Introduction
The incidence of infants with cholestasis is between
1 in 2,500 and 1 in 5,000 newborns worldwide and has become the
leading cause of hospitalization of children with liver diseases. A
variety of factors, such as infection, poisoning, autoimmune
issues, genetic defects, metabolic abnormalities and other
uncertainties, can trigger dysfunction of hepatocytes, reduction of
bile flow, or bile duct obstruction, which may result in
cholestasis (1–3). Delayed treatment may affect the
development of children and result in malnutrition. Part of the
liver may turn fibrotic, resulting in cholestatic cirrhosis
(4). For a long time, the degree of
liver fibrosis could only be determined through liver biopsy.
Non-invasive examination and assessment of liver fibrosis have been
a focus of research in recent years. To date, non-invasive
assessments of cholestasis in infants mainly involve four marker
liver fibrosis assays and liver function assays. Correlation
analyses between the liver fibrosis assays and the liver function
assays have been rarely reported. The present study aimed to
provide a simple, safe and economical method for assessing liver
fibrosis in infants and promote an effective method for early
diagnosis and clinical monitoring of liver fibrosis in infants.
Subjects and methods
Research subjects
Thirty infants (experimental group) with cholestasis
were recruited from the outpatient clinic and admitted to the
Department of Pediatrics at The Third Hospital of Hebei Medical
University (Hebei, China) between May 2012 and December 2013.
Twenty healthy infants who underwent a routine examination at The
Third Hospital of Hebei Medical University were included in the
control group of this study. The grouping was not adjusted for
gender. All infants were ≤18 months of age. Inclusion criteria of
infants with cholestasis were: i) ≤18 months of age, with morbidity
within 6 months after birth; ii) diagnosis of infant cholestasis
based on the recommendation of the American Academy of Pediatrics;
i.e., jaundice in full-term infants with total bilirubin (TBIL)
<85 µmol/l and direct bilirubin (DBIL) >17 µmol/l; or TBIL
>85 µmol/l and DBIL levels >20% of TBIL (5). The study was approved by the medical
ethics committee of The Third Hospital of Hebei Medical University.
Parents of all the infants signed the written informed consent
prior to entering the study.
Methods
Four serum biomarkers for the determination of
liver fibrosis
Radioimmunoassays were used to detect four serum
biomarkers [hyaluronic acid (HA), procollagen type III (PCIII),
laminin (LN), and collagen type IV (cIV)] of liver fibrosis.
Infants were required to fast overnight and 2–3 ml of venous blood
was collected in the early morning. All the blood samples were
placed in pro-coagulant tubes and centrifuged at 1,006 × g for 3
min to isolate the serum for later assays. For blood samples that
could not be measured on the day of collection, serum was isolated
and stored at −20°C, and measurements were completed within a week.
The four biomarkers of liver fibrosis were tested using
radioimmunoassays from Beijing North Institute Biotechnology
(Beijing, China) and measured with a γ-radioimmunoassay counter
(XH-6020; Xi'an Nuclear Instrument Factory, Shaanxi, China).
Experimental procedures strictly followed the manufacturer's
protocols.
Measurement of liver function in the serum
Detection kits for liver function biomarkers
[alanine aminotransferase (ALT), aspartate aminotransferase (AST),
TBIL, DBIL, indirect bilirubin (IBIL), γ-glutamyl transferase
(γ-GT), cholinesterase (CHE) and total bile acids (TBA)] were
purchased from Johnson & Johnson Medical (Shanghai), Ltd.
(Shanghai, China). An automatic biochemical analyzer (AU5400TM;
Olympus Optical Corp., Ltd., Japan) was used for measurements.
Approximately 2–3 ml of venous blood from each patient were
collected in the early morning after fasting overnight and placed
in a pro-coagulant tube, followed by centrifuging at 1,006 × g for
3 min to isolate serum for further assays. Measurements of ALT,
AST, γ-GT, CHE and TBA were based on assays using the initial rate
method and measurements of TBIL, DBIL and IBIL were based on
colorimetric assays.
Statistical analysis
SPSS 19.0 software (SPSS, Inc., Armonk, NY, USA) was
used for data processing and statistical analysis. Statistical
results are presented as mean ± standard deviation. Normally
distributed numerical data were compared using an independent
sample t-test. Comparisons between non-normally distributed
numerical data were performed with a rank sum test. Differences in
each liver function assay and each of the four biomarkers for liver
fibrosis were measured by partial correlation analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Results of liver function tests in
infants with cholestasis
Serum levels of ALT, AST, TBIL, DBIL, IBIL, γ-GT and
TBA in the infants with cholestasis were significantly higher
compared to the healthy infants in the control group (P<0.01 for
each test); while serum levels of CHE in the infants with
cholestasis were significantly lower compared to the healthy
infants (P<0.01; Table I).
 | Table I.Changes of liver function indexes in
infants with cholestasis. |
Table I.
Changes of liver function indexes in
infants with cholestasis.
| Variables | Control group | Infants with
cholestasis | P-value |
|---|
| Total, n | 20 | 30 |
|
| ALT, U/l | 11.900±1.917 | 117.900±261.894 | <0.01 |
| AST, U/l | 29.450±3.602 | 115.567±208.786 | <0.01 |
| TBIL, µmol/l |
7.140±1.632 | 103.970±74.226 | <0.01 |
| DBIL, µmol/l |
2.165±0.555 | 55.057±41.516 | <0.01 |
| IBIL, µmol/l |
4.975±1.212 | 48.247±47.440 | <0.01 |
| γ-GT, U/l | 11.000±1.487 | 169.300±184.600 | <0.01 |
| CHE, KU/l | 9.601±1.171 | 6.639±1.936 | <0.01 |
| TBA, µmol/l | 3.195±2.587 | 49.447±58.214 | <0.01 |
Observation outcomes for the four
biomarkers of liver fibrosis in infants with cholestasis
Among the four biomarkers of liver fibrosis, serum
levels of HA, PCIII and cIV in the infants with cholestasis were
significantly higher compared to the healthy infants (P<0.01 for
each test; Table II).
 | Table II.Variation of the four indicators of
liver fibrosis in infants with cholestasis. |
Table II.
Variation of the four indicators of
liver fibrosis in infants with cholestasis.
| Variables | Control group | Infants with
cholestasis | P-value |
|---|
| Total, n | 20 | 30 |
|
| HA, ng/ml | 81.165±7.319 | 201.273±177.030 | <0.01 |
| PCIII, ng/ml | 141.314±26.609 | 541.877±170.920 | <0.01 |
| LN, ng/ml | 116.209±8.374 | 123.637±19.322 | >0.05 |
| cIV, ng/ml | 78.430±10.205 | 134.371±44.984 | <0.01 |
Correlation analyses between liver
function tests and the four biomarkers of liver fibrosis in infants
with cholestasis
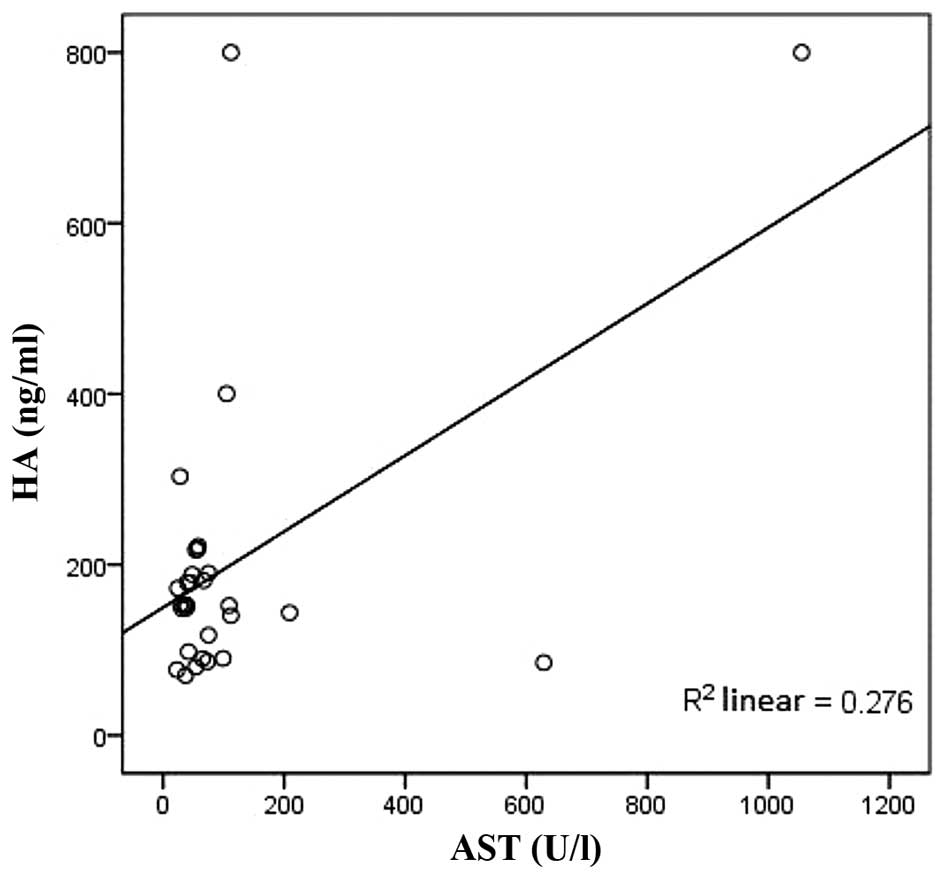
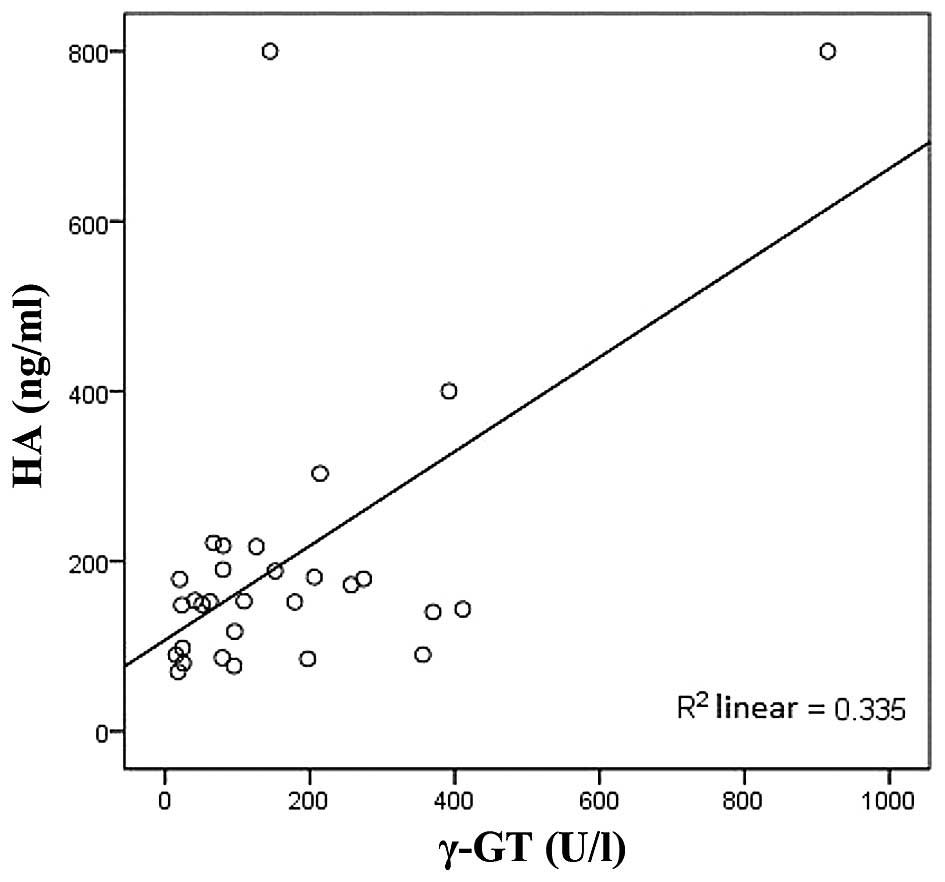
HA was positively correlated with AST and γ-GT
(r=0.736, P<0.01; r=0.599, P<0.01, respectively; Figs. 1 and 2)
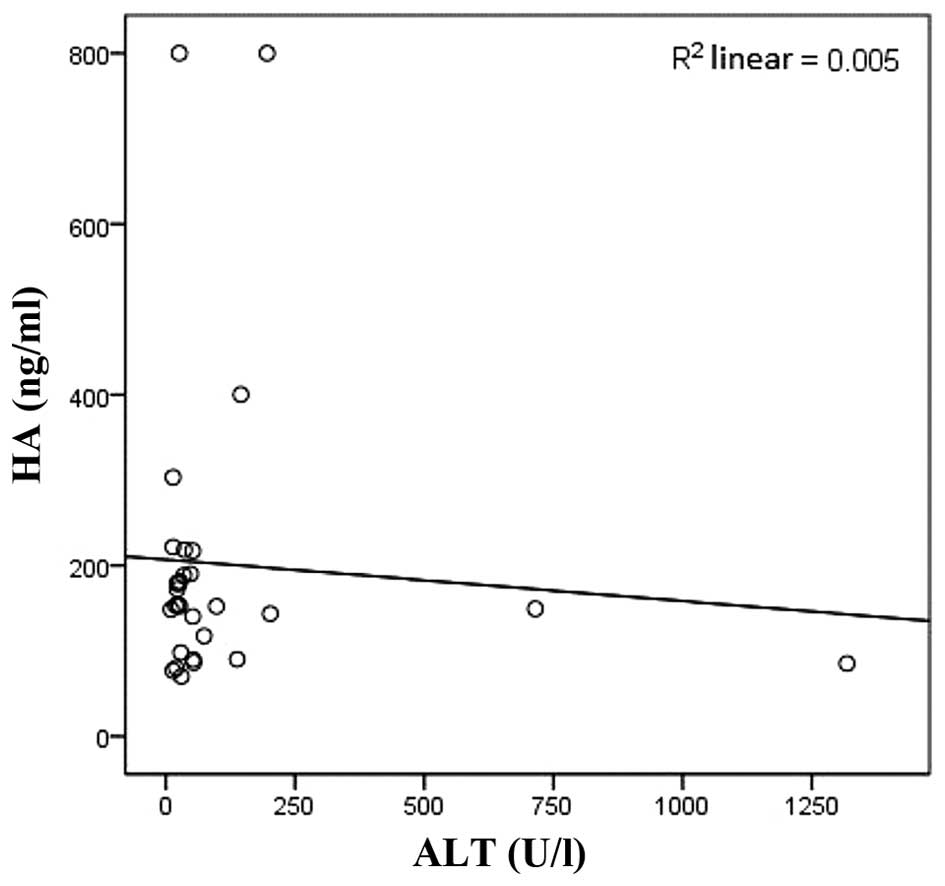
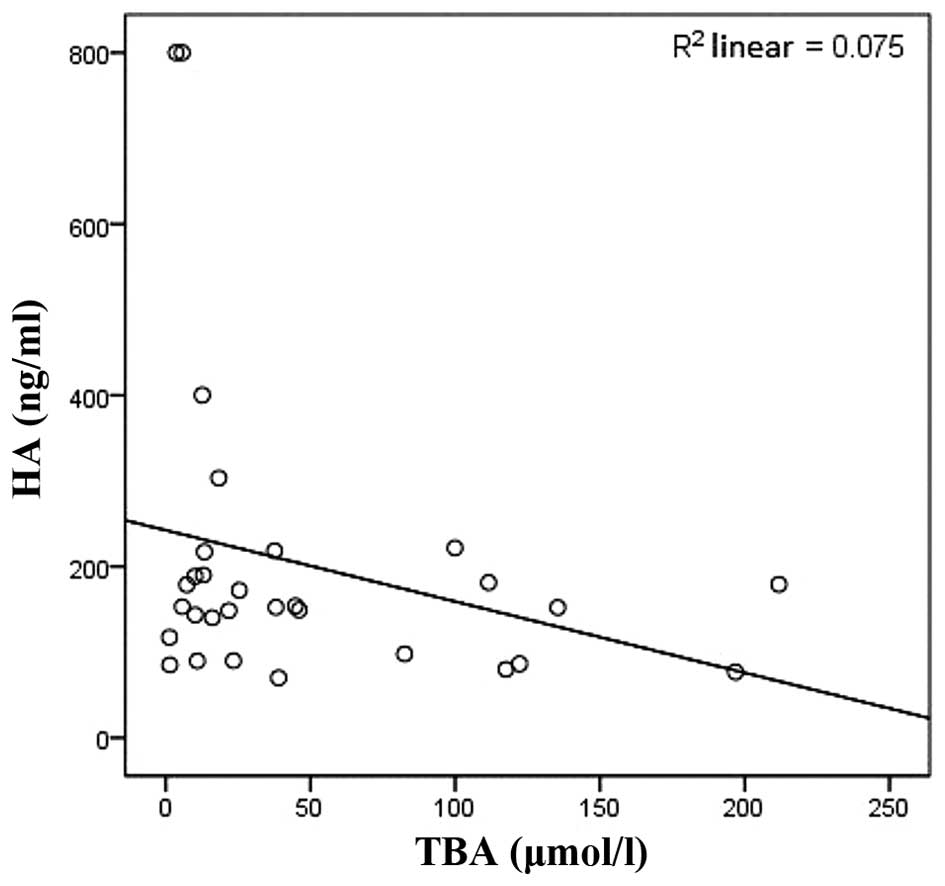
and negatively correlated with ALT, CHE and TBA (r=−0.627,
P<0.01; r=−0.427, P<0.05; r=−0.463, P<0.05, respectively;
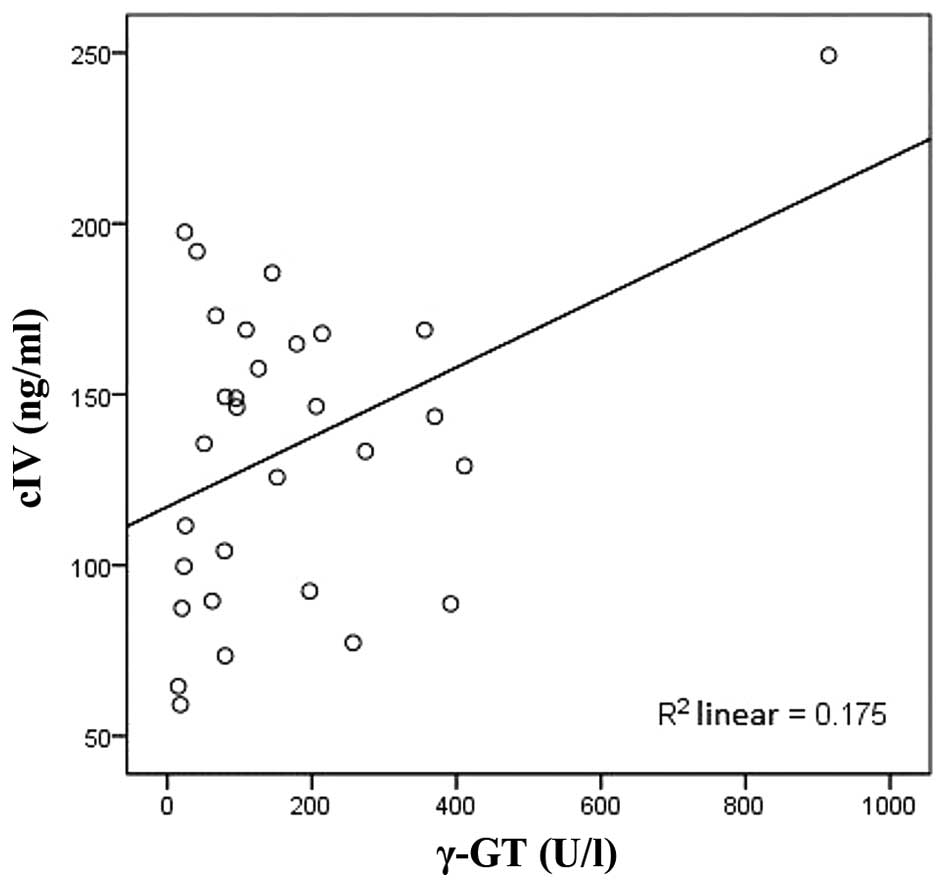
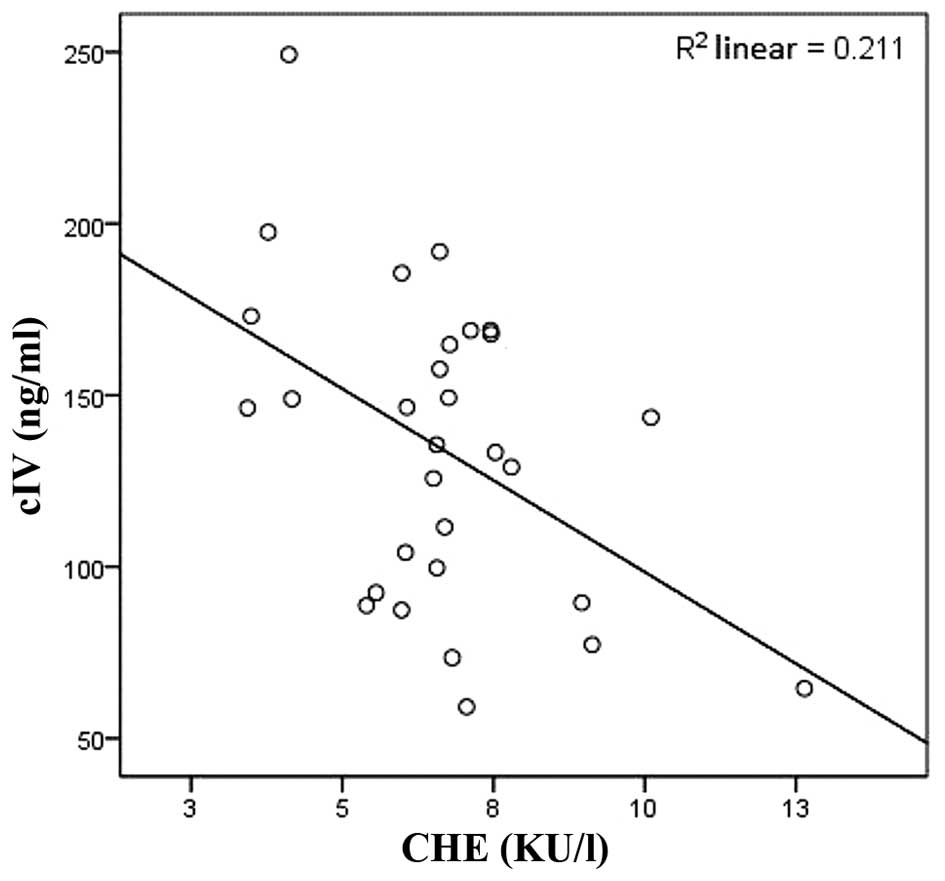
Figs. 3–5). cIV was positively correlated with γ-GT
(r=0.466, P<0.05; Fig. 6) and
negatively correlated with CHE (r=−0.520, P<0.05; Table III; Fig.
7).
 | Table III.Correlation analyses between liver
function markers and the four biomarkers of liver fibrosis in
infants with cholestasis. |
Table III.
Correlation analyses between liver
function markers and the four biomarkers of liver fibrosis in
infants with cholestasis.
|
| HA, ng/ml | PCIII, ng/ml | LN, ng/ml | cIV, ng/ml |
|---|
|
|
|
|
|
|
|---|
| Marker | r | P-value | r | P-value | r | P-value | r | P-value |
|---|
| ALT, U/l | −0.627 | <0.01 |
0.086 | N.S. | −0.350 | N.S. | −0.051 | N.S. |
| AST, U/l |
0.736 | <0.01 | −0.220 | N.S. |
0.342 | N.S. |
0.020 | N.S. |
| TBIL, µmol/l |
0.016 | N.S. | −0.019 | N.S. | −0.154 | N.S. |
0.374 | N.S. |
| DBIL, µmol/l | −0.356 | N.S. |
0.031 | N.S. |
0.156 | N.S. | −0.356 | N.S. |
| IBIL, µmol/l |
0.028 | N.S. |
0.002 | N.S. |
0.168 | N.S. | −0.378 | N.S. |
| γ-GT, U/l |
0.599 | <0.01 | −0.124 | N.S. |
0.237 | N.S. |
0.466 | <0.05 |
| CHE, KU/l | −0.427 | <0.05 |
0.219 | N.S. | −0.189 | N.S. | −0.520 | <0.05 |
| TBA, µmol/l | −0.463 | <0.05 |
0.236 | N.S. | −0.192 | N.S. |
0.077 | N.S. |
Discussion
Infants with cholestasis should be diagnosed early
and receive effective treatment in a timely manner. Delayed
treatment of cholestasis in infants seriously affects their
development, resulting in liver fibrosis. Certain cases may further
develop cholestatic cirrhosis. Therefore, early diagnosis of liver
fibrosis in the infants with cholestasis becomes particularly
important.
Serum HA, PCIII, cIV and LN are four common
biomarkers of liver fibrosis. HA is one of the components of
proteoglycan in the extracellular matrix (ECM) of hepatocytes.
During liver tissue damage, the hepatic stellate cells (HSCs)
synthesize HA and the level of HA increases. In addition, HA
degradation in sinusoidal endothelial cells decreases, resulting in
elevated levels of serum HA (6). The
increased rate of serum HA in liver fibrosis was particularly
associated with the severity of liver lesions. Serum HA can
accurately and sensitively reflect the damages of hepatocytes and
the amount of fibers in the liver (7–9). PCIII are
type III collagen precursors and are significantly associated with
the quantitation of type III procollagen peptide (PCIIIP). These
indicators are valuable in the early diagnosis of liver fibrosis
and significant in the prognosis of chronic liver disease. Serum
levels of PCIII are consistent with the progression of liver
fibrosis and activity. However, serum levels of PCIII have no
specificity with liver fibrosis as they also increase in fibrosis
of other organs. cIV is an important structural component of the
reticular basement membrane. Following its synthesis in
hepatocytes, cIV directly uses the procollagen form to participate
in the composition of ECM. cIV content increases in the early stage
of liver fibrosis, and its conversion rate accelerates during the
progression of liver fibrosis. Eventually, cIV forms a complete
basement membrane with the continuous deposition of LN. Clinical
studies have shown that serum levels of cIV sensitively reflect
damage in hepatocytes and the severity of liver fibrosis. Serum cIV
is a biomarker for early liver fibrosis. LN is a structural
glycoprotein and is mainly distributed in the transparent layer of
the basement membrane (10). During
liver cirrhosis, HSC synthesized LN levels are significantly
increased, which is an important basis for the formation of portal
hypertension. Clinical studies confirmed that serum levels of LN
were positively correlated with the severity of liver cirrhosis.
Detection of serum LN had an important diagnostic value for portal
hypertension. In the present study, despite serum LN, serum levels
of HA, PCIII and cIV in the infants with cholestasis were
significantly higher compared to the healthy infants. However, the
study was a clinical trial, which could not exclude differences in
the course of disease, the living environment and individual
infants, which may have affected the experimental results. These
results demonstrated no significant differences in serum LN between
the infants with cholestasis and the healthy infants. One possible
explanation was that serum LN accumulation in an early stage of
liver cirrhosis was not as significant as in later stages of liver
cirrhosis. Thus, monitoring of serum LN at the early stage of liver
cirrhosis had no significant clinical impact.
Liver biochemical tests are also known as liver
function tests, which are important clinical tests to determine the
presence of liver damage, to evaluate the severity of liver
diseases, to track the progression of liver diseases, and to
determine the treatment efficacies and prognoses of liver disease.
Liver function tests in this study showed that levels of serum ALT,
AST, TBIL, DBIL, IBIL, γ-GT, CHE and TBA in the infants with
cholestasis were significantly higher compared to the healthy
infants, which may be associated with different degrees of damage
in the epithelial cells of bile ducts and hepatocytes following
cholestasis, resulting in damage to normal physiological liver
function and apoptosis.
In order to find simple assessments of liver
fibrosis in infants and promote early diagnosis and clinical
monitoring in infants with different degrees of liver fibrosis, the
present study conducted correlation analyses between four serum
biomarkers of liver fibrosis and liver function markers in infants
with cholestasis. The results showed that serum HA was positively
correlated with serum AST and negatively correlated with serum ALT.
ALT is mainly present in the cytoplasm and is most abundantly
located in liver cells. ALT is a sensitive marker for damage of
hepatocytes. ASTs are mainly present in the cytoplasm and
mitochondria, and are mainly distributed in cardiac muscles,
followed by liver tissues, skeletal muscles and kidneys.
Significant elevation of transaminase is usually observed in acute
damage of hepatocytes. In general, the sensitivity of ALT for
identifying liver damage is higher than AST. However, levels of ALT
and AST were not directly associated with the severity of liver
damage (11). The results of the study
were possibly associated with the relatively long course of the
disease in the infants with cholestasis, which had already passed
the acute phase and in which liver function may have been
completely damaged. In addition, these results may be due to
differences in individuals, different living environments and
experimental error. To further confirm the results, an increased
sample size and classification of the course of the disease are
necessary.
Serum γ-GT is produced in the mitochondria of
hepatocytes. Extrahepatic or intrahepatic obstruction during
cholestasis blocks the excretion of γ-GT, thereby increasing the
serum concentration of γ-GT (12). A
number of studies have confirmed that serum γ-GT is an important
biomarker to identify damage of hepatocytes and the degree of
extrahepatic obstruction of bile ducts (13,14). In the
present study, serum γ-GT was found to be positively and
significantly correlated with serum HA and serum cIV, suggesting
that these three biomarkers were sensitive to cholestatic liver
fibrosis. In addition, the results suggested that synthesis of γ-GT
may be closely associated with the synthesis of HA and cIV. CHE is
synthesized by liver tissue and secreted into blood. Its major
physiological function is to catalyze the hydrolysis of
acetylcholine. When the liver is damaged, synthesis of CHE and CHE
activities are reduced. Reduction of serum CHE levels is closely
associated with the pathological staging of liver fibrosis.
Therefore, serum CHE can be used as a sensitive marker to determine
the inflammatory changes and degree of damage in the liver
parenchyma cells in the patients with chronic hepatitis. The higher
the severity of liver fibrosis, the lower the serum levels of CHE
(15). In the present study,
cholestasis in the infants of the experimental group is a chronic
process that gradually progresses to an advanced stage.
Furthermore, serum CHE has been positively associated with serum
cIV and HA, to a certain extent reflecting close associations
between the synthesis of CHE in the liver, as well as serum
biomarkers (such as cIV and HA) of cholestatic liver fibrosis in
infants. Further studies in the signal transduction pathways for
the biosynthesis of CHE, cIV and HA will help determine if they
share common characteristics. TBA is a bile acid that is not taken
up by hepatocytes during intestinal reabsorption and is partially
released into the blood circulation. Bile obstruction due to
cholestasis, or secretion and/or ingestion impairment of
hepatocytes, increases the serum TBA. A previous study has
confirmed that serum bile acid is specific, sensitive and stable
during liver functional changes (16).
In the present study, serum TBA was negatively associated with
serum HA and was statistically significant. Despite experimental
errors that could not be eliminated, theoretically, the excessive
production and accumulation of TBA may induce inflammation, thereby
activating a series of intracellular signal transduction pathways.
To date, the impact of TBA accumulation on HA synthesis has been
rarely reported. Further studies are necessary in this regard.
Although some of the results in the present study require further
experimental confirmation, the study suggested that the serum
levels of HA, cIV, γ-GT and CHE are sensitive markers for
cholestatic liver fibrosis in infants.
In conclusion, statistically significant differences
were observed for liver function markers (ALT, AST, TBIL, DBIL,
IBIL, γ-GT and TBA) and biomarkers HA, PCIII and cIV of liver
fibrosis between infants with cholestasis and healthy infants.
Serum levels of HA, cIV, γ-GT and CHE are sensitive markers for
cholestatic liver fibrosis in infants.
Acknowledgements
The study was supported by the Key Medical Science
Research Program of Hebei Province (grant no. ZL20140195).
References
|
1
|
Elferink RO and Groen AK: Genetic defects
in hepatobiliary transport. Biochim Biophys Acta. 1586:129–145.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benchimol EI, Walsh CM and Ling SC: Early
diagnosis of neonatal cholestatic jaundice: Test at 2 weeks. Can
Fam Physician. 55:1184–1192. 2009.PubMed/NCBI
|
|
3
|
Dong C and Huang ZH: Diagnosis and
treatment of cholestatic liver disease in infants. Zhonghua
Linchuang Yishi Zazhi. 40:16–18. 2012.
|
|
4
|
Morgan NV, Hartley JL, Setchell KD,
Simpson MA, Brown R, Tee L, Kirkham S, Pasha S, Trembath RC, Maher
ER, et al: A combination of mutations in AKR1D1 and SKIV2L in a
family with severe infantile liver disease. Orphanet J Rare Dis.
8:742013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moyer V, Freese DK, Whitington PF, Olson
AD, Brewer F, Colletti RB and Heyman MB: North American Society for
Pediatric Gastroenterology, Hepatology and Nutrition: Guideline for
the evaluation of cholestatic jaundice in infants: Recommendations
of the North American Society for Pediatric Gastroenterology,
Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 39:115–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee HH, Seo YS, Um SH, Won NH, Yoo H, Jung
ES, Kwon YD, Park S, Keum B, Kim YS, et al: Usefulness of
non-invasive markers for predicting significant fibrosis in
patients with chronic liver disease. J Korean Med Sci. 25:67–74.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Shabrawi MH, El Zein Abedin MY, Omar N,
Kamal NM, Elmakarem SA, Khattab S, El-Sayed HM, El-Hennawy A and
Ali AS: Predictive accuracy of serum hyaluronic acid as a
non-invasive marker of fibrosis in a cohort of multi-transfused
Egyptian children with β-thalassaemia major. Arab J Gastroenterol.
13:45–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nath NC, Rahman MA, Khan MR, Hasan MS,
Bhuiyan TM, Hoque MN, Kabir MM, Raha AK and Jahan B: Serum
hyaluronic acid as a predictor of fibrosis in chronic hepatitis B
and C virus infection. Mymensingh Med J. 20:614–619.
2011.PubMed/NCBI
|
|
9
|
Nobili V, Alisi A, Torre G, De Vito R,
Pietrobattista A, Morino G, De Ville De Goyet J, Bedogni G and
Pinzani M: Hyaluronic acid predicts hepatic fibrosis in children
with nonalcoholic fatty liver disease. Transl Res. 156:229–234.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Timpl R, Rohde H, Robey PG, Rennard SI,
Foidart JM and Martin GR: Laminin - a glycoprotein from basement
membranes. J Biol Chem. 254:9933–9937. 1979.PubMed/NCBI
|
|
11
|
Green RM and Flamm S: AGA technical review
on the evaluation of liver chemistry tests. Gastroenterology.
123:1367–1384. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu D and Huang ZH: Differential
diagnostic value of 5′-NT and γ-GT in infantile hepatitis syndrome
(HIS) and biliary astresia. J Clin Exp Med. 8:16–17. 2009.
|
|
13
|
Tarantino G, Finelli C, Colao A, Capone D,
Tarantino M, Grimaldi E, Chianese D, Gioia S, Pasanisi F, Contaldo
F, et al: Are hepatic steatosis and carotid intima media thickness
associated in obese patients with normal or slightly elevated
gamma-glutamyl-transferase? J Transl Med. 10:502012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tavian D, Degiorgio D, Roncaglia N,
Vergani P, Cameroni I, Colombo R and Coviello DA: A new splicing
site mutation of the ABCB4 gene in intrahepatic cholestasis of
pregnancy with raised serum gamma-GT. Dig Liver Dis. 41:671–675.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang HL: Diagnostic value of serum ChE and
TGF-β combined detection in liver fibrosis. Pract Prev Med.
16:l237–l1238. 2009.
|
|
16
|
Matsui S, Yamane T, Takita T, Oishi Y and
Kobayashi-Hattori K: The hypocholesterolemic activity of Momordica
charantia fruit is mediated by the altered cholesterol- and bile
acid-regulating gene expression in rat liver. Nutr Res. 33:580–585.
2013. View Article : Google Scholar : PubMed/NCBI
|