Introduction
In the living cells of aerobic organisms, reactive
oxygen species (ROS) are continuously produced in various
physiological processes, such as metabolic and other biochemical
reactions. A low level of ROS is essential for maintaining
physiological function and biochemical pathway processing,
including intracellular signaling pathways in cell differentiation,
proliferation, apoptosis (1) and
immunity (2). By contrast, high levels
of ROS can cause oxidative stress damaging biological
macromolecules, such as membrane lipids, proteins and DNA, which
may lead to metabolic malfunctions (3–5).
8-Hydroxy-2′-deoxyguanine (8-OHdG) induces ROS-mediated oxidative
damage by promoting the transversion of G/C to A/T, as it has a
higher affinity for pairing with adenine (A) rather than cytosine
(C) (6). Base excision repair (BER) of
DNA reverses a number of spontaneous and environmentally induced
genotoxic or miscoding base lesions in a process initiated by DNA
glycosylases (7). Human OGG1 (hOGG1)
efficiently repairs the incorrect or damaged bases by removing
8-OHdG, as part of the BER pathway (8).
Previous studies have reported several polymorphisms
in the hOGG1 gene (9,10). In vitro activity assays
confirmed that some of the variants affect hOGG1 expression
resulting in a substantially higher DNA repair activity. Variants
in the hOGG1 gene have been investigated in numerous
diseases (11–13). A number of genetic variants in the
hOGG1 gene may alter the repair function and thus contribute
to the development of ROS-related diseases.
PCOS is one of the most common reproductive
endocrine disorders, affecting 5–10% of women of reproductive age
(14). PCOS is a heterogeneous
syndrome characterized by clinical and/or biochemical androgen
excess, polycystic ovaries and ovulatory dysfunction (15). While the etiology of PCOS remains to be
elucidated, accumulating evidence suggests that chronic low-grade
inflammation strengthens the development of metabolic aberration
and ovarian dysfunction in this disorder (16,17).
Circulating TNF-α levels are elevated in PCOS patients (18). TNF-α is mainly derived from mononuclear
cells (MNCs), and hyperglycemia can further induce MNCs to produce
TNF-α and ROS (18,19). TNF-α and ROS in turn exacerbate the
inflammatory process, resulting in chronic inflammation in PCOS
patients. The hOGG1 gene is associated with decreased
insulin sensitivity (20), a common
feature of certain PCOS patients. Therefore, we hypothesize that a
link between the hOGG1 gene and PCOS may exist.
Materials and methods
Subjects
A total of 865 individuals, consisting of 425 PCOS
patients and 440 non-PCOS control women, were involved in the
present study. All the participants were of Han Chinese origin.
Peripheral venous blood samples were collected at Nanjing Drum
Tower Hospital of Nanjing University, Memorial Hospital of Sun
Yat-Sen University (Nanjing, Jiangsu, China), and at the Department
of Obstetrics and Gynecology of Anhui Medical University (Hefei,
Anhui, China) between 2004 and 2013. The PCOS patients were
diagnosed based on the 2003 Rotterdam Criteria (15). All the controls had normal ovulatory
menstrual cycles and did not show hirsutism or other manifestations
of hyperandrogenism. Serum hormone levels and clinical variables
[including age and body mass index (BMI)] were measured as
previously described (21). The study
was approved by the Ethics Committee of Nanjing University and
informed consent was obtained from each participant.
Polymorphism genotyping analysis in
the hOGG1 gene
Genomic DNA was isolated from peripheral blood
leukocytes using an UltraPure™ Genome DNA kit (SBS Genetech Co.,
Ltd., Shanghai, China) and stored at −80°C. A DNA fragment
containing part of the 5′ untranslated region (UTR) and the full
region of exon 1 was amplified with the primers: Forward, 5′-AGG
AGG TGG AGG AAT TAA GT-3′ and reverse, 5′-GGC TTC TCA GGC TCA GTC
A-3′, as described previously (9,11).
Amplification of DNA sequences, including Ser326Cys polymorphism in
exon 7, was carried out with: Forward primer, 5′-GGA AGG TGC TTG
GGG AAT-3′ and reverse primer, 5′-ACT GTC ACT AGT CTC ACC AG-3′.
Polymorphism chain reaction (PCR) was run in a total volume of 25
µl containing 50 ng of genomic DNA, 6 pmol of each primer, 2.5 µl
short tandem repeat 10X buffer (Promega, Madison, WI, USA) and 0.75
units of GoTaq DNA polymerase (Promega). The PCR protocol was
conducted as follows: Denaturing at 95°C for 5 min, followed by 30
cycles consisting of 30 sec of denaturation at 94°C, 30 sec of
annealing at 60°C for 5′-UTR or 58°C for exon 7, and 30 sec of
extension at 72°C; and a final single extension of 10 min at 72°C.
For 5′-UTR, the genotyping was carried out by direct sequencing on
an ABI 3130 automated sequencer at Nanjing Springen Bio-Technique
Corp. (Nanjing, China). Genotyping for the hOGG1 Ser326Cys
polymorphism was performed by the PCR-restriction fragment length
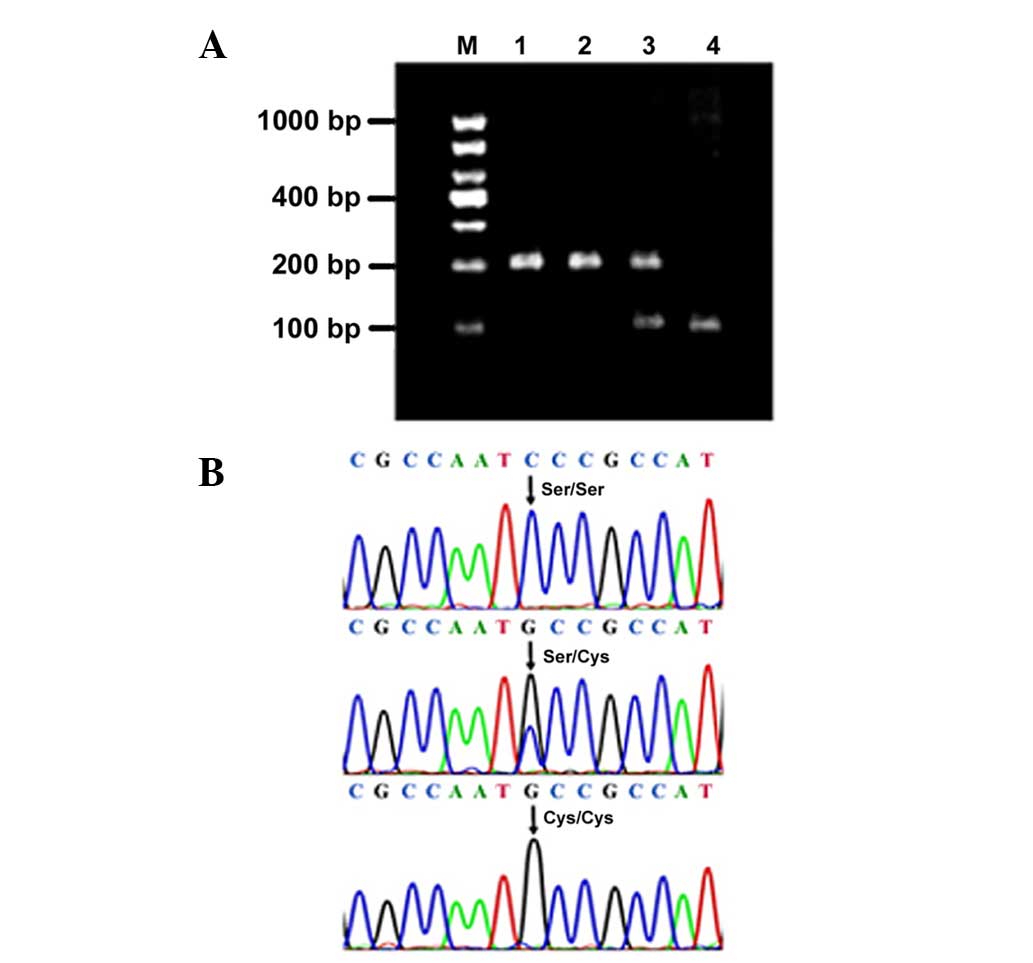
polymorphism (RFLP) assay. Briefly, a 200-base pair (bp) PCR
product was digested at 37°C with Fnu4HI (Thermo Fisher
Scientific, Inc., San Jose, CA, USA) for 16 h resulting in a single
200-bp band for the homozygous Ser/Ser hOGG1 variant, a
single 100-bp band for the homozygous Cys/Cys hOGG1 variant,
and double bands of 200 and 100 bp for the heterozygous Ser/Cys
hOGG1 variant. Digestion was visualized following
electrophoresis on a 3% agarose gel containing ethidium bromide.
Finally, 100 DNA samples were randomly selected for direct sequence
to validate Ser326Cys.
Serum hormone determination
Venous blood samples were collected in the early
follicular phase (days 3–5) of the menstrual cycle for those who
had menstrual cycles and at any time for those who were
amenorrheic. Prior to sample collection, all participants went
through a 12-h overnight fast. Serum estradiol (E2), testosterone
(T), luteinizing hormone (LH) and follicle-stimulating hormone
(FSH) were determined by radioimmunoassay (Beijing North Institute
of Biological Technology of China, Beijing, China and the CIS
Company of France, Gif-sur-Yvette, France). The intra-assay and
inter-assay coefficients of variation were <10% for all the
assays.
Statistical analysis
Fisher's exact test or χ2 test was used
when appropriate to detect the association between genotypic
variants in the hOGG1 gene. The PCOS risks were determined
by the odds ratio (OR) and its corresponding 95% confidence
intervals (CIs). Genotype frequencies for each single-nucleotide
polymorphism (SNP) were determined for Hardy-Weinberg equilibrium
in the control group. The results of serum hormone levels, age and
BMI are reported as the mean ± standard deviation. Genotypic
distribution analysis between PCOS and control was carried out by
Fisher's exact test. Biochemical steroid levels among different
genotypes were compared by analysis of covariance to correct for
age and BMI. All the statistical analysis was performed using the
statistical program SPSS version 17.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics of PCOS and
control subjects
Clinical variables and serum steroid hormone levels
were compared between women with PCOS and controls (Table I). PCOS patients had significantly
higher BMI, testosterone levels and LH/FSH ratios as compared with
healthy control women.
 | Table I.Clinical and endocrine
characteristics of PCOS patients and controls. |
Table I.
Clinical and endocrine
characteristics of PCOS patients and controls.
| Patients | Total, n | Age, years | BMIa, kg/m2 | FSHa, IU/l | LHa, IU/l | LH/FSHa | E2a, pg/ml | Ta, nmol/l |
|---|
| Control | 440 |
33.02±5.39 |
21.95±3.91 |
8.36±8.32 |
5.62±4.86 |
0.75±0.50 |
65.33±58.40 |
2.45±1.75 |
| PCOS | 425 |
26.95±6.79 |
22.96±6.13 |
5.97±3.22 |
11.77±8.96 |
2.26±3.04 |
79.3±69.22 |
2.7±6.02 |
Distribution of the hOGG1 gene
variants
Five variants were screened in a case-control study
that included 425 PCOS patients and 440 age-matched controls by
direct sequencing and/or RFLP (Fig. 1A and
B). Four of the variants were SNP, namely, c.-18G>T,
c.-23A>G, c. −53G>C and Ser326Cys. The genotypic
distributions of the four SNPs were consistent with Hardy-Weinberg
equilibrium in the control group. A rare variation, c.-45G>A,
was only observed in the normal controls with an extremely low
allele frequency (<1%), while another previously reported rare
variation, c.-63G > C, was not detected in the Chinese
population examined in the present study. Of note, the four closely
adjacent variants did not appear to belong to an individual linkage
disequilibrium block, as there were no combined variants in the
whole population investigated.
No significant differences were detected in the
genotype frequency or allele frequency in all five variations
between patients and control (Table
II). There were no differences in the clinical variables and
hormone levels among the different genotypes in all the variants,
except that the FSH level was elevated in the GC genotype (P=0.002)
of c. −53G>C in PCOS patients (Tables
III–VII). Furthermore, the
potential joint effect between the four rare variants and Ser326Cys
was investigated to evaluate the risk of the combined variants in
PCOS. However, no significant association was found in each
individual allele (Table II) or
genotype combinations (data not shown).
 | Table II.Germline hOGG1 variations and
genotype/allele frequencies in the case-control study. |
Table II.
Germline hOGG1 variations and
genotype/allele frequencies in the case-control study.
|
|
| Total, n (%) |
|
|
|---|
|
|
|
|
|
|
|---|
| SNP sites |
Genotype/allele | PCOS (n=425) | Control
(n=440) |
P-valuea | OR (95%
CI)b |
|---|
| c.-53G>C | GC | 10 (2.4) | 5 (1.1) | 0.199 | 2.096
(0.711–6.185) |
|
| GG | 415 (97.6) | 435 (98.9) |
| 1 |
|
| C | 10 (1.2) | 5 (0.6) | 0.201 | 2.083
(0.709–6.121) |
|
| G | 840 (98.8) | 875 (99.4) |
| 1 |
| c.-45G>A | GA | 0 (0.0) | 2 (0.4) | 0.500 | – |
|
| GG | 425 (100.0) | 438 (99.6) |
| – |
|
| A | 0 (0.0) | 2 (0.2) | 0.500 | – |
|
| G | 850 (100.0) | 878 (99.8) |
| – |
| c.-23A>G | AG | 7 (1.6) | 14 (3.2) | 0.185 | 0.510
(0.204–1.275) |
|
| AA | 418 (98.4) | 426 (96.8) |
| 1 |
|
| G | 7 (0.8) | 14 (1.6) | 0.188 | 0.514
(0.206–1.279) |
|
| A | 843 (99.2) | 866 (98.4) |
| 1 |
| c.-18G>T | GT | 13 (3.0) | 20 (4.5) | 0.289 | 0.663
(0.325–1.350) |
|
| GG | 412 (97.0) | 420 (95.5) |
| 1 |
|
| T | 13 (1.5) | 20 (2.3) | 0.294 | 0.668
(0.330–1.351) |
|
| G | 837 (98.5) | 860 (97.7) |
| 1 |
| Ser326Cys | GG | 156 (36.7) | 141 (32.0) |
| 1.314
(0.878–1.966) |
| (C>G) | CG | 205 (48.2) | 223 (50.7) | 0.319 | 1.092
(0.744–1.601) |
|
| CC | 64 (15.1) | 76 (17.3) |
| 1 |
|
| G | 517 (60.8) | 505 (57.4) | 0.146 | 1.153
(0.952–1.397) |
|
| C | 333 (39.2) | 375 (42.6) |
| 1 |
 | Table III.Comparisons of variants at c.-53
G>C for women with or without PCOS in terms of anthropometric
characteristics and serum hormone concentrations. |
Table III.
Comparisons of variants at c.-53
G>C for women with or without PCOS in terms of anthropometric
characteristics and serum hormone concentrations.
|
| Control |
| PCOS |
|
|---|
|
|
|
|
|
|
|---|
| Genotypes | GG | GC | P-value | GG | GC | P-value |
|---|
| Age, years |
32.99±5.35 |
36.67±10.40 | 0.240 |
26.89±6.73 |
29.33±9.35 | 0.384 |
| BMI,
kg/m2 |
21.95±3.92 |
21.54±1.46 | 0.856 |
22.96±6.17 |
22.97±4.83 | 0.994 |
| FSH, IU/l |
8.33±8.33 |
11.75±7.98 | 0.480 |
5.86±2.77 |
10.36±11.51 | 0.002a |
| LH, IU/l |
5.63±4.88 |
4.97±1.79 | 0.815 |
11.72±9.04 |
14.08±4.66 | 0.562 |
| LH/FSH |
0.75±0.50 |
0.48±0.13 | 0.356 |
2.26±3.07 |
2.17±0.99 | 0.947 |
| T, nmol/l |
2.46±1.74 |
0.24±0 | 0.207 |
2.70±6.09 |
2.49±1.16 | 0.938 |
| E2, pg/ml |
65.68±58.51 |
26.83±27.25 | 0.252 |
79.18±69.64 |
84.35±55.60 | 0.869 |
 | Table VII.Comparisons of Ser326Cys (C>G) for
women with or without PCOS in terms of anthropometric
characteristics and serum hormone concentrations. |
Table VII.
Comparisons of Ser326Cys (C>G) for
women with or without PCOS in terms of anthropometric
characteristics and serum hormone concentrations.
|
| Control |
| PCOS |
|---|
|
|
|
|
|
|---|
| Genotypes | CC | CG | GG | P-value | CC | CG | GG | P-value |
|---|
| Age, years |
31.48±2.65 |
33.12±5.03 |
35.88±5.64 | 0.675 |
25.95±8.01 |
28.13±4.03 |
30.88±6.74 | 0.468 |
| BMI,
kg/m2 |
21.46±3.74 |
21.99±2.33 |
21.77±3.74 | 0.897 |
22.56±5.66 |
23.43±4.51 |
22.78±3.78 | 0.649 |
| FSH, IU/l |
7.79±7.49 |
8.68±2.94 |
8.29±4.47 | 0.469 |
5.78±4.23 |
6.13±4.46 |
5.89±2.23 | 0.657 |
| LH, IU/l |
5.67±4.74 |
5.74±4.04 |
4.96±3.74 | 0.658 |
11.59±6.99 |
12.84±9.00 |
8.66±6.74 | 0.371 |
| LH/FSH |
0.78±0.16 |
0.75±0.69 |
0.74±0.23 | 0.865 |
2.31±1.92 |
2.27±1.03 |
1.76±1.89 | 0.582 |
| T, nmol/l |
2.49±1.32 |
2.32±0.19 |
2.45±1.96 | 0.618 |
2.80±1.64 |
2.44±1.96 |
2.68±1.87 | 0.654 |
| E2, pg/ml |
59.90±41.59 |
65.60±48.22 |
66.81±48.73 | 0.413 |
67.16±48.37 |
77.80±58.11 |
81.49±64.77 | 0.200 |
Discussion
PCOS is a complex endocrine disease with no clear
etiology. Accumulating evidence suggests that genetic and
environmental factors contribute to its occurrence and development
(22). Familial clustering of PCOS or
PCOS-related metabolic symptoms indicates a genetic origin
(23,24), although no consensus of the inheritance
mode has been reached. To date, significant attention has been
focused on the genes involved in the androgen biosynthetic pathways
(CYP11, CYP17 and CYP19) and insulin-related
pathways (INS and INSR). However, other genes that
are crucial in maintaining normal metabolic functions, such as
insulin sensitivity preservation may show a link with this
metabolic disease. The hOGG1 gene is critical in BER.
Several polymorphisms in hOGG1 have been identified as
associated with insulin sensitivity and type 2 diabetes mellitus
(T2MD). The underlying role of ROS-induced oxidative stress in PCOS
together with the correlation between ROS and the BER system
provide us with a new insight into the possible causes of PCOS. To
the best of our knowledge, this is the first time that functional
polymorphisms in the hOGG1 gene in Chinese PCOS patients
have been investigated.
The structure and functions of hOGG1 have been well
studied and several of its polymorphisms have been identified.
Specifically, certain polymorphisms in hOGG1 that are
associated with insulin sensitivity or T2MD have been reported,
indicating the functional involvement of hOGG1 in the
maintenance of normal glucose metabolism (9,20,25). Previous studies have reported an
association between oxidative stress and insulin resistance not
only in the context of diabetes, but also in nondiabetic
individuals and in those with metabolic syndromes (26–28).
Consequently, increased generation of ROS in response to oxidative
stress in metabolic syndromes prompted other investigators to focus
on genomic instability and DNA damage that are associated with
hOGG1.
The present study investigated four rare SNPs in
5′-UTR and a common SNP (Ser326Cys) in exon 7 of the hOGG1
gene in a case-control study of PCOS. The 5′-UTR region is known to
modulate gene expression at the post-transcriptional level by
influencing mRNA stability and translational efficiency medicated
by transcription factors (TF) (29,30). The
inactivation or induction of the corresponding TF can modulate the
expression of the hOGG1 gene, thus influencing the activity
of the protein (31,32). Previous studies revealed that certain
types of functional variants in the 5′-UTR of the hOGG1 gene
are capable of increasing the risks of diseases, including cancer
(9,11,33,34). The present finding suggests that there
was no significant correlation between the variants in hOGG1
and the PCOS risk. Although the FSH level was elevated in the GC
genotype of c. −53G>C in PCOS patients, it is difficult to
provide any reasonable explanation for this phenomenon. The
polymorphism Ser326Cys in the hOGG1 gene is shown to be
associated with OGG1 activity in in vitro and in vivo
studies, and it is suggested that the 326Cys allele may pose a
higher risk of 8-oxoG formation in DNA (35,36). The
associations between the hOGG1 Ser326Cys polymorphism and
various diseases have been extensively investigated. However,
conflicted results, even in the same disease, have been reported
(37,38). The present study does not support an
association of this polymorphism with PCOS susceptibility. Given
the low allele frequency of the variants in 5′-UTR of hOGG1,
further studies with a larger sample size are required to confirm
these findings.
In conclusion, hOGG1 is not an independent
risk factor for PCOS development, and no putative linkage to the
syndrome could be established.
Acknowledgements
The authors are grateful to all the members who
participated in the present study. This study was supported by
grants from the National Natural Science Foundation of China (no.
81170541) and the Natural Basic Research Program of China (no. 973
program 2010CB 945103).
References
|
1
|
Ghosh J and Myers CE: Inhibition of
arachidonate 5-lipoxygenase triggers massive apoptosis in human
prostate cancer cells. Proc Natl Acad Sci USA. 95:13182–13187.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yin GY, Yin YF and He XF: Effect of
zhuchun pill on immunity and endocrine function of elderly with
kidney-yang deficiency. Zhongguo Zhong Xi Yi Jie He Za Zhi.
15:601–603. 1995.(In Chinese). PubMed/NCBI
|
|
3
|
Stadtman ER and Berlett BS: Reactive
oxygen-mediated protein oxidation in aging and disease. Drug Metab
Rev. 30:225–243. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chopra S and Wallace HM: Induction of
spermidine/spermine N1-acetyltransferase in human cancer cells in
response to increased production of reactive oxygen species.
Biochem Pharmacol. 55:1119–1123. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wells PG, Kim PM, Laposa RR, Nicol CJ,
Parman T and Winn LM: Oxidative damage in chemical teratogenesis.
Mutat Res. 396:65–78. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shibutani S, Takeshita M and Grollman AP:
Insertion of specific bases during DNA synthesis past the
oxidation-damaged base 8-oxodG. Nature. 349:431–434. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krokan HE, Nilsen H, Skorpen F, Otterlei M
and Slupphaug G: Base excision repair of DNA in mammalian cells.
FEBS Lett. 476:73–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
David SS, O'Shea VL and Kundu S:
Base-excision repair of oxidative DNA damage. Nature. 447:941–950.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun C, Liu X, Zhang H, Guo W, Cai Z, Chen
H, Zhang K, Zhu D and Wang Y: Functional polymorphism of
hOGG1 gene is associated with type 2 diabetes mellitus in
Chinese population. Mol Cell Endocrinol. 325:128–134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kohno T, Shinmura K, Tosaka M, Tani M, Kim
SR, Sugimura H, Nohmi T, Kasai H and Yokota J: Genetic
polymorphisms and alternative splicing of the hOGG1 gene,
that is involved in the repair of 8-hydroxyguanine in damaged DNA.
Oncogene. 16:3219–3225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Wang J, Guo W, Liu X, Sun C, Cai
Z, Fan Y and Wang Y: Two functional variations in 5′-UTR of
hoGG1 gene associated with the risk of breast cancer in
Chinese. Breast Cancer Res Treat. 127:795–803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Marchand L, Donlon T, Lum-Jones A,
Seifried A and Wilkens LR: Association of the hOGG1 Ser326Cys
polymorphism with lung cancer risk. Cancer Epidemiol Biomarkers
Prev. 11:409–412. 2002.PubMed/NCBI
|
|
13
|
Coppedè F, Migheli F, Ceravolo R, Bregant
E, Rocchi A, Petrozzi L, Unti E, Lonigro R, Siciliano G and
Migliore L: The hOGG1 Ser326Cys polymorphism and Huntington's
disease. Toxicology. 278:199–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ehrmann DA: Polycystic ovary syndrome. N
Engl J Med. 352:1223–1236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
consensus workshop group: Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome (PCOS). Hum Reprod. 19:41–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelly CC, Lyall H, Petrie JR, Gould GW,
Connell JM and Sattar N: Low grade chronic inflammation in women
with polycystic ovarian syndrome. J Clin Endocrinol Metab.
86:2453–2455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benson S, Janssen OE, Hahn S, Tan S, Dietz
T, Mann K, Pleger K, Schedlowski M, Arck PC and Elsenbruch S:
Obesity, depression, and chronic low-grade inflammation in women
with polycystic ovary syndrome. Brain Behav Immun. 22:177–184.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gonzalez F, Thusu K, Abdel-Rahman E,
Prabhala A, Tomani M and Dandona P: Elevated serum levels of tumor
necrosis factor alpha in normal-weight women with polycystic ovary
syndrome. Metabolism. 48:437–441. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
González F, Minium J, Rote NS and Kirwan
JP: Hyperglycemia alters tumor necrosis factor-alpha release from
mononuclear cells in women with polycystic ovary syndrome. J Clin
Endocrinol Metab. 90:5336–5342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CL, Hsieh MC, Hsin SC, Lin HY, Lin
KD, Lo CS, Chen ZH and Shin SJ: The hOGG1 Ser326Cys gene
polymorphism is associated with decreased insulin sensitivity in
subjects with normal glucose tolerance. J Hum Genet. 51:124–128.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia Y, Che Y, Zhang X, Zhang C, Cao Y,
Wang W, Xu P, Wu X, Yi L, Gao Q, et al: Polymorphic CAG repeat in
the androgen receptor gene in polycystic ovary syndrome patients.
Mol Med Rep. 5:1330–1334. 2012.PubMed/NCBI
|
|
22
|
Goodarzi MO, Dumesic DA, Chazenbalk G and
Azziz R: Polycystic ovary syndrome: Etiology, pathogenesis and
diagnosis. Nat Rev Endocrinol. 7:219–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lunde O, Magnus P, Sandvik L and Høglo S:
Familial clustering in the polycystic ovarian syndrome. Gynecol
Obstet Invest. 28:23–30. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hague WM, Adams J, Reeders ST, Peto TE and
Jacobs HS: Familial polycystic ovaries: A genetic disease? Clin
Endocrinol (Oxf). 29:593–605. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Daimon M, Oizumi T, Toriyama S, Karasawa
S, Jimbu Y, Wada K, Kameda W, Susa S, Muramatsu M, Kubota I, et al:
Association of the Ser326Cys polymorphism in the OGG1 gene with
type 2 DM. Biochem Biophys Res Commun. 386:26–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee KU: Oxidative stress markers in Korean
subjects with insulin resistance syndrome. Diabetes Res Clin Pract.
54(Suppl 2): S29–S33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sentí M, Tomás M, Fitó M, Weinbrenner T,
Covas MI, Sala J, Masiá R and Marrugat J: Antioxidant paraoxonase 1
activity in the metabolic syndrome. J Clin Endocrinol Metab.
88:5422–5426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
González F, Rote NS, Minium J and Kirwan
JP: Reactive oxygen species-induced oxidative stress in the
development of insulin resistance and hyperandrogenism in
polycystic ovary syndrome. J Clin Endocrinol Metab. 91:336–340.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van der Velden AW and Thomas AA: The role
of the 5′ untranslated region of an mRNA in translation regulation
during development. Int J Biochem Cell Biol. 31:87–106. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bashirullah A, Cooperstock RL and Lipshitz
HD: Spatial and temporal control of RNA stability. Proc Natl Acad
Sci USA. 98:7025–7028. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee MR, Kim SH, Cho HJ, Lee KY, Moon AR,
Jeong HG, Lee JS, Hyun JW, Chung MH and You HJ: Transcription
factors NF-YA regulate the induction of human OGG1 following
DNA-alkylating agent methylmethane sulfonate (MMS) treatment. J
Biol Chem. 279:9857–9866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Youn CK, Kim SH, Lee DY, Song SH, Chang
IY, Hyun JW, Chung MH and You HJ: Cadmium down-regulates human OGG1
through suppression of Sp1 activity. J Biol Chem. 280:25185–25195.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Xiao N, Guo W, Wu Y, Cai Z, He Q,
Zhang L, Chen X, Sun C, Wang J, et al: The hOGG1 gene 5′-UTR
variant c.-53G>C contributes to the risk of gastric cancer but
not colorectal cancer in the Chinese population: The functional
variation of hOGG1 for gastric cancer risk. J Cancer Res Clin
Oncol. 137:1477–1485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JC, Ka IH, Lee YM, Koo KH, Kim HC, Yu
CS, Jang SJ, Kim YS, Lee HI and Lee KH: MYH, OGG1, MTH1, and APC
alterations involved in the colorectal tumorigenesis of Korean
patients with multiple adenomas. Virchows Arch. 450:311–319. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamane A, Kohno T, Ito K, Sunaga N, Aoki
K, Yoshimura K, Murakami H, Nojima Y and Yokota J: Differential
ability of polymorphic OGG1 proteins to suppress mutagenesis
induced by 8-hydroxyguanine in human cell in vivo. Carcinogenesis.
25:1689–1694. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hill JW and Evans MK: Dimerization and
opposite base-dependent catalytic impairment of polymorphic S326C
OGG1 glycosylase. Nucleic Acids Res. 34:1620–1632. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie H, Xia K, Rong H and Chen X: Genetic
polymorphism in hOGG1 is associated with triple-negative breast
cancer risk in Chinese Han women. Breast. 22:707–712. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding DP, Zhang Y and He XF: Lack of
association between hOGG1 Ser326Cys polymorphism and breast cancer
susceptibility in European population. Breast Cancer Res Treat.
129:1023–1026. 2011. View Article : Google Scholar : PubMed/NCBI
|