Introduction
Skeletal disorders are degenerative diseases causing
progressive disability that are becoming more prevalent in society.
Accurate analysis is key for an optimum achievable diagnosis;
however, gene expression studies are currently limited as
mineralized tissue prevents the study at the molecular level of
embedded bone cells. Remodelling is extremely important for
skeletal integrity. Bone cells, such as osteoblasts, osteocytes and
osteoclasts, act to confer a dynamic equilibrium between bone
formation and bone resorption (1).
Molecular changes occurring in important processes, such as
differentiation, or associated with pharmacological responses, are
often studied in vitro using bone cells derived from
calvariae or femour.
However, these approaches imply digestion and
artifactual conditions, such as incubation in media supplemented
with sera and growth factors. As a result, in spite of versatility,
even well-conducted studies may deliver imprecise information.
Until now, histomorphometric studies performed to
evaluate bone microarchitecture describe the quality and integrity
of bone tissue (2,3). By contrast, this method does not allow a
correct and timely molecular analysis of skeletal changes during
the bone remodeling under endogenous and exogenous stimuli.
The possibility to study molecular changes directly
in bone tissue appears intriguing and useful. However, in order to
perform studies of gene expression associated with bone tissue, it
is important to promptly isolate RNA preserving the quality and
integrity.
Current RNA isolation methods from bone tissue are
based on multiple steps approaches conducted at low temperatures
using liquid nitrogen (4) or beads
maintained at temperatures near freezing (5). The quality of RNA isolated by these means
is good, but the process is time consuming and the extraction steps
have to be performed at an extremely low temperature. The latter
aspect, often limits the possibility to isolate RNA in sterile
conditions and to prevent RNA contamination.
In order to isolate RNA in a simple and fast manner
in a sterile cabinet, a new method that prevents RNA degradation
and contamination with a great feasibility for numerous
laboratories was developed in the present study.
In addition, the RNA obtained by this fast
single-step method (FSSM) was assayed by analysing the expression
of osteoblastic (Runx2, Alp and Sparc) and
osteoclastic (Tnfrsf11 and Ctsk) genes, and the
results were compared with the data obtained using a traditional
method (TM) for RNA isolation.
Materials and methods
Animals
In total, 10 3-month-old female C57BL/6 mice with a
body weight of 25–30 g were obtained from Charles River
Laboratories Italia (Calco, Italy). All the mice were housed under
similar conditions. The mice were fed a standard rodent diet
containing 0.97% calcium, 0.85% phosphorus, 1,045 IU/kg vitamin D3,
22.5% protein, 5.5% fat and 52% carbohydrate, and were provided
access to tap water ad libitum.
The animal procedures were approved by the local
government authorities (University of Padova, Italy) and were
conducted in accordance with the accepted standards of humane
animal care, in accordance with the EU Directive 2010/63/EU.
Tibia bones were harvested and the embedded tissue
removed. For each mouse, two tibias were harvested and one tibia
for each method was used.
RNA extraction by the FSSM
Soft tissue was removed, the tibia was cut into
slices <0.2 cm and submerged in RNAlater (Qiagen, Milano, Italy)
solution accordingly to manufacturer's protocol, and the samples
were incubated overnight at 4°C. On the following day, RNAlater was
removed and the bone was stored at −80°C until RNA isolation.
For isolation, the RNeasy Protect Mini kit (Qiagen)
was used with certain modifications and isolation was performed at
room temperature.
The slices were thawed at room temperature and
simultaneously disrupted and homogenized with a T10 basic
ULTRA-TURRAX at 8,000 × g in the presence of 600 µl of RLT buffer
containing β-mercaptoethanol in the same microtube used for
storage. Subsequently, the lysate was placed into a QIAshredder
spin column on a 2-ml collection tube and centrifuged for 2 min at
full speed. The following steps were performed according to the
manufacturer's protocol with a DNAse treatment.
RNA extraction by a TM
Total RNA was extracted from each pellet using the
RNeasy Mini kit with DNAse treatment. The tibia bones were
harvested from mice and any attached tissue was removed prior to
the addition of RNAlater solution and stored at 4°C. After 24 h the
RNAlater was eliminated and the samples were stored at −80°C until
RNA extraction. Frozen samples were crushed using a mortar and
pestle in liquid nitrogen as previously described (4) and homogenized in RLT Lysis buffer. The
RNA isolation was subsequently performed according to the
manufacturer's protocol.
RNA evaluation and reverse
transcription
The yield and quality of RNA were analysed using the
RNA 6000 Nano LabChip kit (Agilent 2100 bioanalyzer; Agilent
Technologies Inc., Santa Clara, CA, USA) or the spectrophorometer
GeneQuant 1300 (GE Healthcare Europe, GmbH, Freiburg, Germany).
First-strand cDNA was generated using the High-Capacity cDNA
Archive kit, with random hexamers, (Applied Biosystems PE; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. RT product was aliquoted in equal volumes
and stored at −80°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed in a total volume of 50 µl
containing 1X TaqMan Universal PCR Master mix, no AmpErase UNG and
5 µl of cDNA; gene-specific primers and probe sets for each gene
(Runx2, Mm00501584-m1; Alp, Mm01187117-m1;
Sparc, Mm00486332-m1; Tnfrsf11a, Mm00437132_m1;
Ctsk, Mm00484039_m1) were obtained from Assay-on-Demand Gene
Expression Products (Applied Biosystems; Thermo Fisher Scientific,
Inc.). In order to normalise the results, two reference genes that
belong to different categories: β-actin (structural gene;) and
GAPDH (metabolism-related gene) were used [mouse ACTB (Actin, β)
endogenous control, cat. no. 4352341E; and mouse GAPD (GAPDH)
endogenous control, cat. no. 4352339E, Applied Biosystems; Thermo
Fisher Scientific, Inc., respectively].
Amplifications included 10 min at 95°C (AmpliTaq
Gold activation), followed by 40 cycles at 95°C for 15 sec and at
60°C for 1 min. Thermocycling and signal detection were performed
with the ABI Prism 7300 Sequence Detector (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Signals were detected according to
the manufacturer's protocol. Cq values were analysed
using TaqMan SDS analysis software and triplicate Cq
values were averaged.
Relative gene expression levels were calculated for
each sample following normalization and by the ΔΔCq
method for comparing relative fold expression differences. The data
are reported as mRNA fold expression.
Statistical analysis
Analysis of variance followed by Bonferroni as
post-hoc analysis were used and the results are expressed as mean ±
standard error of the mean. P<0.05 was considered to indicate a
statistically significant difference. Analyses were applied to
experiments carried out at least three times. Statistical analyses
were performed using SPSS for windows, version 16.0 (SPSS, Inc.,
Chicago, IL, USA).
Results and Discussion
Molecular approaches could provide highly relevant
information regarding the basis of degenerative diseases with
important consequences on the prevention and treatment. The
possibility to obtain specific and sensible tools for diagnosis and
follow up of degenerative diseases represents a relevant endpoint
to improve and facilitate the managements of patients affected by
these diseases. Several degenerative diseases involve the skeletal
tissue, a deeper analysis of the bone quality would open the
possibility to personalize and improve therapies. However,
calcified deposits prevent a direct nucleic acids extraction, which
complicates the analysis.
Recently, it has been shown that zoledronate, a
potent amino-bisphosphonate, is able to affect peri-implant bone
formation (6) but the molecular
mechanisms involved in this process are lacking.
A number of studies that aimed to understand the
molecular mechanisms of bone remodeling were performed in in
vitro models where it is easier to obtain RNA compared to in
bone tissue (7,8). However, culture conditions, including
medium and sera, have several variations.
The analysis of bone safety with pharmacological
treatment is generally evaluated for histomorphometric studies
(9). This approach, even if well
performed, is not suitable for evaluating molecular changes
occurring in osteoblastic and osteoclastic cells during bone
remodeling. More in general, the methods used for RNA extraction
from bone tissue are time consuming or require certain precautions
to avoid molecular degradation (such as performing RNA extraction
at low temperatures). However, usually the extraction steps are
performed in a sterile cabinet where the presence of ice could
cross contaminate the samples.
On the basis of this finding, a simple and
standardised method to extract RNA for bone tissue could represent
a useful tool for molecular studies in this field.
To compare RNAs prepared either following the method
developed in our laboratory (FSSM) or a traditional one (TM), 10
mice were sacrificed. For each mouse 1 tibia was used for FSSM and
the other for TM. Yield (reported in µg) and quality were compared
by considering the ratio of absorbances
(A260/280 and
A260/230), the rRNA ratio (28S/18S) and the
RNA integrity number (RIN) (Table I).
The results demonstrated that the two methods produced a good yield
of RNA and the RINs, and therefore quality, were high. As compared
to previous methods, FSSM does not use a multistep approach
(4) and avoids treatment with beads or
temperatures below freezing.
 | Table I.RNA yield and quality for RNA obtained
by FSSM and TM. |
Table I.
RNA yield and quality for RNA obtained
by FSSM and TM.
| Method | Yield, µg |
A260/280 |
A260/230 | rRNA ratio
(28S/18S) | RIN |
|---|
| TM | 18.22±2.09 | 1.98±0.13 | 1.95±0.18 | 1.84±0.21 | 9.23±0.81 |
| FSSM | 18.55±1.48 | 2.01±0.01 | 1.97±0.14 | 1.96±0.19 |
9.5±0.22 |
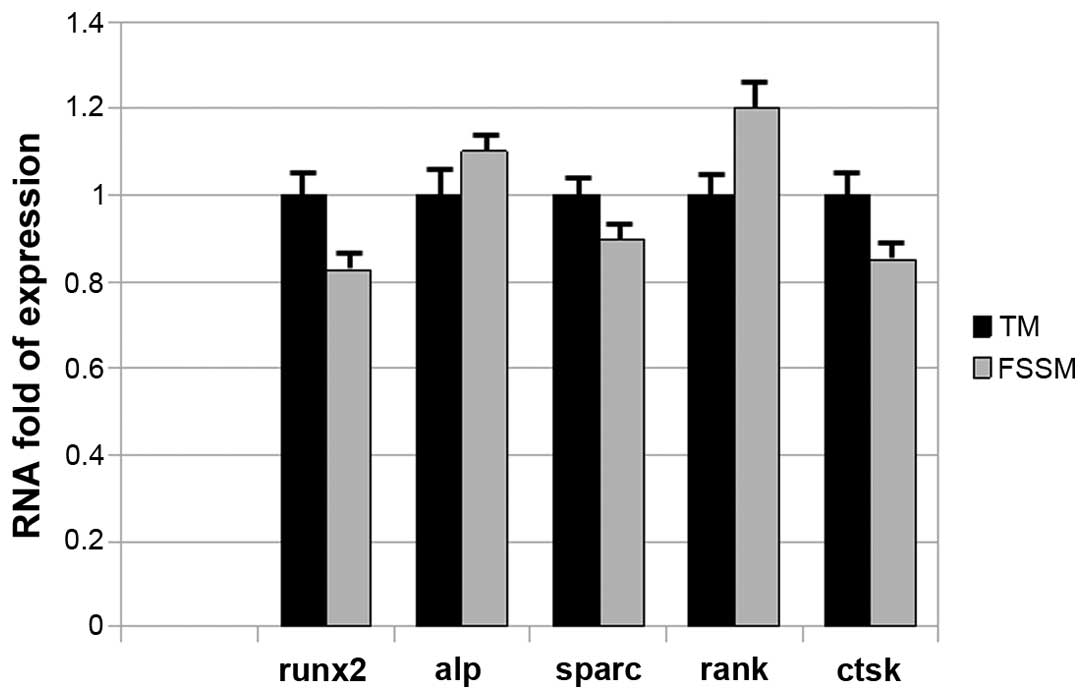
Secondly, whether RNA obtained by FSSM is suitable
for gene expression studies was examined and RT-qPCR assays were
performed. The gene expression analyses were performed using RNA
obtained by the two aforementioned different methods. RNA (1 µg)
was transcribed and 20 ng of cDNA was amplified. The relative
expression of osteoblastic (Runx2, Sparc and
Alp) and osteoclastic (Tnfrsf11 and Ctsk)
genes, calculated in 10 preparations for each assay, is reported in
Fig. 1. In particular, as a calibrator
the mean Cq for each gene obtained from the cDNA of TM
was used and normalised for the mean of the corresponding
Cq of the reference genes, and the fold-change in
expression was calculated using ΔΔCq of the sample
obtained by FSSM. The Runx2, Sparc and Alp
genes associated with osteoblastic lineage, and the Tnfrsf11
and Ctsk genes associated with osteoclastic lineage were
chosen, as they are important targets to skeletal tissue. The
fold-change of expression that was obtained by amplifying cDNA
either for osteogenic or osteoclastic genes was comparable between
the TM and FSSM.
In conclusion, molecular studies in bone tissue are
limited due to the difficulty in purifying a high quality of RNA.
To bypass this, a single-step method was performed and this
technique was compared with a TM. The FSSM carried out in the
present study showed a good result demonstrating that it is
possible to purify bone RNA in a simply way in order to perform
gene expression studies in skeletal diseases.
The FSSM performed in the present study is a
convenient and rapid protocol to obtain a high quality of bone RNA.
This method could simplify the molecular characterization of bone
bioptic samples allowing gene expression studies of skeletal
tissue.
Acknowledgements
The authors would like to thank Dr Samuele Cheri for
help with the reagent preparations.
Glossary
Abbreviations
Abbreviations:
|
FSSM
|
fast single-step method
|
|
TM
|
traditional method
|
References
|
1
|
Lemaire V, Tobin FL, Greller LD, Cho CR
and Suva LJ: Modeling the interactions between osteoblast and
osteoclast activities in bone remodeling. J Theor Biol.
229:293–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dalle Carbonare L, Ballanti P, Bertoldo F,
Valenti MT, Giovanazzi B, Giannini S, Realdi G and Lo Cascio V:
Trabecular bone microarchitecture in mild primary
hyperparathyroidism. J Endocrinol Invest. 31:525–530. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dalle Carbonare L, Valenti MT, Bertoldo F,
Zanatta M, Zenari S, Realdi G, Lo Cascio V and Giannini S: Bone
microarchitecture evaluated by histomorphometry. Micron.
36:609–616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantila Roosa SM, Liu Y and Turner CH:
Gene expression patterns in bone following mechanical loading. J
Bone Miner Res. 26:100–112. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carter LE, Kilroy G, Gimble JM and Floyd
ZE: An improved method for isolation of RNA from bone. BMC
Biotechnol. 12:52012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kettenberger U, Ston J, Thein E, Procter P
and Pioletti DP: Does locally delivered Zoledronate influence
peri-implant bone formation? - Spatio-temporal monitoring of bone
remodeling in vivo. Biomaterials. 35:9995–10006. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khedgikar V, Kushwaha P, Gautam J, et al:
Withaferin A: A proteasomal inhibitor promotes healing after injury
and exerts anabolic effect on osteoporotic bone. Cell Death Dis.
4:e7782013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu M, Hesse E, Morvan F, et al: Zfp521
antagonizes Runx2, delays osteoblast differentiation in vitro, and
promotes bone formation in vivo. Bone. 44:528–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Recker RR, Ste-Marie LG, Chavassieux P,
McClung MR and Lundy MW: Bone safety with risedronate:
Histomorphometric studies at different dose levels and exposure.
Osteoporos Int. 26:327–337. 2015. View Article : Google Scholar : PubMed/NCBI
|