Introduction
Glioma is one of the most common tumors of the
central nervous system, and its high invasiveness leads to a high
fatality rate (1). Chemotherapy is one
of the most common clinical treatments; however, as a result of the
existence of the blood tumor barrier (BTB), the effects of
chemotherapy are limited (2);
therefore, how to selectively open BTB without damage to the normal
blood brain barrier is an urgent problem in the brain tumor
treatment. Recent studies show that interleukin-1β (IL-1β) could
selectively increase the BTB permeability (3), but the exact mechanism remains to be
elucidated.
Antitumor drugs cross the BTB into the brain tumors
by two pathways: The paracellular and transcellular pathways
(4). Due to different physical and
chemical properties of drugs, the vast majority of antineoplastic
drugs cross the brain tumor tissue by the transcellular pathway
(5). IL-1β has been shown to induce
transcellular transport (6),
suggesting that IL-1β induced an increase in BTB permeability that
may be caused by vesicular transport rather than via the opening of
endothelial tight junctions.
Caveolae participate in cell transport, metabolism
and signal transduction. In the biochemical process, the caveolins
protein family has a key role (7).
Caveolin-1 is the main structural protein of
caveolae, which has an extremely important role in the activation
and positioning of cell signaling molecules for vesicle rupture,
endocytosis, fusion and exocytosis (8). Studies have shown that the protein
expression level of caveolin-1 associated with BBB permeability
regulation (9). Recently, a study has
shown that vascular endothelial growth factor (VEGF) can be
combined with caveolae to form a ‘small hole’ in the endothelial
cell membrane, thereby promoting the transmembrane transport of
biomolecules. IL-1β could induce the production of VEGF in stellate
cells (10). Based on the
aforementioned, we hypothesize that IL-1β could enhance
transcellular transport of brain tumor microvascular through
regulating the expression of caveolin-1, and this process can be
mediated by VEGF.
To test the hypothesis, a model of rat C6 glioma was
established, and investigated the effects of IL-1β to enhance BTB
permeability by Evans blue (EB). In addition, whether IL-1β had an
effect on caveolin-1 protein expression in brain tumor tissues and
whether VEGF regulates this process by western blots and
immunohistochemistry methods was investigated.
Materials and methods
Preparation of the rat C6 glioma
model
The clean level male Wistar rats (200–220 g) were
purchased from the Laboratory Animal Center of North China
University of Science and Technology (Hebei, China). All the animal
experiments were conducted in accordance with the National
Institute of Health Guide for the Care and Use of Laboratory
Animals, in addition to the policies of the North China University
of Science and Technology and Chinese authority. First, 10% chloral
hydrate (3.5 ml/kg, intraperitoneal injection) anesthesia was used
in the rats, and subsequently 1×106 C6 cells were injected into the
intracranial using a Hamilton syringe. Using a stereotaxic
instrument, the coordinates were a location on the right side of
the brain that targets the caudate nucleus, the coordinates of
anterior fontanelle before 1 mm and sagittal suture immediately
next to 3 mm. Intracranial tumor formation was ~2 weeks after
transplantation into the intracranial C6 cells.
Treatment of tumor-burdened rats
Tumor-burdened rats were randomized into two groups:
Control and IL-1β. For the control group, the rats were treated
with saline solution. For the IL-1β group, IL-1β (3.7 ng/kg/min)
was infused into tumor-burdened rat brain via the common carotid
artery for 30, 60 and 120 min, respectively.
Measurement of BTB permeability by EB
seepage quantity
First, rats were injected 2% EB (2 ml/kg) via the
tail vein for 2 h, rats in the control and IL-1β groups were
anesthetized with chloral hydrate and the brain with a tumor was
weighed. Subsequently, brain tumor tissue was immersed in formamide
solution (1 ml/100 mg) at 60°C for 24 h.
The optical density value was determined by
spectrophotometry (at 620 nm) to assess EB of the supernatant.
Western blot analysis of caveolin-1
and VEGF
The influence of IL-1β on caveolin-1 and VEGF
protein expression levels were analyzed by western blot analysis.
The protein homogenates of the brain tissue were prepared by
homogenization in 10 volumes of pyrolysis buffer, and
centrifugation at 17,000 × g for 1 h. The soluble protein content
was determined by the Coomassie G250 binding method. The protein
lysate was placed on a 12% SDS-polyacrylamide gel fraction (each
sample was 12 µg/lane), and subsequently transferred to a
nitrocellulose membrane (Merck Millipore, Darmstadt, Germany). The
membranes were blocked in blocking buffer overnight at 4°C. The
samples were incubated with rabbit polyclonal antibodies
anti-caveolin-1 (cat. no. HYK-1453R; 1:400; Abcam, Cambridge, UK)
and anti-VEGF (cat. no. sc-152; 1:400; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) were incubated for 2 h. Caveolin-1 and
VEGF bands were visualized using the enhanced chemiluminescene (ECL
kit; Santa Cruz Biotechnology, Inc.).
Immunohistochemistry analysis of
caveolin-1
The glioma tissues in the control and IL-1β groups
at 15 min after infusion were fixed with 4% paraformaldehyde to
carry out the immunohistochemical investigation. The sections were
immunohistochemically stained with the donkey polyclonal antibody
anti-caveolin-1 (diluted 1:100; Santa Cruz Biotechnology, Inc.)
following standard procedures.
Statistical analysis
All the statistical analyses were performed with
computer software (SigmaStat; SPSS, Inc., Chicago, IL, USA). All
the data are expressed as mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Assessment of BTB permeability by
EB
Brain glioma tissue was stained in blue, while
normal tissue did not stain. EB content in the glioma tissue was
significantly increased in the IL-1β group at 60-min infusion
compared with the control group (40.4±1.9 and 17.2±0.8 µg/g,
respectively; P<0.05), as shown in Fig.
1.
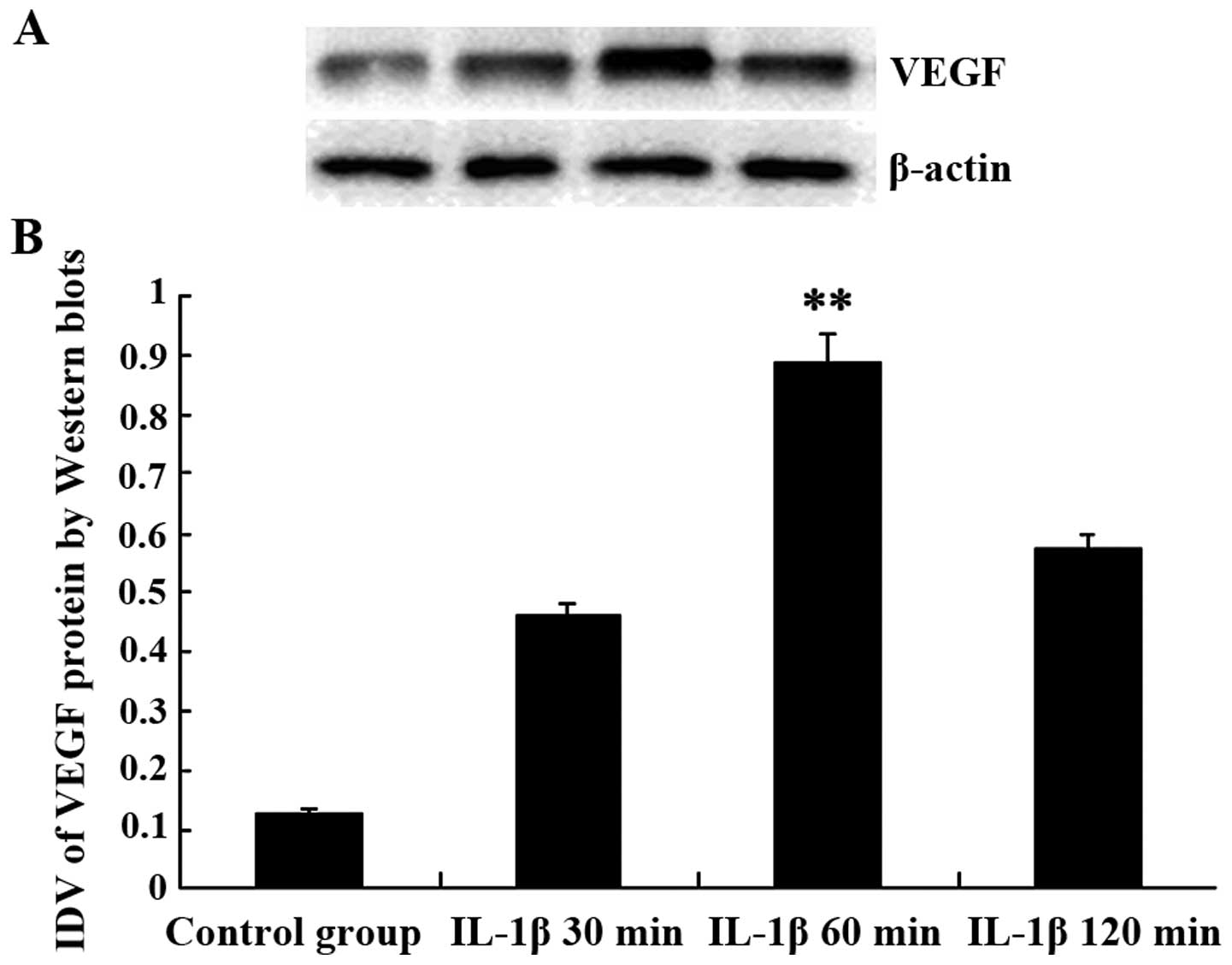
IL-1β increases the expression of VEGF
in tumor capillaries
VEGF expression in the rat brain glioma tissues in
the control group was lower compared to the IL-1β group. Compared
with the control group, the expression of VEGF protein was
increased significantly at IL-1β group. The integrated density
value (IDV) of VEGF at control, 30, 60 and 120 min groups were
0.127±0.011, 0.462±0.015, 0.895±0.013 and 0.574±0.062,
respectively, as shown in Fig. 2.
IL-1β increases the expression of
caveolin-1 in the rat brain glioma model
Compared with the control group, the expression of
caveolin-1 protein was increased significantly in the IL-1β
infusion group. The IDV of caveolin-1 in the control, 30, 60 and
120 min groups were 0.369±0.019, 1.158±0.041, 1.365±0.078 and
1.172±0.084, respectively, as shown in Fig. 3.
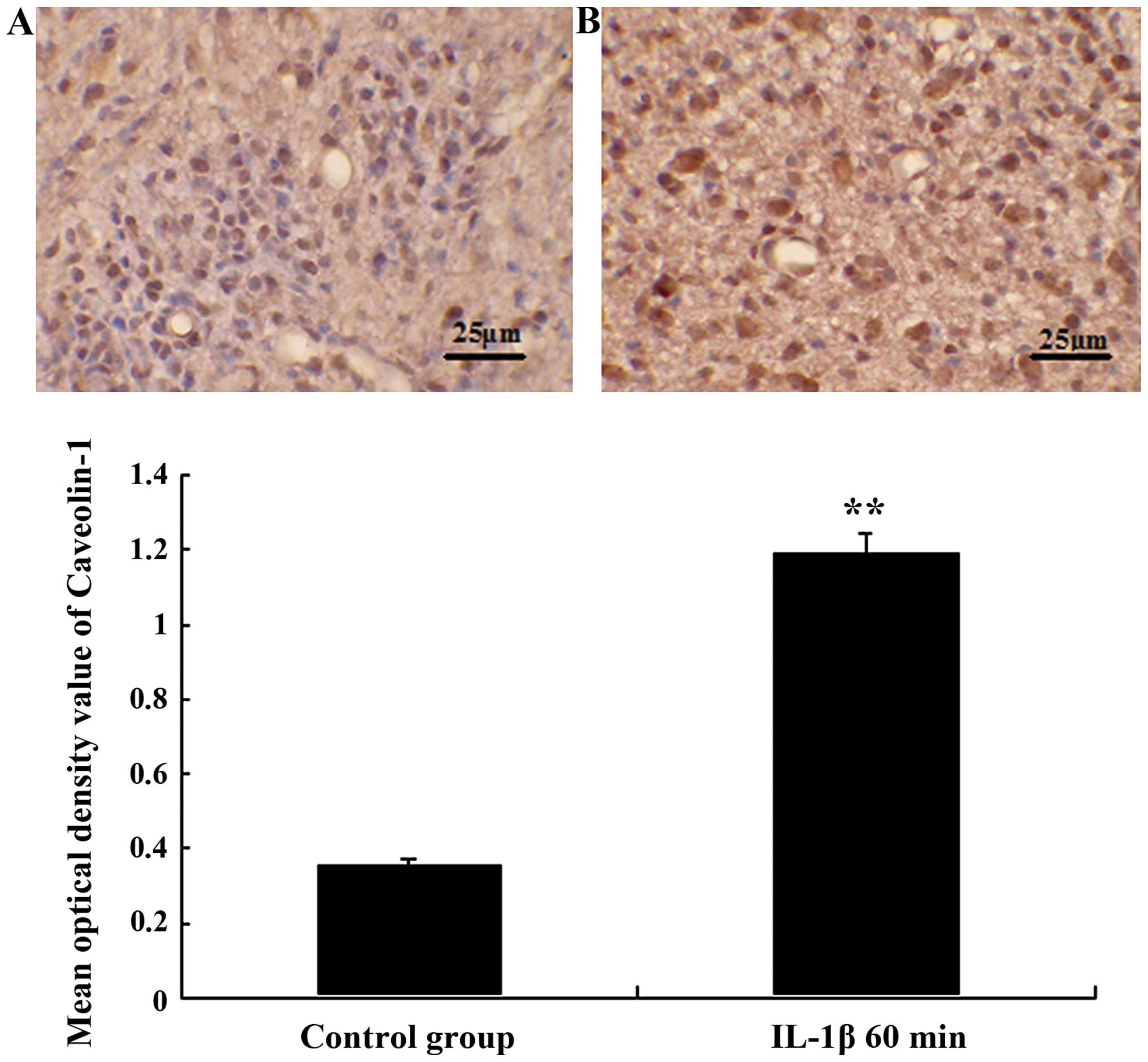
Expression of caveolin-1 in tumor
capillaries and cells following the IL-1β infusion
Caveolin-1-like immunoreactivity showed that
caveolin-1 protein expression was further enhanced in tumor
capillaries and tumor cells following the IL-1β infusion, and
reaches its maximum after IL-1β infusion for 60 min. The mean
optical density values of caveolin-1 were 0.192±0.021 and
0.359±0.018, respectively, as shown in Fig. 4.
Discussion
Malignant glioma is the most common type of brain
tumor. The blood brain barrier (BBB) is the main factor limiting
its drug treatment (1,11). Selective destruction of BBB, using
antitumor drugs through the BBB, is a promising therapy for
invasive glioma.
BBB consists of capillary endothelial cells, the
basement membrane, pericytes and astrocytic foot processes. There
are close continuous tight junctions between the brain capillary
endothelial cells. Our previous studies demonstrated that IL-1β, a
cell factor, could increase BTB permeability (12). However, the exact mechanism of the
increase of IL-1β-induced BTB permeability remains to be
elucidated.
The study by Allan and Rothwell (13) demonstrated that the main functional
role of IL-1β is in the vascular endothelial cells. VEGF has an
extremely important role in the proliferation, migration and
formation of blood vessels (14–18). Our
experimental results show that IL-1β could induce the expression of
VEGF in tumor capillaries; the peak appears at 60 min after
infusion and subsequently decreased. This trend is consistent with
the temporal changes in the permeability of BTB. Those results
suggest that VEGF may mediate the process of BTB permeability by
IL-1β; however, the associated mechanism requires further
investigation.
Caveolin-1 is the symbolic protein of the caveolae,
and has an important role in maintaining the shape, structure and
function of caveolae, particularly the endocytosis of endothelial
cells (19). Endocytosis of
endothelial cells did not occur following knockout caveolin-1
(20). Furthermore, caveolin-1 is
associated with the transport of BBB, and its protein expression is
associated with the increase in BBB permeability (9,21). To
clarify the mechanism of the IL-1β-induced BTB permeability
increase, in the present study, IL-1β was shown to increase the
expression of caveolin-1 protein, and the maximum expression level
appeared at 60 min after IL-1β infusion. The permeability of BTB
increased following IL-1β infusion and its peak also appeared at 60
min, which corresponds with the increased expression of caveolin-1
and VEGF. Those results suggest that VEGF is a key signaling
molecules in IL-1β increased BTB permeability.
In conclusion, the mechanism of the IL-1β increase
in BTB permeability is extremely complex. The present results show
that the VEGF/caveolin-1 signaling pathways may be one of its
mechanisms. These results suggest that the mechanism of the IL-1β
increase of BTB permeability may be through the VEGF increase in
the endocytosis of cerebral microvascular endothelial cells.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. 81101912 and 81201048), the
Hebei Province Science and Technology Support Program (grant no.
152777189), the Hebei Province Administration of Traditional
Chinese Medicine (grant no. 2014195) and the Hebei Province
Department of Health and Family Planning Commission (grant no.
20150491).
References
|
1
|
Drappatz J, Schiff D, Kesari S, Norden AD
and Wen PY: Medical management of brain tumor patients. Neurol
Clin. 25:1035–1071, ix. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laquintana V, Trapani A, Denora N, Wang F,
Gallo JM and Trapani G: New strategies to deliver anticancer drugs
to brain tumors. Expert Opin Drug Deliv. 6:1017–1032. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sadowska GB, Chen X, Zhang J, Lim YP,
Cummings EE, Makeyev O, Besio WG, Gaitanis J, Padbury JF, Banks WA
and Stonestreet BS: Interleukin-1β transfer across the blood-brain
barrier in the ovine fetus. J Cereb Blood Flow Metab. 35:1388–1395.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Komarova Y and Malik AB: Regulation of
endothelial permeability via paracellular and transcellular
transport pathways. Annu Rev Physiol. 72:463–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salama NN, Eddington ND and Fasano A:
Tight junction modulation and its relationship to drug delivery.
Adv Drug Deliv Rev. 58:15–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dohgu S, Fleegal-DeMotta MA and Banks WA:
Lipopolysaccharide-enhanced transcellular transport of HIV-1 across
the blood-brain barrier is mediated by luminal microvessel IL-6 and
GM-CSF. J Neuroinflammation. 8:1672011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quest AF, Gutierrez-Pajares JL and Torres
VA: Caveolin-1: An ambiguous partner in cell signalling and cancer.
J Cell Mol Med. 12:1130–1150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun SW, Zu XY, Tuo QH, Chen LX, Lei XY, Li
K, Tang CK and Liao DF: Caveolae and caveolin-1 mediate endocytosis
and transcytosis of oxidized low density lipoprotein in endothelial
cells. Acta Pharmacol Sin. 31:1336–1342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nag S, Venugopalan R and Stewart DJ:
Increased caveolin-1 expression precedes decreased expression of
occludin and claudin-5 during blood-brain barrier breakdown. Acta
Neuropathol. 114:459–469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ben Menachem, Zidon O, Ben Menahem Y, Ben
Hur T and Yirmiya R: Intra- hippocampal transplantation of neural
precursor cells with transgenic over- expression of IL-1 receptor
antagonist rescues memory and neurogenesis impairments in an
Alzheimer's disease model. Neuropsychopharmacology.
13:27362013.
|
|
11
|
Hayashi Y, Yoshida Y and Hamada J:
Blood-tumor barrier in malignant brain tumor. No Shinkei Geka.
34:983–999. 2006.(In Japanese). PubMed/NCBI
|
|
12
|
Qin LJ, Xue YX, Gu YT, Zhang ZY, Zhang T
and Sun N: Effect and mechanisms of interleukin-1β in process of
opening the blood-brain barrier by bradykinin. Chinese
Pharmacological Bulletin. 1:58–61. 2012.
|
|
13
|
Allan SM and Rothwell NJ: Cytokines and
acute neurodegeneration. Nat Rev Neurosci. 2:734–744. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davies DC: Blood-brain barrier breakdown
in septic encephalopathy and brain tumours. J Anat. 200:639–646.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faehling M, Kroll J, Föhr KJ, Fellbrich G,
Mayr U, Trischler G and Waltenberger J: Essential role of calcium
in vascular endothelial growth factor A-induced signaling:
Mechanism of the antiangiogenic effect of carboxyamidotriazole.
FASEB J. 16:1805–1807. 2002.PubMed/NCBI
|
|
16
|
Argaw AT, Asp L, Zhang J, Navrazhina K,
Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, et al:
Astrocyte-derived VEGF-A drives blood-brain barrier disruption in
CNS inflammatory disease. J Clin Invest. 122:2454–2468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei M, Li H, Huang H, Xu D, Zhi D, Liu D
and Zhang Y: Increased expression of EMMPRIN and VEGF in the rat
brain after gamma irradiation. J Korean Med Sci. 27:291–299. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim J and Jung Y: Different expressions of
AQP1, AQP4, eNOS, and VEGF proteins in ischemic versus non-ischemic
cerebropathy in rats: Potential roles of AQP1 and eNOS in
hydrocephalic and vasogenic edema formation. Anat Cell Biol.
44:295–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lajoie P and Nabi IR: Regulation of
raft-dependent endocytosis. J Cell Mol Med. 11:644–653. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Drab M, Verkade P, Elger M, Kasper M, Lohn
M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, et al:
Loss of caveolae, vascular dysfunction, and pulmonary defects in
caveolin-1 gene-disrupted mice. Science. 293:2449–2452. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhong Y, Smart EJ, Weksler B, Couraud PO,
Hennig B and Toborek M: Caveolin-1 regulates human immunodeficiency
virus-1 Tat-induced alterations of tight junction protein
expression via modulation of the Ras signaling. J Neurosci.
28:7788–7796. 2008. View Article : Google Scholar : PubMed/NCBI
|