Introduction
Idiopathic membranous nephropathy (IMN) is an
autoimmune glomerulonephropathy, the pathogenesis of which remains
unknown. Pathologically, IMN is characterized by immune complex
deposition in glomerular basement membrane (GBM) epithelial cells
with diffuse thickening of the GBM (1). IMN is one of the main causes for primary
nephrotic syndrome (NS) in adults (2),
accounting for 9.89–13.3% of all glomerular nephritis cases in
mainland China (3,4).
The onset of IMN is insidious and the prognosis
varies significantly. IMN can be spontaneously cured in 25–50%
patients, while immunosuppressant therapy may fail in other
patients, in whom the condition may progress gradually to end-stage
renal disease (ESRD) (5–7). According to the classic view based on
early studies, male gender, age >50 years, hypertension, the
presence of ultra-large quantities of proteinuria (>10 g/24 h),
and elevation of serum creatinine (SCr) in the beginning of the
disease onset are factors unfavorably affecting the prognosis
(8). However, the reliability of this
conclusion has been challenged by increasing evidence in recent
years (9). As all these studies used a
2-fold increase in SCr within 2 years as the surrogate end-point of
ESRD, the sensitivity to prognostic judgment of patients with
chronic kidney disease (CKD) is inadequate (9).
The latest study of the chronic kidney disease
prognosis consortium (CKD-PC) identified that a 30% decrease in the
estimated glomerular filtration rate (eGFR) within 2 years was a
better surrogate end-point (9). In the
present study, this observation end-point was used to follow-up
patients with IMN confirmed by clinical pathology in the Kidney
Institute of the Chinese People's Liberation Army (Shanghai,
China), between February 2011 and August 2012, to explore the
correlation between the clinicopathological parameters and the
prognosis in IMN patients.
Patients and methods
Patient selection
Included in the present study were 73 IMN patients
aged between 18 and 80 years who presented with glomerular
membranous nephropathy, as confirmed by renal biopsy, and received
treatment, and whose SCr was <445 µmol/l at the time of
recruitment. Exclusion criteria were secondary factors (autoimmune
diseases, hepatitis B and C, tumors, excessive exposure to organic
solvents and undergoing treatment with some medicines that
potentially cause secondary membranous nephropathy), patients with
complicated diabetes mellitus, and prealbumin <200 mg/l.
Clinical parameters
Gender, age, blood pressure (BP), 24-h urine
protein, serum albumin, blood urea nitrogen (BUN), SCr, eGFR as
estimated by the chronic kidney disease epidemiology collaboration
(CKD-EPI) equation (10), and cystatin
C (Cyst C) of all the included patients were recorded.
Pathological parameters
All the renal biopsy specimens were routinely
examined by optical microscopy, immunofluorescence microscopy and
electron microscopy. The renal pathology was in accordance with the
Ehrenreich criteria. Immunoglobulin G (IgG), IgM, IgA, C3, C4, Clq
and fibrin-related antigen were detected by immunofluorescence
staining. The percentage of glomerular sclerosis (% GS), severity
of tubulointerstitial injury, the type, intensity and location of
immunoglobulin and complement deposition were observed by
immunofluorescence, and the location of immune complex deposition
was observed by electron microscopy. Tubulointerstitial injury area
(TIA) was defined as 4 grades. Tubular atrophy, interstitial
fibrosis, interstitial edema, interstitial inflammation and acute
tubular injury were each graded semiquantitatively on a scale of
0–3+ based on the % cortical area affected (absent, ≤25,
26–50 and >50%, respectively) (11).
Treatment
For the initial treatment, all the patients received
angiotensin receptor blocker (ARB) therapy following biopsy, with
the dose adjusted depending on BP. A 6-month course of treatment
with prednisone acetate (0.5 mg/kg per day) and cyclophosphamide
(1.0 g/m) was implemented in patients who presented with one of the
following three conditions 6 months after ARB therapy (12): i) 24-h urine protein >4 g that
remained at a level of 50% higher than the baseline without showing
a declining tendency; ii) the existence of severe, disabling or
life-threatening clinical symptoms associated with NS; and iii) a
≥30% elevation in SCr within 6–12 months after confirmation of the
diagnosis, but eGFR not <25–30 ml/min/1.73 m2, and
these changes were not due to NS complications.
Follow-up records
Follow-up records included 24-h quantitative
proteinuria, serum albumin, BUN and SCr. Complete remission (CR)
was defined as urine protein <0.4 g/24 h and serum albumin
>35 g/l for ≥1 month; partial remission (PR) was defined as
urine protein decreasing by >50% and quantitative proteinuria
<3.5 g/24 h and serum albumin >30 g/l for ≥1 month; no
response (NR) was defined as persistent proteinuria within the
range of nephropathy without reaching the criterion of urine
protein for PR.
End-point events
A 30% decrease in eGFR or entry of ESRD within 2
years was set as the observation end-point. Patients who entered
the end-point or those who did not enter the end-point during the
2-year follow-up period were assigned to two groups.
Statistical analysis
All the measurement data were treated using SPSS
19.0 (SPSS, Inc., Chicago, IL, USA). Comparison between the two
groups was performed using the χ2 test, and P<0.05
was considered to indicate a statistically significant difference.
Statistically significant clinicopathological parameters were
tested by stepwise multivariate logistic regression analysis, and
P<0.05 was considered to indicate a statistically significant
difference. The cumulative risk was analyzed by Kaplan-Meier curve,
and the risk difference was tested by log-rank method, using
P<0.05 as statistical significance.
Ethical approval
All the participants signed their written consent to
participate in the study. The protocol was approved by the Ethics
Committee of the Second Military Medical University (Shanghai,
China).
Results
General data
Finally, 73 IMN patients were enrolled in the study,
including 44 (60.3%) males and 29 (39.7%) females ranging in age
from 18 to 80 years with a mean age of 52.8±15.4 years at the time
of enrollment. The mean follow-up duration after renal biopsy was
15.35±5.68 months, ranging from 3 to 25.5 months with a median of
16 months.
Severe hypoproteinemia (albumin <25 g/l) was
observed in 35 (47.9%) patients, large quantities of proteinuria
(>3.5 g/l) in 30 (41.1%), microscopic hematuria in 15 (20.5%),
accompanying hypertension in 23 (31.5%), and eGFR <90
ml/min/1.73 m2 at the time of renal biopsy in 26 (35.6%)
patients. Pathological stages I and II were predominant, accounting
for 94.5%. GS occurred as an accompanying sign in 35 (47.9%)
patients. TIA was >25% in 8 patients.
Follow-up status
All the IMN patients received ARB therapy during the
follow-up periods, including 55 patients who received prednisone
acetate and cyclophosphamide. Following treatment, 28 (38.4%)
patients achieved CR, 21 (28.8%) achieved PR, and 24 (32.9%)
patients achieved NR in terms of urine protein. During the 2-year
follow-up period, 28 (38.4%) patients reached the observation
end-point (all reached a 30% decrease in eGFR, and none reached
ESRD), and the remaining 45 (61.6%) did not reach the observation
end-point.
Comparison of the baseline data between the two
groups showed significant differences in the following six
clinicopathological parameters: Age, ≥60 years; SCr, >97 µmol/l;
EPI-eGFR, <90 ml/min/1.73 m2; BUN, >5.6 mmol/l;
serum albumin <25 g/l; and TIA, >25% (Table I). There was no significant difference
between the two groups in terms of the pathological stage (Table I) and the remission rate of urine
protein following treatment during the follow-up period (Table II).
 | Table I.Comparison of baseline data between
patients who reached and did not reach the observation
end-point. |
Table I.
Comparison of baseline data between
patients who reached and did not reach the observation
end-point.
| Observation
index | Non end-point group
(n=45), n (%) | End-point group
(n=28), n (%) | P-value |
|---|
| Age ≥60 years | 11 (22.4) | 15 (53.6) | 0.012 |
| Male | 26 (57.8) | 18 (64.3) | 0.581 |
| Hypertension | 12 (26.7) | 11 (39.3) | 0.259 |
| Blood urea nitrogen
>5.6 mmol/l | 15 (33.3) | 16 (57.1) | 0.045 |
| Serum creatinine
>97 µmol/l | 4 (8.9) | 9
(32.1) | 0.012 |
| Serum albumin <25
g/l | 17 (37.8) | 18 (64.3) | 0.027 |
| Proteinuria >3.5
g/24 h | 20 (44.4) | 10 (35.7) | 0.461 |
| Estimated glomerular
filtration rate <90 ml/min·1.73 m2 | 12 (26.7) | 14 (50.0) | 0.043 |
| Serum cystatin C
>0.86 mg/l | 24 (53.3) | 12 (42.9) | 0.348 |
| Glomerular
sclerosis | 20 (44.4) | 15 (53.6) | 0.448 |
| Tubulointerstitial
injury area >25% | 2 (4.4) | 6
(21.4) | 0.024 |
| Pathological
stage |
|
| 0.299 |
| I | 11 (24.4) | 6
(21.4) |
|
| II | 33 (73.3) | 19 (67.9) |
|
|
III | 1 (2.2) | 3
(10.7) |
|
 | Table II.Comparison of the therapeutic effect
and the use of immunosuppressants between the two groups. |
Table II.
Comparison of the therapeutic effect
and the use of immunosuppressants between the two groups.
| Observation
index | Non end-point group
(n=45), n (%) | End-point group
(n=28), n (%) | P-value |
|---|
| Complete
remission | 20 (44.4) | 8
(28.6) | 0.175 |
| Partial
remission | 14 (31.1) | 7
(25.0) | 0.575 |
| No response | 11 (24.4) | 13 (46.4) | 0.052 |
| Immunosuppressant
use | 32 (71.1) | 23 (82.1) | 0.288 |
Multivariate logistic regression
analysis
Multivariate logistic regression analysis of the
aforementioned six variants showing statistically significant
differences between the two groups demonstrating that age ≥60
years, serum albumin <25 g/l and TIA >25% were three
independent risk factors predicting the occurrence of the end-point
event in IMN patients (Table
III).
 | Table III.Multivariate logistic regression
analysis on idiopathic membranous glomerulonephritis progression to
the observation end-point. |
Table III.
Multivariate logistic regression
analysis on idiopathic membranous glomerulonephritis progression to
the observation end-point.
| Observation
index | OR value | P-value |
|---|
| Tubulointerstitial
injury area >25% | 6.724 | 0.044 |
| Serum albumin
<25 g/l | 3.195 | 0.034 |
| Age ≥60 years | 3.471 | 0.025 |
| Blood urea nitrogen
>5.6 mmol/l | 0.046 | 0.830 |
| Serum creatinine
>97 µmol/l | 2.157 | 0.142 |
| eGFR <90
ml/min·1.73 m2 | 0.021 | 0.884 |
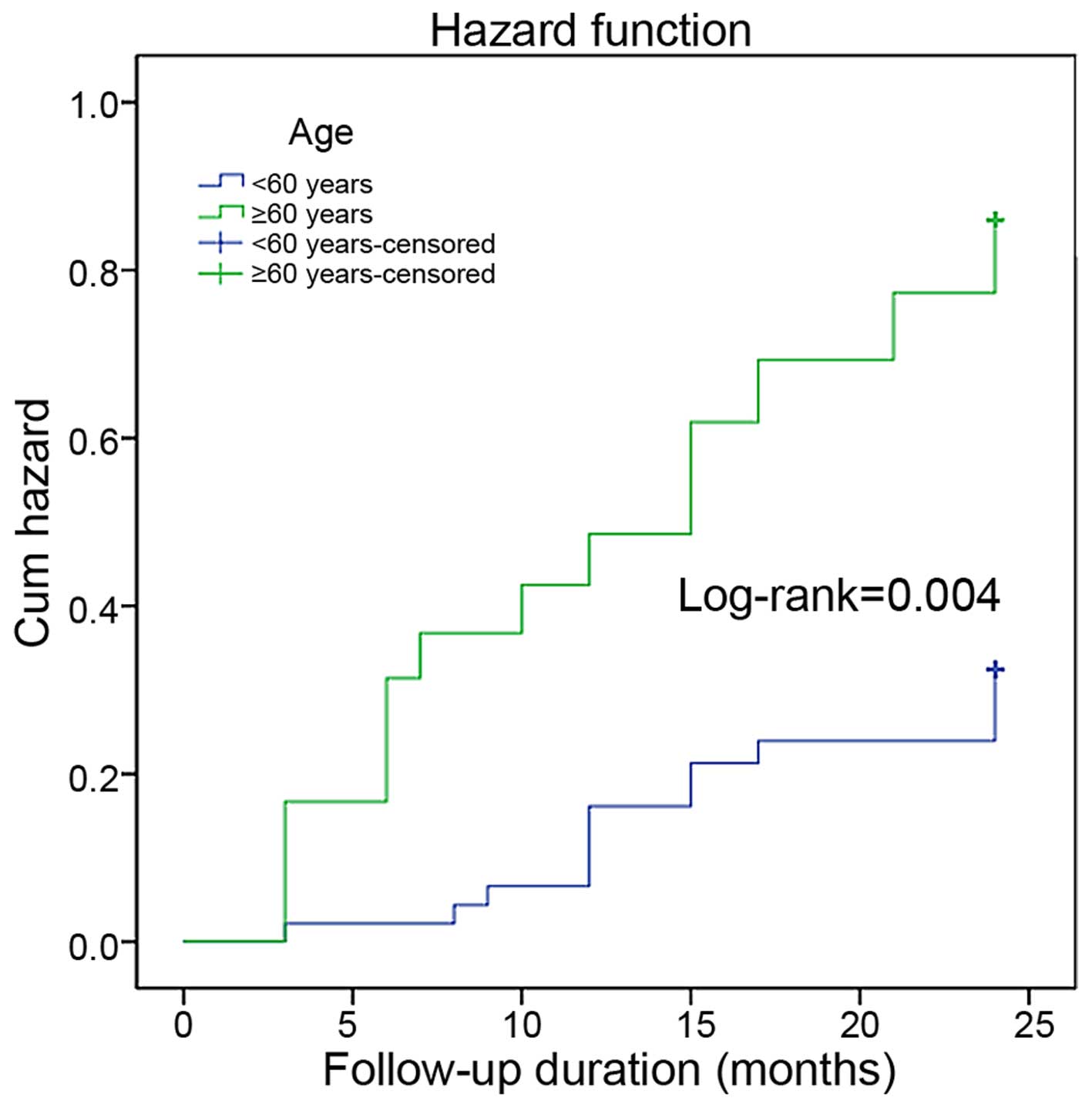
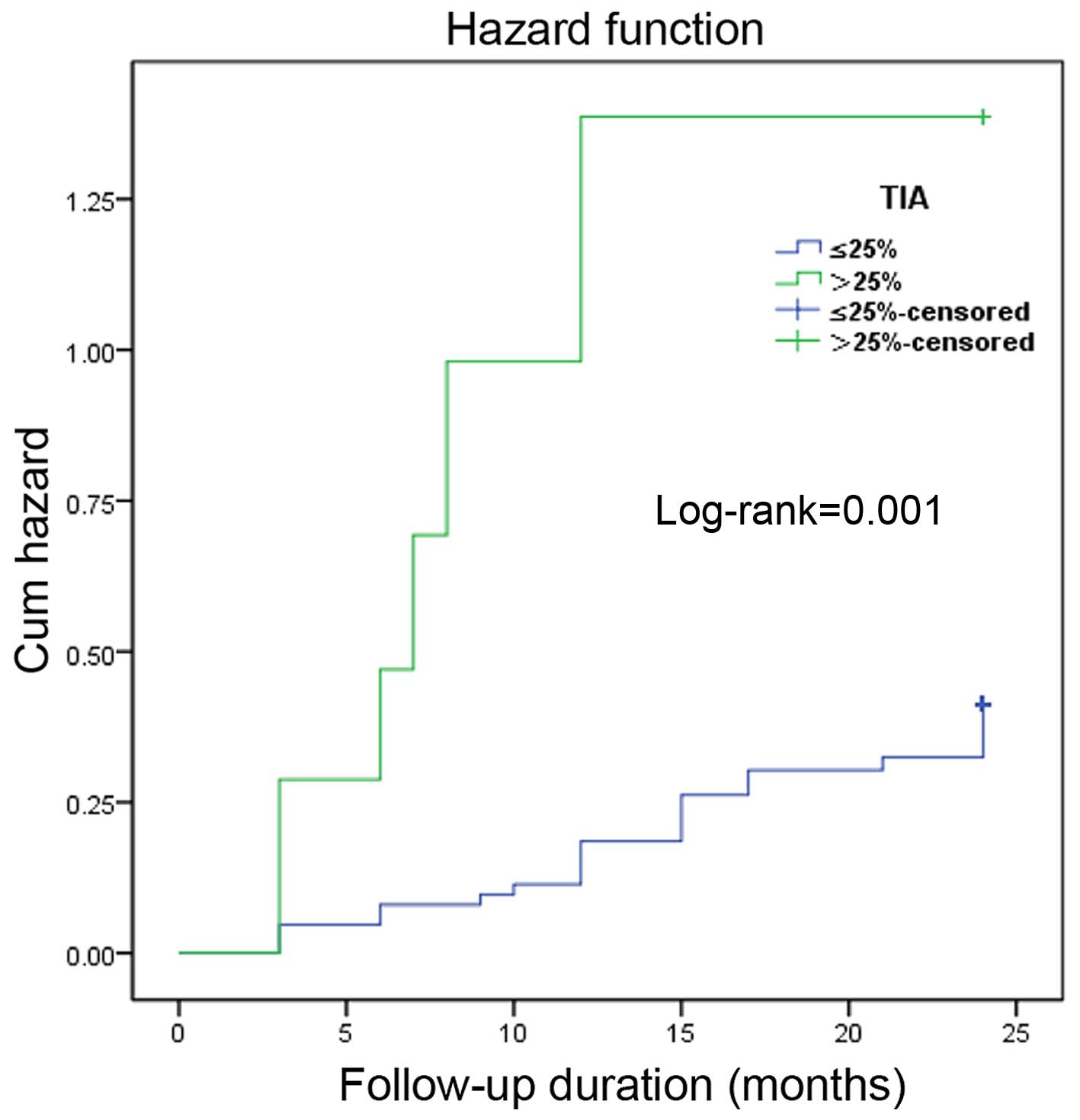
Kaplan-Meier curve showed that the occurrence rate
of the end-point event was significantly higher in IMN patients
with age ≥60 years, serum albumin <25 g/l and TIA >25%
(log-rank P=0.004, P=0.024 and P=0.001) (Figs. 1–3).
Discussion
IMN is the most common cause of primary NS in adults
(13). The proportion of IMN in
primary glomerulonephritis has increased markedly in recent years,
accounting for ~20% of patients receiving renal puncture biopsy
(14). The clinical prognosis varies
greatly: Approximately one-third of the patients can achieve
spontaneous remission, and approximately one-third of the patients
remain proteinuric with a relatively stable renal function.
Long-term follow-up studies have demonstrated that the remaining
30% of the patients may progress to ESRD or succumb within 5–10
years (15–18). However, there is not sufficient
research on risk factors affecting the prognosis of IMN. The study
results from different centers are largely contradictory, and the
results obtained from Asian populations alone are not all the same
(19–21). The possible reason for these
discrepancies may be due to the fact that all these studies used
the 2-fold increase in SCr as the surrogate end-point. Knowing that
the phenomenon of 2-fold increase in SCr is an index with poor
sensitivity, Coresh et al (9)
conducted a meta-analysis on SCr levels in 28 cohorts of 1.5
million subjects and identified that in CKD patients with either
poor (eGFR <60 ml/min/1.73 m2) or good (eGFR ≥60
ml/min/1.73 m2) baseline renal function, the percentage
of patients with a 2-fold increase in SCr within 2 years was
extremely low, accounting for <1 and 0.1%, respectively. These
figures are by far lower than the actual occurrence. Therefore,
this index should not be used as the end-point index to predict the
occurrence of ESRD, as it may distort the research results.
According to the suggestion of the CKD-PC, the
criterion of a 30% decrease in eGFR within 2 years was used in the
present study as the surrogate end-point. The result showed that
its sensitivity was 5–10-fold as high as that of a 2-fold increase
in SCr. During the 2-year follow-up period, only 6 patients (8.2%)
were detected whose SCr increased by 2-fold, while 28 (38.4%)
patients reached this end-point (a 30% decrease in eGFR within 2
years) which is consistent with the study in the literature that
approximately one-third of the IMN patient would progress to
ESRD.
It was found in the present study that age ≥60
years, SCr >97 µmol/l, EPI-eGFR <90 ml/min/1.73
m2, BUN >5.6 mmol/l, serum albumin <25 g/l, and
TIA >25% are all factors that may increase the risk of end-point
event occurrence. However, only age ≥60 years, serum albumin <25
g/l and TIA >25% are independent risk factors of end-point event
occurrence.
Shiiki et al (19) analyzed the data of 949 Japanese IMN
patients and identified that age ≥60 years was the independent risk
factor for ESRD, which is consistent with the present result. The
risk of end-point event occurrence in patients >60 years
increased by 3.471-fold in the present study. An earlier study
(22) also supported this finding. An
older age is a common risk factor for all patients with chronic
glomerulonephritis (23). This may be
the result of the synergic effect of decreased renal function
associated with physiological aging and the disease itself.
The majority of studies believe that
tubulointerstitial injury is an important factor predicting the
deteriorating progression of renal function in IMN patients
(24,25). Wehrmann et al (24) followed up 334 IMN patients for 5.2
years and revealed that the degree of tubulointerstitial
pathological change was the only factor associated with the
prognosis. Zuo et al (21)
observed 217 IMN patients and found that a change in chronic
tubulointerstitial was a high-risk factor contributing to the
aggravation and progression of the disease to ESRD. Troyanov et
al (26) also reported that the
prognosis was poorer in IMN patients whose renal biopsy reported
tubulointerstitial pathological change, which is similar to the
present study finding. The stepwise multivariate logistic
regression analysis in the present study showed that the risk of
end-point event recurrence in patients with chronic TIA >25%
increased by 6.724-fold.
There is no definite conclusion regarding the impact
of proteinuria on the prognosis of IMN patients. Studies in the
20th century (27,28) reported that the long-term prognosis was
extremely good in IMN patients without proteinuria within the range
of nephropathy, in whom the 10-year kidney survival was as high as
90–100%, which is different from the conclusion drawn from recent
studies. Hladunewich et al (29) reported that the prognosis may also be
poor in patients whose proteinuria did not reach the range of NS.
Polanco et al (30) found that
the prognosis may be good in patients whose proteinuria reached 12
g/24 h. The present study did not find that proteinuria within the
range of NS had any significant influence on the prognosis;
however, the risk of end-point event occurrence in patients with
serum albumin <25 g/l increased by 3.195-fold. Kaneko et
al (31) identified that
biological antioxidant potential (BAP) was closely correlated with
the serum albumin concentration in patients with idiopathic
nephrotic syndrome, and decreased BAP may aggravate the
inflammatory response. Roche et al (32) also found that serum albumin is the most
abundant antioxidant in the living body, and therefore
hypoalbuminemia may reduce the ability of the body to repair
oxidative stress. As this mechanism also exists in IMN patients,
hypoalbuminemia may be the consequence of IMN and the cause that
promotes the progression of nephropathy.
In conclusion, since 1993, the US Food and Drug
Administration has accepted the use of a 2-fold increase in SCr
against the baseline value as the surrogate end-point of
progression in chronic nephropathy (33). However, subsequent clinical practices
have reported that this criterion is inadequate in various aspects.
When a 30% decrease in eGFR within 2 years could be used as the
surrogate end-point, the follow-up period and experimental cost
could be reduced markedly, and the experiment efficiency could also
be improved (9). Taking the above
reasons into account, this criterion was used as the end-point in
this study and found that age >60 years, the serum albumin
concentration <25 g/l, and a higher score of chronic
tubulointerstitial injury are independent risk factors predicting
the progression of IMN patients to ESRD, thus providing an
alternative reference for assessing the prognosis of renal disease
patients.
Acknowledgements
The present study was supported by a grant from the
National Science Foundation of China to X-Z.Z. (no. 81270765).
References
|
1
|
Kerjaschki D and Farquhar MG: The
pathogenic antigen of Heymann nephritis is a membrane glycoprotein
of the renal proximal tubule brush border. Proc Natl Acad Sci USA.
79:5557–5561. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heymann W, Hackel DB, Harwood S, Wilson SG
and Hunter JL: Production of nephrotic syndrome in rats by Freund's
adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med.
100:660–664. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou FD, Zhao MH, Zou WZ, Liu G and Wang
H: The changing spectrum of primary glomerular diseases within 15
years, A survey of 3331 patients in a single Chinese centre.
Nephrol Dial Transplant. 24:870–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li LS and Liu ZH: Epidemiologic data of
renal diseases from a single unit in China: Analysis based on
13,519 renal biopsies. Kidney Int. 66:920–923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marx BE and Marx M: Prediction in
idiopathic membranous nephropathy. Kidney Int. 56:666–673. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hogan SL, Muller KE, Jennette JC and Falk
RJ: A review of therapeutic studies of idiopathic membranous
glomerulopathy. Am J Kidney Dis. 25:862–875. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glassock RJ: Diagnosis and natural course
of membranous nephropathy. Semin Nephrol. 23:324–332. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wasserstein AG: Membranous
glomerulonephritis. J Am Soc Nephrol. 8:664–674. 1997.PubMed/NCBI
|
|
9
|
Coresh J, Turin TC, Matsushita K, Sang Y,
Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, et
al: CKD Prognosis Consortium: Decline in estimated glomerular
filtration rate and subsequent risk of end-stage renal disease and
mortality. JAMA. 311:2518–2531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: CKD-EPI (chronic kidney disease epidemiology
collaboration): A new equation to estimate glomerular filtration
rate. Ann Intern Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stokes MB, Valeri AM, Markowitz GS and
D'Agati VD: Cellular focal segmental glomerulosclerosis Clinical
and pathologic features. Kidney Int. 70:1783–1792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beck L, Bomback AS, Choi MJ, Holzman LB,
Langford C, Mariani LH, Somers MJ, Trachtman H and Waldman M: KDOQI
US commentary on the 2012 KDIGO clinical practice guideline for
glomerulonephritis. Am J Kidney Dis. 62:403–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hofstra JM, Fervenza FC and Wetzels JF:
Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol.
9:443–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan X, Xu J, Ren H, Zhang W, Xu Y, Shen P,
Li X, Wang W, Chen X, Wu P, et al: Changing spectrum of
biopsy-proven primary glomerular diseases over the past 15 years: A
single-center study in China. Contrib Nephrol. 181:22–30. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schieppati A, Mosconi L, Perna A, Mecca G,
Bertani T, Garattini S and Remuzzi G: Prognosis of untreated
patients with idiopathic membranous nephropathy. N Engl J Med.
329:85–89. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Polanco N, Gutiérrez E, Rivera F,
Castellanos I, Baltar J, Lorenzo D and Praga M: Grupo de Estudio de
las Enfermedades Glomerulares de la Sociedad Española de Nefrología
(GLOSEN): Spontaneous remission of nephrotic syndrome in membranous
nephropathy with chronic renal impairment. Nephrol Dial Transplant.
27:231–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McQuarrie EP, Stirling CM and Geddes CC:
Idiopathic membranous nephropathy and nephrotic syndrome, Outcome
in the era of evidence-based therapy. Nephrol Dial Transplant.
27:235–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshimoto K, Yokoyama H, Wada T, Furuichi
K, Sakai N, Iwata Y, Goshima S and Kida H: Pathologic findings of
initial biopsies reflect the outcomes of membranous nephropathy.
Kidney Int. 65:148–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiiki H, Saito T, Nishitani Y, Mitarai T,
Yorioka N, Yoshimura A, Yokoyama H, Nishi S, Tomino Y, Kurokawa K,
et al: Research Group on Progressive Renal Diseases in Japan:
Prognosis and risk factors for idiopathic membranous nephropathy
with nephrotic syndrome in Japan. Kidney Int. 65:1400–1407. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eriguchi M, Oka H, Mizobuchi T, Kamimura
T, Sugawara K and Harada A: Long-term outcomes of idiopathic
membranous nephropathy in Japanese patients treated with low-dose
cyclophosphamide and prednisolone. Nephrol Dial Transplant.
24:3082–3088. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zuo K, Wu Y, Li SJ, Xu F, Zeng CH and Liu
ZH: Long-term outcome and prognostic factors of idiopathic
membranous nephropathy in the Chinese population. Clin Nephrol.
79:445–453. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zent R, Nagai R and Cattran DC: Idiopathic
membranous nephropathy in the elderly, A comparative study. Am J
Kidney Dis. 29:200–206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garg AX, Kiberd BA, Clark WF, Haynes RB
and Clase CM: Albuminuria and renal insufficiency prevalence guides
population screening, Results from the NHANES III. Kidney Int.
61:2165–2175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wehrmann M, Bohle A, Bogenschütz O,
Eissele R, Freislederer A, Ohlschlegel C, Schumm G, Batz C and
Gärtner HV: Long-term prognosis of chronic idiopathic membranous
glomerulonephritis. An analysis of 334 cases with particular regard
to tubulo-interstitial changes. Clin Nephrol. 31:67–76.
1989.PubMed/NCBI
|
|
25
|
Ponticelli C, Zucchelli P, Passerini P,
Cagnoli L, Cesana B, Pozzi C, Pasquali S, Imbasciati E, Grassi C,
Redaelli B, et al: A randomized trial of methylprednisolone and
chlorambucil in idiopathic membranous nephropathy. N Engl J Med.
320:8–13. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Troyanov S, Roasio L, Pandes M, Herzenberg
AM and Cattran DC: Renal pathology in idiopathic membranous
nephropathy, A new perspective. Kidney Int. 69:1641–1648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Honkanen E, Törnroth T, Grönhagen-Riska C
and Sankila R: Long-term survival in idiopathic membranous
glomerulonephritis, Can the course be clinically predicted? Clin
Nephrol. 41:127–134. 1994.PubMed/NCBI
|
|
28
|
Ponticelli C, Passerini P, Altieri P,
Locatelli F and Pappalettera M: Remissions and relapses in
idiopathic membranous nephropathy. Nephrol Dial Transplant. 7(Suppl
1): 85–90. 1992.PubMed/NCBI
|
|
29
|
Hladunewich MA, Troyanov S, Calafati J and
Cattran DC: Metropolitan Toronto Glomerulonephritis: Registry The
natural history of the non-nephrotic membranous nephropathy
patient. Clin J Am Soc Nephrol. 4:1417–1422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Polanco N, Gutiérrez E, Covarsí A, Ariza
F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C,
Pons S, et al: Grupo de Estudio de las Enfermedades Glomerulares de
la Sociedad Española de Nefrología: Spontaneous remission of
nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc
Nephrol. 21:697–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaneko K, Kimata T, Tsuji S, Shimo T,
Takahashi M and Tanaka S: Serum albumin level accurately reflects
antioxidant potentials in idiopathic nephrotic syndrome. Clin Exp
Nephrol. 16:411–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roche M, Rondeau P, Singh NR, Tarnus E and
Bourdon E: The antioxidant properties of serum albumin. FEBS Lett.
582:1783–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lewis EJ, Hunsicker LG, Bain RP and Rohde
RD: The Collaborative Study, Group. The effect of
angiotensin-converting-enzyme inhibition on diabetic nephropathy. N
Engl J Med. 329:1456–1462. 1993. View Article : Google Scholar : PubMed/NCBI
|