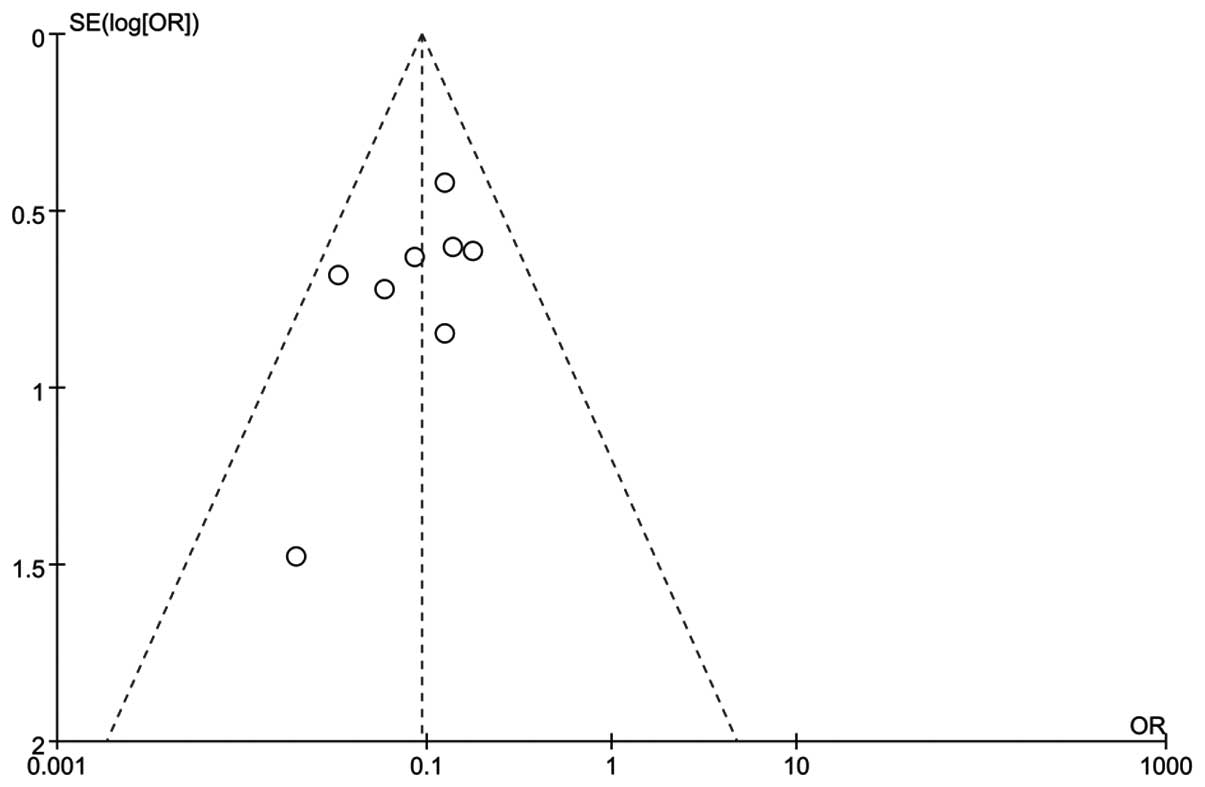
Publication bias and sensitivity analysis
In order to test whether the final result of this
meta-analysis was affected by individual study and gauge the
stability of the results, a sensitivity analysis was conducted
(Fig. 5). The pooled OR in the
meta-analysis was not effect by single study. The result of Egger's
regression test showed the asymmetrical distribution in the funnel
plot in the positive rate of MMP-2 between high and low Gleason
score groups (Egger's test, t=−2.00).
Discussion
Worldwide, the incidence of PCa ranked second in all
male malignant tumors (18). In the
USA, the incidence of PCa has been higher compared to lung cancer,
and it became the first tumor hazard in male health. In Asia, the
incidence of PCa is much lower compared to Europe, America and
other developed countries. However, with the development of the
economy and technology, and changes to life and eating habits, the
incidence of PCa is in a growing trend in recent years, and the
growth rate is higher than the developed countries in Europe and
America.
Early PCa often has no symptoms, and it is always
diagnosed in the advanced stage following the appearance of
symptoms (19). It will lose some
selectivity for treatment, and the prognosis is poor. Therefore,
how to early diagnose of PCa and monitor the prognosis of PCa is
extremely important.
Clinical tumor-node-metastasis (TNM) staging of PCa
and the malignant degree, Gleason score of PCa risk grade, and the
prognosis have an important reference value. In PCa TNM staging,
lymph node metastasis of judgment depends mainly on the computed
tomography (CT) scanning, magnetic resonance imaging (MRI) or
biopsy. Open or laparoscopic lymph node dissection is the gold
standard for N staging. However, the specificity of CT, MRI and
lymph node biopsy are invasive examinations, which certain patients
find it difficult to accept. Currently, PSA is widely applied in
the clinic, and it has an important role in the diagnosis of PCa;
however, when the PSA value is in the 4–10 ng/ml gray zone, it will
reduce the sensitivity and specificity of PSA.
MMPs are ECM-degrading enzymes belonging to a family
of zinc- and calcium-dependent endopeptidases. They are important
for cancer invasion and metastasis (20) and they have a significant role for ECM
degradation (21,22). MMP-2 is one of the MMP families. It has
a critical role in tumor growth and metastasis (23–25). There
are studies that have identified that the MMP-2 content in the
serum is associated with the grading and malignant degree of PCa
(26,27). Certain studies observed that MMP-2 may
have the potential to be used as a molecular marker for PCa
(7). MMP-2 may be used as a predictor
of PCa (8). In addition, certain
studies showed an increased or decreased expression level of MMP-2
through the regulation of an associated MMP-2 pathway, and tumor
cell invasion could be promoted or inhibited in PCa cells (28).
Recently, the association between MMP-2 expression
and PCa has been investigated by certain studies. However, there
remain certain disputes regarding the result, and the sample sizes
are small. Additionally, there is no meta-analysis reporting the
association of MMP-2 expression and PCa. Therefore, the present
meta-analysis was conducted to explore the association between
MMP-2 expression and PCa patients.
There are a total of 8 studies, including 498 cases
and 177 controls, in this meta-analysis. Between the BPH and PCa
groups, there was no significant heterogeneity (I2=0%,
P=0.55), and the pooled OR was 0.09 (95% CI, 0.06–0.15; Z=10.58;
P<0.00001), indicating that MMP-2 overexpression was associated
with PCa. The pooled OR with its 95% CI indicated that the
expression of MMP-2 increased with the increase of the PCa Gleason
score. Subgroup analysis showed that the expression of MMP-2 was
positively correlated with clinical stage in PCa patients. Although
the expression of MMP-2 was not significantly correlated with serum
PSA, MMP-2 may be a potential marker in PCa patients. It may be
used in early diagnosis and the evaluation of prognosis regarding
PCa.
Although a comprehensive search was performed, along
with rigorous statistical data analysis, certain limitations
remain. Firstly, the literature that was searched may predominantly
accept their positive results, leading to expansion of the results
of the meta-analysis. Secondly, certain studies were not included
in the meta-analysis due to the non-uniform data standards.
Thirdly, only studies written in Chinese or English were included
in the meta-analysis even though there was no language restriction.
Fourthly, the majority of the included studies are based on the
population of Asian countries.
In conclusion, taken together the present
meta-analysis showed that MMP-2 was highly expressed in PCa
patients compared with BPH patients. The expression of MMP-2 was
closely correlated with Gleason score and clinical stages in PCa
patients. Therefore, MMP-2 can serve as an indicator in PCa
patients. However, there are also certain limitations. The
additional studies should explore the prognostic value of serum
MMP-2 in PCa patients and clarify the prognostic significance of
MMP-2 expression in PCa patients.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81370699). The authors
acknowledge the reviewers for their comments on this study.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nemeth JA, Yousif R, Herzog M, Che M,
Upadhyay J, Shekarriz B, Bhagat S, Mullins C, Fridman R and Cher
ML: Matrix metalloproteinase activity, bone matrix turnover, and
tumor cell proliferation in prostate cancer bone metastasis. J Natl
Cancer Inst. 94:17–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lokeshwar BL: MMP inhibition in prostate
cancer. Ann NY Acad Sci. 878:271–289. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song H, Li Y, Lee J, Schwartz AL and Bu G:
Low-density lipoprotein receptor-related protein 1 promotes cancer
cell migration and invasion by inducing the expression of matrix
metalloproteinases 2 and 9. Cancer Res. 69:879–886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srivastava P, Lone TA, Kapoor R and Mittal
RD: Association of promoter polymorphisms in MMP2 and TIMP2 with
prostate cancer susceptibility in North India. Arch Med Res.
43:117–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
dos Reis ST, Villanova FE, Andrade PM,
Pontes J Jr, de Sousa-Canavez JM, Sañudo A, Antunes AA, Dall'oglio
MF, Srougi M and Leite Moreira KR: Matrix metalloproteinase-2
polymorphism is associated with prognosis in prostate cancer. Urol
Oncol. 28:624–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trudel D, Fradet Y, Meyer F, Harel F and
Têtu B: Membrane-type-1 matrix metalloproteinase, matrix
metalloproteinase 2, and tissue inhibitor of matrix proteinase 2 in
prostate cancerIdentification of patients with poor prognosis by
immunohistochemistry. Hum Pathol. 39:731–739. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong WD, Han ZD, He HC, Bi XC, Dai QS,
Zhu G, Ye YK, Liang YX, Qin WJ, Zhang Z, et al: CD147, MMP-1, MMP-2
and MMP-9 protein expression as significant prognostic factors in
human prostate cancer. Oncology. 75:230–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia ZY, Ma M and Yu NN: Expression and
significance of COX-2 and MMP-2 in prostate cancer. Shandong Med J.
50:52–53. 2010.
|
|
12
|
Ma Z: Expression of CD147 and MMP-2 in
prostate cancer tissues and the correlation study of it. Tianjin.
9–12. 2014.(In Chinese).
|
|
13
|
Li DD: The expression and significance of
MMP-2 and TIMP-2 in benign and malignant prostatic lesions.
Tianjin. 9–11. 2013.(In Chinese).
|
|
14
|
Escaff S, Fernández JM, González LO,
Suárez A, González-Reyes S, González JM and Vizoso FJ: Comparative
study of stromal metalloproteases expression in patients with
benign hyperplasia and prostate cancer. J Cancer Res Clin Oncol.
137:551–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XY, Hong BF, Chen GF, Lu YL and
Zhong M: Significance of MMP2 and MMP9 expression in prostate
cancer. Zhonghua Nan Ke Xue. 11359–361. (364)2005.(In Chinese).
PubMed/NCBI
|
|
16
|
Wu YD, Liu BQ, Zhang XP, et al: Detection
osteopontin and matrix metalloproteinase-2 in prostate cancer.
Zhengzhou Da Xue Xue Bao Yi Xue Ban. 40:654–656. 2005.(In
Chinese).
|
|
17
|
Wu J and Zhang C: Expression of VEGF MMP-2
and MVD in prostatic cancer and its relation to clinic pathology.
Mod Med Health. 25:3203–3204. 2009.
|
|
18
|
Center MM, Jemal A, Lortet-Tieulent J,
Ward E, Ferlay J, Brawley O and Bray F: International variation in
prostate cancer incidence and mortality rates. Eur Urol.
61:1079–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Ji GY, Li XM, et al: The
influence of mass screening for prostate cancer on the diagnostic
status of the clinical prostate cancer. Chin J Urol. 25:103–105.
2004.
|
|
20
|
Amălinei C, Căruntu ID, Giuşcă SE and
Bălan RA: Matrix metalloproteinases involvement in pathologic
conditions. Rom J Morphol Embryol. 51:215–228. 2010.PubMed/NCBI
|
|
21
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion metastasis, and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
25
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gohji K, Fujimoto N, Hara I, Fujii A,
Gotoh A, Okada H, Arakawa S, Kitazawa S, Miyake H, Kamidono S, et
al: Serum matrix metalloproteinase-2 and its density in men with
prostate cancer as a new predictor of disease extension. Int J
Cancer. 79:96–101. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Shi J, Feng J, Klocker H, Lee C
and Zhang J: Type IV collagenase (matrix metalloproteinase-2 and
−9) in prostate cancer. Prostate Cancer Prostatic Dis. 7:327–332.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lovaas JD, Zhu L, Chiao CY, Byles V,
Faller DV and Dai Y: SIRT1 enhances matrix metalloproteinase-2
expression and tumor cell invasion in prostate cancer cells.
Prostate. 73:522–530. 2013. View Article : Google Scholar : PubMed/NCBI
|