Introduction
Fas is a type I transmembrane protein that can
transform the apoptotic signal into susceptible target cells
through the binding of the Fas ligand (FasL), belonging to the
tumor necrosis factor/nerve growth factor family (1–3). The
Fas/FasL signaling pathway is one of the well-characterised
extrinsic pathways of apoptosis. While FasL combines with the death
receptor Fas, Fas binds to the death domain (DD) of Fas-associated
protein with DD (FADD) and recruits procaspase-8 to construct the
death-inducing signaling complex through the death effector domain
of FADD. Apoptosis is a programmed process of normal cell death,
which is regulated by genes and has a crucial role in maintaining
physiological homeostasis (4). The out
of control regulation of apoptosis could make a contribution to the
pathogenesis of malignancies, such as ovarian cancer, breast cancer
and neuroblastoma (5–7). Among haematological cancers, Fas-mediated
apoptosis was presented in multiple myeloma, Burkitt's lymphoma and
acute myeloid leukemia (AML) (8–11).
AML is one of the hematological malignancies caused
by the malignant proliferation of myeloid protocells. In China, an
incidence of 1.62/100,000 has been reported and the mortality of
AML has been ranked as sixth place among the malignancies (12). The possible risk influence involved in
AML includes occupational, environmental, lifestyle or genetic
factors, although the specific etiology of the majority of leukemia
remains to be elucidated (13–15). The results of previous studies
demonstrated that the expression of Fas has been presented at cell
surfaces of AML, suggesting that Fas-mediated apoptosis is likely
to associate with the pathogenesis of AML (16,17).
Furthermore, a high-level expression of Fas was found in the
myeloblasts and the lack of functional Fas signaling was reported
to have a crucial role in several subtypes of AML (18). This evidence potentially provides the
proof of the association between Fas-mediated apoptosis and the
pathological mechanisms of AML.
Significant evidence has suggested that the Fas gene
was regulated by a number of genetic components positioned in the
5-upstream promoter region of the gene, particularly within the
transcription factor binding sites (19). The Fas gene has been reported to be
highly polymorphic from the dbSNP databases (http://www.ncbi.nlm.nih.gov/projects/SNP/). However,
two potential functional polymorphisms have been most extensively
studied, one involves an A-to-G substitution at the −670 nucleotide
position in the enhancer region (Fas-670A>G, rs1800682) and the
other a G-to-A allele mutation at the −1377 position within the
silencer region, which alters the SP-1 transcription factor-binding
site (Fas-1377G>A, rs2234767) (20). They were reported to reduce Fas
expression (20,21). The composition of the Fas-670
polymorphism is the mutant homozygous genotype Fas-670G/G,
heterozygous genotype Fas-670G/A and the wild homozygote
Fas-670A/A. The statistical data of the wild homozygote among the
healthy population ranged from 25.5 to 43.6%, and the rate of
mutant homozygote Fas-670G/G reached 12%, whereas the frequency
range of the heterozygous Fas-670A/G was 44.2–60.5% (22,23).
However, previous results regarding the association
between the Fas gene polymorphism and risk of AML are
controversial. Only 2 studies discussed the linkage between the Fas
gene polymorphism and the risk of AML. One of them confirmed that
the mutations in the Fas promoter were associated with increasing
the risk of AML in the Caucasian population, whereas the other
verified that the variations of the Fas gene were not associated
with the AML risk in Koreans (21,24). In
addition, other studies reported that there was no connection
between the variation of Fas gene and malignancies (25,26).
To explore the association between the mutation of
the Fas gene and the risk of AML, a case-control study was
conducted to test the frequency of the polymorphism at
Fas-670A>G among patients with AML and the healthy population in
China.
Materials and methods
Study subject
The present case-control study comprised 98 newly
diagnosed AML patients and 2,014 healthy individuals. The classical
morphological criteria of the French-American-British
classification was used to establish diagnosis. Confirmed
histopathology of the AML patients was consecutively recruited from
The First Affiliated Hospital of Guangxi Medical University
(Nanning, Guangxi, China) between 2011 and 2014. The individuals of
the control group were randomly selected from the files of the
Fangchenggang Area Male Health and Examination Survey (FAMHES), and
they exhibited no history or diagnosis of carcinoma, as described
previously (27). All the individuals
were not related ethnicities and all the data were collected in
southern China. A standardized questionnaire was issued regarding
demographics and lifestyle, such as age, smoking and drinking, and
the feedback was collected for analysis. The individuals who had
smoked at least one cigarette per day lasting over half a year,
either formerly or currently, were defined as smokers; others were
non-smokers. The individuals who drank at least once a week lasting
over half a year, either formerly or currently, were defined as
drinkers; others were non-drinkers. The study was approved by the
Ethics and Human Subject Committee of the First Affiliated Hospital
of Guangxi Medical University, and all specimens were collected
following informed consents.
DNA extraction and single nucleotide
polymorphism (SNP) genotyping
Bone marrow (2 ml) from each AML patient was drawn
into Vacutainer tubes containing EDTA and stored at −80°C, and 2 ml
of peripheral blood from the control subjects was preserved using
the same procedure. Subsequently, genomic DNA was isolated using
the DNA isolation kit (Aidlab Biotechnologies, Beijing, China) and
preserved at −80°C. SNP genotyping of AML was carried out using the
SNaPshot Multiplex kit (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and the primers were shown as
follows: Forward, ATAGCTGGGGCTATGCGATTTG and reverse,
GTTGGGGAGGTCTTGAAGGAGA. The genotyping information of the controls
was extracted from the FAMHES database and the Omni 1 platform
(Illumina, Inc., San Diego, CA, USA) was used for genotyping. The
methods used were as previously described (28).
Identification of eligible
studies
Studies on the association between the −670G/A
polymorphism in the Fas gene and susceptibility of AML were
selected by a comprehensive search (last study obtained was from
August 2015) with English language only selected in the PubMed,
EMBASE and Wanfang databases. The following search terms and their
synonyms were used: ‘Fas’, ‘-670 G/A’ or ‘CD95’, ‘polymorphism’ or
‘mutation’, and ‘acute myeloid leukemia’ or ‘AML’. The cited
references were also individually searched for the eligible
studies.
Selection and exclusion criteria
The studies were identified according to the
following criteria: i) The study must use a case-control design;
ii) the study clarified the association between the −670G/A
polymorphism in the Fas genes and the risk for AML; iii) presented
adequate genotyping data (numbers of GG, GA and AA genotypes in the
case and control groups, respectively) to estimate the odds ratio
(OR) with the corresponding 95% confidence interval (CI); iv)
genotype distributions were in Hardy-Weinberg equilibrium (HWE)
among controls (P>0.05), and v) the study used human subjects.
Studies meeting any of the following items should be excluded: i)
Insufficient data; ii) the study used animal or cell subjects or
subjects were children; iii) the study was based on other diseases,
such as acute lymphoblastic leukaemia or myelodysplastic syndromes
and the prognosis used medicine; and iv) duplicated studies.
Data extraction
Original data was extracted carefully from all the
eligible studies by Ying Huang, according to the above selection
and exclusion criteria to ensure the accuracy of the retrieved
information, and the result was reviewed by a third investigator,
Zhenfang Liu. Our research team extensively discussed and resolved
the discrepancies. The following information was collected for each
study: First author, year of publication, ethnicity, sample size of
cases and controls, study period, and genotype distributions for
cases and controls.
Statistical analysis
The differences of demographic variables between the
cases and controls were derived using Pearson's χ2 test.
The logistic regression was used to estimate the associations of
polymorphisms and AML risk measured by ORs and 95% CIs. The pooled
OR was estimated based on the individual ORs. It was evaluated for
i) the dominant model (rare allele carriers versus common
homozygous carriers); ii) recessive model (rare homozygous carriers
versus common allele carriers); iii) codominant model (heterozygous
versus common homozygous carriers and rare homozygous versus common
homozygous), and iv) additive model (minor allele versus major
allele) (29). To estimate the P-value
among different studies, the χ2 based Q-test and
I2 statistics [with values between 0 and 100%, with
higher values leading to greater heterogeneity (no heterogeneity,
I2, 0–25%; moderate heterogeneity, I2,
25–50%; significant heterogeneity, I2, 50–75%; and
extreme heterogeneity, I2, 75–100%)] were used for
checking the heterogeneity (30,31).
P>0.05 for the Q-test or I2<50% demonstrates an
absence of heterogeneity across studies. In this case, the
fixed-effects model (the Mantel-Haenszel method) was used.
Otherwise, the OR of each study was calculated by the
random-effects model (the DerSimonian and Laird method). It assumes
that the random-effects model indicates substantial diversity and
evaluates the within-study sampling error and between-study
variation (32). The assumption of the
fixed-effects model is that all the studies are assessing the same
underlying effect and consider only the within-study variance
(33). Due to the analysis of 3
studies only, a subgroup analysis was not performed. Furthermore, a
sensitivity analysis was employed to assess the stability of the
results. The estimation of the potential publication bias was
conducted using funnel plots and Begg's rank correlation test
(34). HWE of the control groups was
tested by the χ2 test for goodness of fit. All the
P-values were two-sided. All the statistical analyses were
performed with STATA 12.0 and SPSS 16.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Characteristics of AML patients and
controls
The frequency of significance of the characteristics
among cases and controls are presented in Table I. Cases and controls appeared to be
adequately matched for age. The median age of cases was 38.3 years
(range, 14–76 years) and that of the controls was 37.5 years
(range, 20–69 years; P=0.57). The gender rate of the controls was
significantly different from that of cases as the controls were not
collected to match with cases regarding gender (P<0.01).
Similarly, the rate of smoking and drinking status was
significantly different from that of the cases (all P<0.01). In
a subgroup analysis of non-smokers and non-drinkers, there were
significant differences between the age and gender rates of the
controls and that of the cases (P=0.02 and P<0.01,
respectively). Two patients presented with an undifferentiated
leukemia (M1: n=2), 18 with an immature granulocytic leukemia (M2:
n=18), 78 with a monocytic leukemia (M4: n=51; M5: n=25; M6: n=2)
and 2 were unknown.
 | Table I.Distribution of the characteristics
of acute myeloid leukemia patients and the controls. |
Table I.
Distribution of the characteristics
of acute myeloid leukemia patients and the controls.
|
Characteristics | Patients
(n=98) | Controls
(n=2,014) | P-value |
|---|
| Median age, years
(range) | 38.3 (14–76) | 37.5 (20–69) |
0.57 |
|
Non-smoker and
non-drinker | 37.1 (14–76) | 41.5 (20–69) |
0.02 |
| Male, n (%) | 50 (51.0) | 2,014
(100.0) | <0.01 |
|
Non-smoker and
non-drinker | 22 (22.4) |
161
(100.0) | <0.01 |
| History of smoking,
n (%) |
|
|
|
|
Smoker | 24 (24.5) | 1,107 (55.0) | <0.01 |
|
Non-smoker | 74 (75.5) |
907 (45.0) |
|
| History of
drinking, n (%) |
|
|
|
|
Drinker | 17 (17.3) | 1,721 (85.5) | <0.01 |
|
Non-drinker | 81 (82.7) |
293 (14.5) |
|
Association between the Fas-670A>G
polymorphism and AML risk
Among the control group, genotype distributions were
in HWE (P>0.05). Allele and genotype frequencies of the
Fas-670A>G polymorphism among the cases and controls and their
association with AML risk are presented in Table II. The heterozygous GA genotype was
the most frequent in patients with AML (54.1%), whereas 28.6%
exhibited the GG genotype, and 17.3% were homozygous AA. There were
no significant differences from those among the controls (50.3,
28.2 and 21.5%, respectively). An unconditional logistic regression
model was used to adjust for age. Compared to the wild homozygous
AA genotype, the manifestation of variant alleles (heterozygous and
variant homozygous genotypes) did not influence the risk of
developing AML (GA vs. AA: OR, 1.34; 95% CI, 0.77–2.35; P=0.30; GG
vs. AA: OR, 1.27; 95% CI, 0.69–2.35; P=0.45; GA+GG vs. AA: OR,
1.32; 95% CI, 0.77–2.25; P=0.31). Similarly, there was no
association between the Fas-670 polymorphism and the risk for AML
following adjusting for age, smoking and drinking (Table II). Subsequently, a further subgroup
analysis among non-smokers and non-drinkers was conducted. The
Fas-670A>G genetic variants did not show a statistically
significant influence on the susceptibility of an individual to AML
(GA vs. AA: OR, 0.59; 95% CI, 0.25–1.40; P=0.23; GG vs. AA: OR,
0.91; 95% CI, 0.46–1.77; P=0.77; GA+GG vs. AA: OR, 1.31; 95% CI,
0.51–3.39; P=0.58) (Table III).
 | Table II.Allele and genotype frequencies of
Fas-670A>G polymorphism among patients and controls and their
association with acute myeloid leukemia risk following
adjustment. |
Table II.
Allele and genotype frequencies of
Fas-670A>G polymorphism among patients and controls and their
association with acute myeloid leukemia risk following
adjustment.
| Genotype | Patients (n=98), n
(%) | Controls (n=2,014),
n (%) | ORa (95% CI) | P-value | ORb (95% CI) | P-value |
|---|
| AA | 17 (17.3) | 433
(21.5) | 1.00 | 0.58 | 1.00 | 0.79 |
| GA | 53 (54.1) | 1,013 (50.3) | 1.34
(0.77–2.35) | 0.30 | 1.21
(0.66–2.21) | 0.55 |
| GG | 28 (28.6) | 568
(28.2) | 1.27
(0.69–2.35) | 0.45 | 1.25
(0.64–2.45) | 0.51 |
| AA+GA | 81 (82.7) | 1,581 (78.5) | 1.32
(0.77–2.25) | 0.31 | 1.22
(0.69–2.18) | 0.50 |
 | Table III.Allele and genotype frequencies of
the Fas-670A>G polymorphism among non-smokers and non-drinkers
and their association with acute myeloid leukemia risk following
adjustment. |
Table III.
Allele and genotype frequencies of
the Fas-670A>G polymorphism among non-smokers and non-drinkers
and their association with acute myeloid leukemia risk following
adjustment.
| Genotype | Patients (n=68), n
(%) | Controls (n=161), n
(%) | ORa (95%CI) | P-value |
|---|
| AA | 11 (16.2) | 38
(23.6) | 1.00 | 0.46 |
| GA | 37 (54.4) | 83
(51.6) | 0.59
(0.25–1.40) | 0.23 |
| GG | 20 (29.4) | 40
(24.8) | 0.91
(0.46–1.77) | 0.77 |
| AA+GA | 57 (83.8) | 123 (76.4) | 1.31
(0.51–3.39) | 0.58 |
Characteristics of the eligible
studies
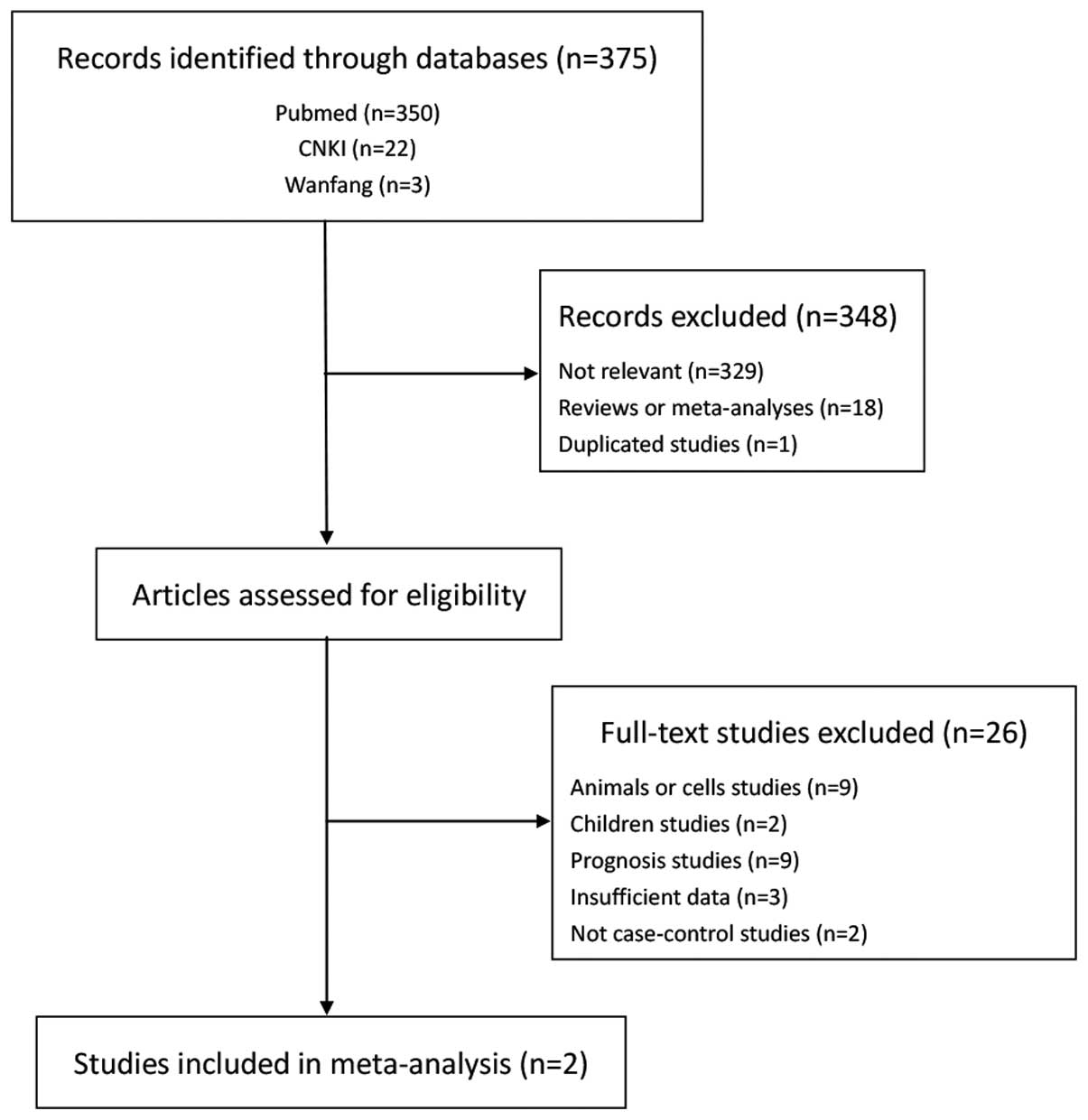
A total of 375 studies were retrieved through the
initial search. Following screening by titles and abstracts, 348
studies were eliminated based on the inclusion and exclusion
criteria (Fig. 1). Subsequent to
browsing the full articles, 9 studies that used animals or cells as
subjects, 2 that studied children and 9 that were regarding the
prognosis were excluded. Five were excluded due to the deficiency
of raw statistical data and they were not case-control studies.
Finally, with the inclusion of the present experimental results,
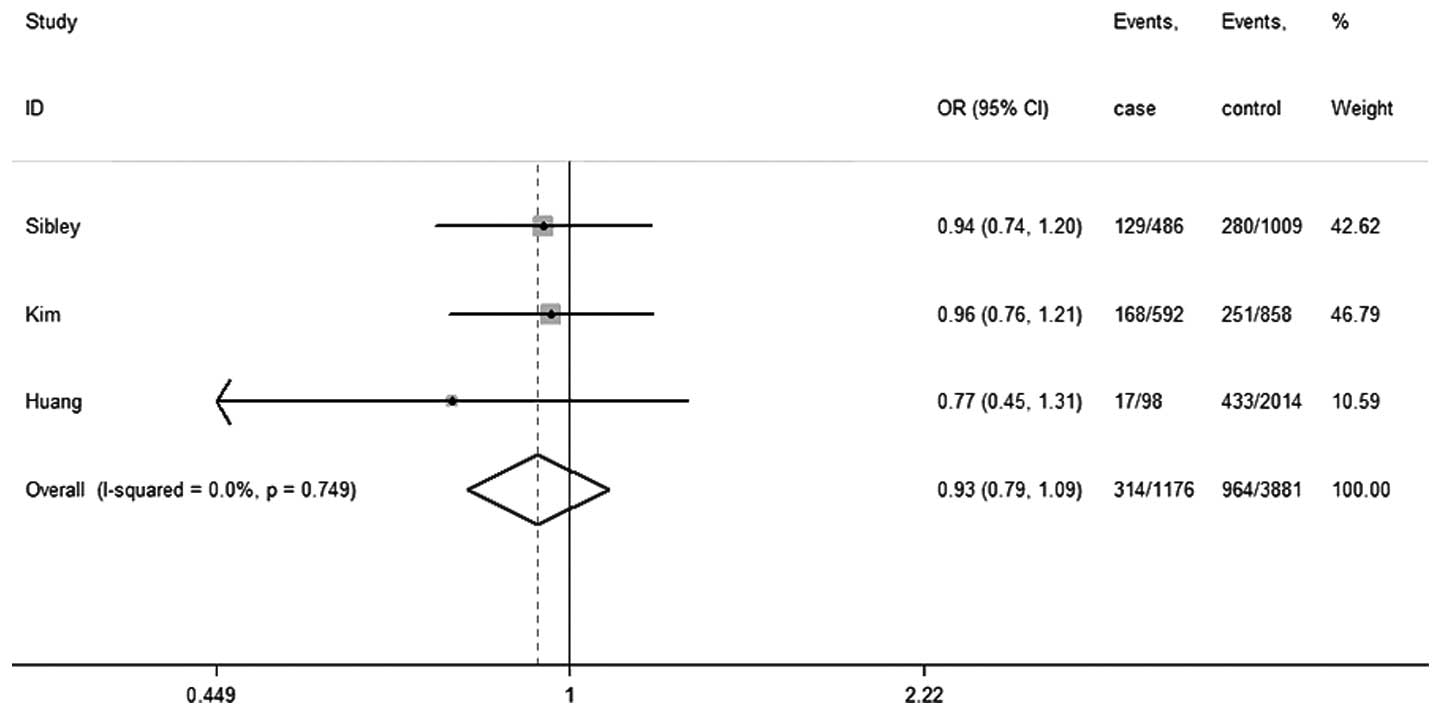
only 3 studies were included in this meta-analysis (21,24). The
characteristics of each study and the distributions of the Fas gene
polymorphism among patients and controls are summarized in Table IV. Three separate studies consisted of
2 Asian population studies and 1 Caucasian population study. There
were 3 case-control studies with 1,144 AML cases and 3,806 controls
concerning the association between the Fas-670A/G polymorphism and
the risk of AML. Two of these studies, including the present study,
were hospital-based and one was population-based (21). Their genotype distributions among the
controls were in agreement with HWE.
 | Table IV.Characteristics of each study and the
forest plot for the association of the Fas polymorphism with the
risk of acute myeloid leukemia in the dominant model (AA vs.
GA+GG). |
Table IV.
Characteristics of each study and the
forest plot for the association of the Fas polymorphism with the
risk of acute myeloid leukemia in the dominant model (AA vs.
GA+GG).
|
|
|
|
| Cases, n (%) | Controls, n
(%) |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| First author,
year | Country | Ethnicity | Case/control,
n | AA | AA+GA | AA | AA+GA | Weight, % | OR (95% CI) | (Refs.) |
|---|
| Sibley, 2003 | UK | Caucasian | 454/934 | 129 | 357 | 280 |
729 |
42.6 | 0.94
(0.74–1.20) | (21) |
|
|
|
|
| (28.4) | (78.6) | (30.0) | (78.1) |
|
|
|
| Kim, 2010 | Korea | Asian | 592/858 | 168 | 424 | 251 |
607 |
46.8 | 0.96
(0.76–1.21) | (24) |
|
|
|
|
| (28.4) | (71.6) | (29.3) | (70.7) |
|
|
|
| Present study,
2015 | China | Asian |
98/2,014 | 17 | 81 | 433 | 1,581 |
10.6 | 0.77
(0.45–1.31) |
|
|
|
|
|
| (17.4) | (82.7) | (21.5) | (78.5) |
|
|
|
| Total |
|
|
| 314 |
| 370 |
| 100.0 | 0.93
(0.79–1.09) |
|
Quantitative data analysis
No significant between-study heterogeneity was
detected across studies, and thus the analyses were conducted using
the fixed-effects models. Under the dominant model, the summary ORs
and 95% CIs for the Fas-670A/G polymorphism are shown in Table IV and its forest plot is shown in
Fig. 2. The results illustrated that
the Fas-670 polymorphism had no statistically evident association
with the risk of AML (OR, 0.93; 95% CI, 0.79–1.09;
Pheterogeneity=0.75; I2=0.0%). Similar
consequences were observed in other groups (G vs. A: OR, 1.01; 95%
CI, 0.91–1.12; Pheterogeneity=0.79; I2=0.0%; GG
vs. AA: OR, 1.01; 95% CI, 0.82–1.24; Pheterogeneity=0.70;
I2=0.0%; GA vs. AA: OR, 1.12; 95% CI, 0.94–1.32;
Pheterogeneity=0.81; I2=0.0%; recessive model:
OR, 0.94; 95% CI, 0.79–1.12; Pheterogeneity=0.85;
I2=0.0%) (forest plot not shown). Owing to the
deficiency of raw data, a subgroup analysis regarding non-smokers
and non-drinkers could not be performed.
Sensitivity analyses
A sensitivity analysis was carried out by sequential
removal of each individual eligible study to estimate the stability
of the combined results and the source of the heterogeneity. The
results indicated that the consequences were not materially altered
by any single study (data not shown).
Publication bias
No clear asymmetry appeared to be shown in the
Begg's funnel plot in all the genetic models, suggesting deficiency
of publication bias (AA vs. GA+GG: P=0.30). In addition, the
Egger's test was conducted to provide statistical evidence for the
lack of publication bias (AA vs. GA+GG: P=0.06).
Discussion
The Fas gene is situated in chromosome 0q24.1,
involving 9 exons, 8 introns and a promoter (35). Fas is the apoptotic signal transmission
reporter that is a type of transmembrane protein. When combined
with its natural ligand FasL, it can initiate the apoptosis process
by the death signal cascade (3). Thus,
genetic polymorphisms in the Fas have an association with
susceptibility to malignancies such as epithelial ovarian cancer or
squamous cell carcinoma of the larynx and hypopharynx (5,36). Among
them, the polymorphism involving the A/G alleles at the −670
nucleotide position in the promoter region (Fas-670A>G,
rs1800682) is one of the common mutations. It is located within the
signal transducers and activators of transcription 1 (STAT1)
binding element [interferon (IFN)-γ-activated sequence] and results
from an ATCCG (G/A) AA substitution, which may have a functional
gene regulation (20). In a new
meta-analysis containing 52 case-control studies, there was no
association between the Fas-670A>G polymorphism and risk of
cancers, including cervical cancer, gastric cancer, breast cancer
and lung cancer. However, there was a significantly decreased risk
in prostate cancers and melanoma for the GG+AG vs. AA comparison
model in the Fas-670A>G polymorphism subgroup analysis, and a
significantly increased risk was observed among those of the
African population for the GG+GA vs. AA comparison model (37). This demonstrates that the Fas-670
polymorphism may have an association with certain types of cancer.
Furthermore, recent results regarding the association between the
Fas gene polymorphism and the risk of AML are controversial.
Therefore, the present study conducted a case-control study and a
meta-analysis to explore the association between the Fas-670A>G
polymorphism and the risk of AML.
However, the present results showed that the
polymorphism at position −670 bp of the Fas gene had no association
with the risk of developing AML. In accordance with the previous
studies in the United Kingdom and Korea (21,24), on the
basis of analysis of 98 case patients and 2,014 matched normal
controls, it was revealed that there was no significant connection
between the Fas-670A>G polymorphism and the risk of AML in the
Chinese population. Similarly, in a subgroup analysis of
non-smokers and non-drinkers, there were no significant differences
between the Fas-670A>G polymorphism and the risk of AML. The
present study, however, only included 98 cases and 2,014 controls,
which was too small to confirm our proposed hypothesis. To solve
this problem, a further meta-analysis was conducted. The
meta-analysis, including 1,144 cases and 3,806 controls from 2
published case-control studies and one group of statistics from the
present study, investigated the connection between a potentially
functional mutation within the Fas promoter region and risk of AML.
The results demonstrated that there was no evidence of the risk of
AML associated with the Fas gene polymorphism at position −670 bp.
No significant association was observed with AML under any genetic
model. A subgroup analysis regarding non-smokers and non-drinkers
could not be performed owing to the absence of raw data. The
deficiency of data for statistical analysis may cause a serious
confounding bias. To minimize the likelihood bias, a detailed
protocol was conducted prior to starting the study, and a
meticulous search for eligible studies was carried out and used
explicit methods for data extraction and statistical analysis. To
the best of our knowledge, this is the first meta-analysis to
investigate whether the Fas-670A>G genetic polymorphism was
associated with the risk of AML. The result of a new study
exploring the connection between Fas-670 polymorphism and
susceptibility to leukemia was similar to the present study
(38).
There are certain reasons that may explain the
present result of the meta-analysis. In the first instance, the
Fas-670A>G polymorphism has no association with the risk of AML
as the phosphorylation of transcription factor STAT1 is activated
through the MEK/ERK pathway instead of the Fas/FasL pathway. The
activated STAT1 has a crucial role in the process of drug-induced
differentiation of AML cells (39,40).
Previous studies have indicated that Fas expression can be
upregulated by IFN-γ stimulation through STAT1 activation in
monocytes isolated from −670AA genotype subjects, but not from
subjects with the −670GG genotype (41,42). Sibley
et al (21) reported that there
was a similar capacity in −670A and −670G alleles to combine STAT1.
However, STAT1 is a dasatinib off-target effect protein, which
participates in the differentiation process and its phosphorylation
is activated by the MEK/ERK pathway in AML (43). Therefore, we can assume that genetic
mutations of the Fas/FasL pathway have no association with the risk
of AML, although the Fas-670A>G polymorphism is located within
the STAT1 binding element. In addition, the Fas/FasL pathway may be
obstructed by pathological immune cells in AML. Previous studies
have reported that immune cells in malignancies have aberrant Fas
and FasL genes, which induced downregulated Fas and upregulated
FasL expressions (44–50). Target cells killed by cytotoxic T
lymphocytes (CTLs) and natural killer cells are one of the
mechanisms of apoptosis in the immune system, and the Fas/FasL
pathway mediates the immune effect of CLTs (51–53).
However, certain studies have revealed that the rate of regulatory
T cells (Tregs) in peripheral blood and bone marrow of newly
diagnosed AML patients was significantly higher than that in the
healthy controls and it changed with their physical condition; for
example, it decreased when the patients were in complete remission
and it increased again when they relapsed (54–56). Zhou
et al (57) revealed that Tregs
could significantly suppress the proliferation and migration of
CTLs. Therefore, Tregs in AML may inhibit Fas-induced apoptosis so
that the Fas-670A>G genetic polymorphism cannot reflect the
development of AML.
According to the results of the present
meta-analysis, we can presume a hypothesis that the Fas-670G allele
substitution may serve to confer a molecular interaction to the
action of the Fas-1377A allele. Although there is no significant
association independently between the Fas-670A>G genetic
polymorphism and the risk of AML, Sibley et al (21) found that −670A, with the presence of
−1377A, increased the risk of AML significantly more than −670G.
Look et al (58) reported the
evidence for the transcriptional synergy between STAT1 and SP1, and
this interaction may serve to confer an additional composition of
specificity for transcription. They concluded that the recruitment
of STAT1 to promotion may be conducted by SP1, and SP1 may serve as
a better linkage of STAT1 action to the basal transcription
complex. Considering the above evidence, we can presume that the
guanine substitution at the −670 bp position in the Fas gene may
serve to confer a molecular interaction to the action of Fas-1377A
allele; however, further studies are required to detect the
association between the downstream protein of the Fas-670A>G
polymorphism and AML risk.
However, the present study has certain limitations.
Firstly, it has been reported that there are certain copy number
variations in the Fas gene sequence from the dbVar database
(http://www.ncbi.nlm.nih.gov/dbvar),
which could affect the results of SNP regarding the association
between gene polymorphism and the disease. Secondly, there are
mismatches between the patients and the control subjects with
regards to the rate of gender and history of drinking alcohol and
smoking. In the meta-analysis, further subgroup analysis by age,
gender and different populations could not be conducted due to the
insufficient detailed information. Furthermore, it has been
reported that a small number of studies could affect the results
and more relevant studies are required to draw a more accurate
conclusion. Finally, a potential English language bias may exist as
English literature only was selected, and there may be differences
between English language literature and others. Therefore, whether
a genetic polymorphism exists in the incidence of AML between
regions could not be concluded.
In conclusion, the present study suggested that
there was no significant association independently between the
Fas-670A>G polymorphism and the risk of AML. To reach a
definitive conclusion, more well-designed studies with larger
samples are required to identify an association between this
polymorphism and the risk of AML.
Acknowledgements
The present study was supported by grants from the
Guangxi Natural Science Foundation (nos. 2010GXNSFA013181 and
2010GXNSFB013064), Guangxi Science Fund for Distinguished Young
Scholars (no. 2012GXNSFFA060009), and National Natural Science
Foundation of China (nos. 81160072 and 81560028).
Glossary
Abbreviations
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Nagata S: Apoptosis regulated by a death
factor and its receptor: Fas ligand and Fas. Philos Trans R Soc
Lond B Biol Sci. 345:281–287. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Itoh N, Yonehara S, Ishii A, Yonehara M,
Mizushima S, Sameshima M, Hase A, Seto Y and Nagata S: The
polypeptide encoded by the cDNA for human cell surface antigen Fas
can mediate apoptosis. Cell. 66:233–243. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zornig M, Hueber A, Baum W and Evan G:
Apoptosis regulators and their role in tumorigenesis. Biochim
Biophys Acta. 1551:F1–F37. 2001.PubMed/NCBI
|
|
5
|
Li Y, Hao YL, Kang S, Zhou RM, Wang N and
Qi BL: Genetic polymorphisms in the Fas and FasL genes are
associated with epithelial ovarian cancer risk and clinical
outcomes. Gynecol Oncol. 128:584–589. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Zheng Z, Yu W, Lin H, Cui B and
Cao F: Polymorphisms of the FAS and FASL genes and risk of breast
cancer. Oncol Lett. 3:625–628. 2012.PubMed/NCBI
|
|
7
|
Han W, Zhou Y, Zhong R, Wu C, Song R, Liu
L, Zou L, Qiao Y, Zhai K, Chang J, et al: Functional polymorphisms
in FAS/FASL system increase the risk of neuroblastoma in Chinese
population. PloS One. 8:e716562013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Westendorf JJ, Lammert JM and Jelinek DF:
Expression and function of Fas (APO-1/CD95) in patient myeloma
cells and myeloma cell lines. Blood. 85:3566–3576. 1995.PubMed/NCBI
|
|
9
|
Shima Y, Nishimoto N, Ogata A, Fujii Y,
Yoshizaki K and Kishimoto T: Myeloma cells express Fas
antigen/APO-1 (CD95) but only some are sensitive to anti-Fas
antibody resulting in apoptosis. Blood. 85:757–764. 1995.PubMed/NCBI
|
|
10
|
Gutierrez MI, Cherney B, Hussain A,
Mostowski H, Tosato G, Magrath I and Bhatia K: Bax is frequently
compromised in Burkitt's lymphomas with irreversible resistance to
Fas-induced apoptosis. Cancer Res. 59:696–703. 1999.PubMed/NCBI
|
|
11
|
Pordzik S, Petrovici K, Schmid C, Kroell
T, Schweiger C, Köhne CH and Schmetzer H: Expression and prognostic
value of FAS receptor/FAS ligand and TrailR1/TrailR2 in acute
myeloid leukemia. Hematology. 16:341–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chongli Y and Xiaobo Z: Anemia CESGoLaA:
Incidence Survey of Leukemia in China in 1986. Chinese Journal of
Hematology. 10:618–621. 1986.
|
|
13
|
Kasim K, Levallois P, Abdous B, Auger P
and Johnson KC: Lifestyle factors and the risk of adult leukemia in
Canada. Cancer Causes Control. 16:489–500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong O, Harris F, Yiying W and Hua F: A
hospital-based case-control study of acute myeloid leukemia in
Shanghai: Analysis of personal characteristics, lifestyle and
environmental risk factors by subtypes of the WHO classification.
Regul Toxicol Pharmacol. 55:340–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Filippini T, Heck JE, Malagoli C, Del
Giovane C and Vinceti M: A review and meta-analysis of outdoor air
pollution and risk of childhood leukemia. J Environ Sci Health C
Environ Carcinog Ecotoxicol Rev. 33:36–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Min YJ, Lee JH, Choi SJ, Chi HS, Lee JS,
Kim WK and Lee KH: Prognostic significance of Fas (CD95) and TRAIL
receptors (DR4/DR5) expression in acute myelogenous leukemia. Leuk
Res. 28:359–365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iijima N, Miyamura K, Itou T, Tanimoto M,
Sobue R and Saito H: Functional expression of Fas (CD95) in acute
myeloid leukemia cells in the context of CD34 and CD38 expression:
Possible correlation with sensitivity to chemotherapy. Blood.
90:4901–4909. 1997.PubMed/NCBI
|
|
18
|
Komada Y, Zhou YW, Zhang XL, Xue HL, Sakai
H, Tanaka S, Sakatoku H and Sakurai M: Fas receptor (CD95)-mediated
apoptosis is induced in leukemic cells entering G1B compartment of
the cell cycle. Blood. 86:3848–3860. 1995.PubMed/NCBI
|
|
19
|
Nunobiki O, Ueda M, Toji E, Yamamoto M,
Akashi K, Sato N, Izuma S, Torii K, Tanaka I, Okamoto Y and Noda S:
Genetic polymorphism of cancer susceptibility genes and HPV
infection in cervical carcinogenesis. Patholog Res Int.
2011:3640692011.PubMed/NCBI
|
|
20
|
Huang QR, Morris D and Manolios N:
Identification and characterization of polymorphisms in the
promoter region of the human Apo-1/Fas (CD95) gene. Mol Immunol.
34:577–582. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sibley K, Rollinson S, Allan JM, Smith AG,
Law GR, Roddam PL, Skibola CF, Smith MT and Morgan GJ: Functional
FAS promoter polymorphisms are associated with increased risk of
acute myeloid leukemia. Cancer Res. 63:4327–4330. 2003.PubMed/NCBI
|
|
22
|
Sun T, Zhou Y, Li H, Han X, Shi Y, Wang L,
Miao X, Tan W, Zhao D, Zhang X, et al: FASL-844C polymorphism is
associated with increased activation-induced T cell death and risk
of cervical cancer. J Exp Med. 202:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kordi Tamandani DM, Sobti RC and Shekari
M: Association of Fas-670 gene polymorphism with risk of cervical
cancer in North Indian population. Clin Exp Obstet Gynecol.
35:183–186. 2008.PubMed/NCBI
|
|
24
|
Kim HJ, Jin XM, Kim HN, Lee IK, Park KS,
Park MR, Jo DY, Won JH, Kwak JY, Kim HJ, et al: Fas and FasL
polymorphisms are not associated with acute myeloid leukemia risk
in Koreans. DNA Cell Biol. 29:619–624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai HC, Lin WY, Lin YW, Chang CC, Yu MH,
Chen CC and Chu TY: Genetic polymorphisms of FAS and FASL
(CD95/CD95L) genes in cervical carcinogenesis: An analysis of
haplotype and gene-gene interaction. Gynecol Oncol. 99:113–118.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park SH, Choi JE, Kim EJ, Jang JS, Lee WK,
Cha SI, Kim CH, Kam S, Kim DS, Park RW, et al: Polymorphisms in the
FAS and FASL genes and risk of lung cancer in a Korean population.
Lung Cancer. 54:303–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan A, Gao Y, Yang X, Zhang H, Qin X, Mo
L, Peng T, Xia N and Mo Z: Low serum osteocalcin level is a
potential marker for metabolic syndrome: Results from a Chinese
male population survey. Metabolism. 60:1186–1192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang X, Sun J, Gao Y, Tan A, Zhang H, Hu
Y, Feng J, Qin X, Tao S, Chen Z, et al: Genome-wide association
study for serum complement C3 and C4 levels in healthy Chinese
subjects. PLoS Genet. 8:e10029162012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tian J, Pan F, Li J, Ma Y, Cen H, Pan HF,
Pan YY and Ye DQ: Association between the FAS/FASL polymorphisms
and gastric cancer risk: A meta-analysis. Asian Pac J Cancer Prev.
13:945–951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
William GC: The combination of estimates
from different experiments. Biometrics. 10:101–129. 1954.
View Article : Google Scholar
|
|
32
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
34
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Behrmann I, Walczak H and Krammer PH:
Structure of the human APO-1 gene. Eur J Immunol. 24:3057–3062.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Gao J, Li Y, Zhao X, Gao W, Peng
L, Yan D, Liu L, Li D, Wei L, et al: Functional polymorphisms in
FAS and FASL contribute to risk of squamous cell carcinoma of the
larynx and hypopharynx in a Chinese population. Gene. 524:193–196.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu Y, He B, Li R, Pan Y, Gao T, Deng Q,
Sun H, Song G and Wang S: Association of the polymorphisms in the
Fas/FasL promoter regions with cancer susceptibility: A systematic
review and meta-analysis of 52 studies. PloS One. 9:e900902014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, He Y, Lu X, Zeng Z, Tang C, Xue T
and Li Y: Association between Fas/FasL polymorphism and
susceptibility to leukemia: A meta-analysis. Int J Clin Exp Med.
8:3817–3824. 2015.PubMed/NCBI
|
|
39
|
Gianni M, Terao M, Fortino I, LiCalzi M,
Viggiano V, Barbui T, Rambaldi A and Garattini E: Stat1 is induced
and activated by all-trans retinoic acid in acute promyelocytic
leukemia cells. Blood. 89:1001–1012. 1997.PubMed/NCBI
|
|
40
|
Battle TE and Frank DA: STAT1 mediates
differentiation of chronic lymphocytic leukemia cells in response
to Bryostatin 1. Blood. 102:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kanemitsu S, Ihara K, Saifddin A, Otsuka
T, Takeuchi T, Nagayama J, Kuwano M and Hara T: A functional
polymorphism in fas (CD95/APO-1) gene promoter associated with
systemic lupus erythematosus. J Rheumatol. 29:1183–1188.
2002.PubMed/NCBI
|
|
42
|
Farre L, Bittencourt AL, Silva-Santos G,
Almeida A, Silva AC, Decanine D, Soares GM, Alcantara LC Jr, Van
Dooren S, Galvão-Castro B, et al: Fas 670 promoter polymorphism is
associated to susceptibility, clinical presentation and survival in
adult T cell leukemia. J Leukoc Biol. 83:220–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fang Y, Zhong L, Lin M, Zhou X, Jing H,
Ying M, Luo P, Yang B and He Q: MEK/ERK dependent activation of
STAT1 mediates dasatinib-induced differentiation of acute myeloid
leukemia. PloS One. 8:e669152013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu J, Wilson J, He J, Xiang L, Schur PH
and Mountz JD: Fas ligand mutation in a patient with systemic lupus
erythematosus and lymphoproliferative disease. J Clin Invest.
98:1107–1113. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang J, Zheng L, Lobito A, Chan FK, Dale
J, Sneller M, Yao X, Puck JM, Straus SE and Lenardo MJ: Inherited
human caspase 10 mutations underlie defective lymphocyte and
dendritic cell apoptosis in autoimmune lymphoproliferative syndrome
type II. Cell. 98:47–58. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chun HJ, Zheng L, Ahmad M, Wang J, Speirs
CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, et al:
Pleiotropic defects in lymphocyte activation caused by caspase-8
mutations lead to human immunodeficiency. Nature. 419:395–399.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kennedy NJ, Kataoka T, Tschopp J and Budd
RC: Caspase activation is required for T cell proliferation. J Exp
Med. 190:1891–1896. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Alam A, Cohen LY, Aouad S and Sékaly RP:
Early activation of caspases during T lymphocyte stimulation
results in selective substrate cleavage in nonapoptotic cells. J
Exp Med. 190:1879–1890. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Su H, Bidère N, Zheng L, Cubre A, Sakai K,
Dale J, Salmena L, Hakem R, Straus S and Lenardo M: Requirement for
caspase-8 in NF-kappaB activation by antigen receptor. Science.
307:1465–1468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Newton K, Harris AW, Bath ML, Smith KG and
Strasser A: A dominant interfering mutant of FADD/MORT1 enhances
deletion of autoreactive thymocytes and inhibits proliferation of
mature T lymphocytes. EMBO J. 17:706–718. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rouvier E, Luciani MF and Golstein P: Fas
involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J
Exp Med. 177:195–200. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Suda T and Nagata S: Purification and
characterization of the Fas-ligand that induces apoptosis. J Exp
Med. 179:873–879. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kagi D, Vignaux F, Ledermann B, Bürki K,
Depraetere V, Nagata S, Hengartner H and Golstein P: Fas and
perforin pathways as major mechanisms of T cell-mediated
cytotoxicity. Science. 265:528–530. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Szczepanski MJ, Szajnik M, Czystowska M,
Mandapathil M, Strauss L, Welsh A, Foon KA, Whiteside TL and
Boyiadzis M: Increased frequency and suppression by regulatory T
cells in patients with acute myelogenous leukemia. Clin Cancer Res.
15:3325–3332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ustun C, Miller JS, Munn DH, Weisdorf DJ
and Blazar BR: Regulatory T cells in acute myelogenous leukemia: Is
it time for immunomodulation? Blood. 118:5084–5095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang W and Xu Y: Clinical significance of
Treg cell frequency in acute myeloid leukemia. Int J Hematol.
98:558–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou Q, Munger ME, Highfill SL, Tolar J,
Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, et
al: Program death-1 signaling and regulatory T cells collaborate to
resist the function of adoptively transferred cytotoxic T
lymphocytes in advanced acute myeloid leukemia. Blood.
116:2484–2493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Look DC, Pelletier MR, Tidwell RM, Roswit
WT and Holtzman MJ: Stat1 depends on transcriptional synergy with
Sp1. J Biol Chem. 270:30264–30267. 1995. View Article : Google Scholar : PubMed/NCBI
|