Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease that is characterized by chronic invasive arthritis. The
major pathological changes that occur during RA include synovitis
and the resulting articular cartilage and bone damage, which
ultimately lead to articular deformation. Without systematic
treatment, RA will develop into a disability within 10 years in
~45% of patients (1). Although the
pathogenesis of RA has not been fully elucidated, it has been
widely accepted that synovial fibroblasts (SFs) have an important
role in the loss of cartilage tissue integrity (2–4). In healthy
joints, the synovial lining at the border to the joint cavity
consists of 1–3 cell layers, predominantly containing SFs and
macrophages. The physiological functions of SFs are to provide the
joint cavity and the adjacent cartilage with plasma protein and
hyaluronic acid as a lubricant. SFs also produce matrix components,
such as collagen and a series of matrix-degrading enzymes, to
participate in the continuous matrix remodeling (5,6). However, in
RA, the synovial lining thickness increases to 10–15 cell layers
(7,8),
and the SFs are activated to become RASFs, which damage the
cartilage by producing inflammatory cell factors and causing matrix
degradation (9). Therefore, an
improved understanding of the characteristics of SFs will be
important for identifying the contribution of this specific cell
type to the pathogenesis of RA.
To obtain large quantities of SFs from mice in
vitro has been challenging in fundamental studies of RA. As
large rodents, such as rats (10) and
rabbits (11), provide a rich source
of synovial tissues, it is easier to culture SFs in vitro.
However, mice have a small volume of intra-articular synovium
tissues, and therefore, it is difficult to obtain large quantities
of SFs. A primary culture system for mouse SFs has not been
established and remains a challenge, thus increasing the difficulty
associated with performing relevant studies and impeding research
investigating articular diseases. In the present study, an improved
culture method for SFs was established in which after 10 days of
culturing, the cells retained their original characteristics of
constitutive expression of vimentin (12), cluster of differentiation 90.2 (CD90.2)
(13), intracellular adhesion molecule
1 (ICAM-1) (14) and vascular cell
adhesion molecule 1 (VCAM-1) (15).
This method provides a high-yield and pure SFs population.
Materials and methods
Animals and ethics statement
The 10-week-old C57BL/6 mice were purchased from the
Animal Laboratory of Southern Medical University (Guangzhou,
Guangdong, China; license no. SCXK 2011-0015). The study was
performed in accordance with the ‘Guide for the Care and Use of
Laboratory Animals’ published by the US National Institutes of
Health (NIH, publication no. 85-23, revised 1996). The experiments
were approved by the Institutional Animal Care and Use Committee of
Southern Medical University.
Tools and reagents
Microsurgery scissors and forceps were purchased
from Jiaxing Moore Trade Co. (Jiaxing, China), 0.2-µm syringe
filters were purchased from Jet Bio-filtration (Guangzhou, China),
and 1.5-ml Eppendorf tubes, 60-mm Petri dishes, 3-ml plastic
pipettes and 75-cm2 flasks were purchased from Corning
(Corning, NY, USA). Penicillin-streptomycin, Dulbecco's modified
Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained
from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Type
IV collagenase, Tween-20, Triton X-100, phosphate-buffered saline
(PBS) and bovine serum albumin (BSA) were purchased from Sigma
Aldrich (St. Louis, MO, USA). Purified rat anti-mouse CD106 (cat.
no. 553330), purified rat immunoglobulin G2a (IgG2a), κ isotype
control (cat. no. 553927), FITC goat anti-rat Ig secondary antibody
(cat. no. 554016), phycoerythrin (PE) hamster anti-mouse CD54 (cat.
no. 553253), PE hamster IgG1 κ isotype control (cat. no. 553972),
biotin rat anti-mouse CD90.2 (cat. no. 553011), biotin rat IgG2b, κ
isotype control (cat. no. 553987) and PE-streptavidin (cat. no.
554061) were obtained from BD Pharmingen (Franklin Lakes, NJ, USA),
vimentin rabbit monoclonal antibody (Alexa Flour 488 Conjugate;
cat. no. 9854) was obtained from Cell Signaling Technology
(Beverly, MA, USA).
Preparation of reagents
Culture medium was DMEM supplemented with 1% of
penicillin-streptomycin and 10% of FBS. For 1% type IV collagenase,
100 mg of type IV collagenase was reconstituted in 10 ml of PBS,
filter sterilized with a 0.22-µm filter and 1 ml aliquots were
frozen at −20°C. For PBS with Tween-20 (PBST), 100 µl of Tween-20
was diluted in 100 ml of PBS (1:1,000) and stored at room
temperature. This reagent should be used within 2 months or
prepared immediately before use. For 2% BSA, 2 g of BSA was
dissolved in 100 ml of PBS.
Isolation of synovial tissues
Mice were sacrificed by cervical dislocation and
immersed in 75% alcohol for 2 min for sterilization. Under a
stereomicroscope (Olympus, Tokyo, Japan), the skin of the hind
limbs was removed and the synovial tissues around the hip joints
were obtained using microsurgery scissors and forceps (white
sponge; Fig. 1A). During this
procedure, attention was focused on eliminating the ‘egg-yolk’-like
yellow oval substance (insert in Fig.
1B). The synovium is transferred to a 60-mm Petri dish
containing 2 ml of DMEM.
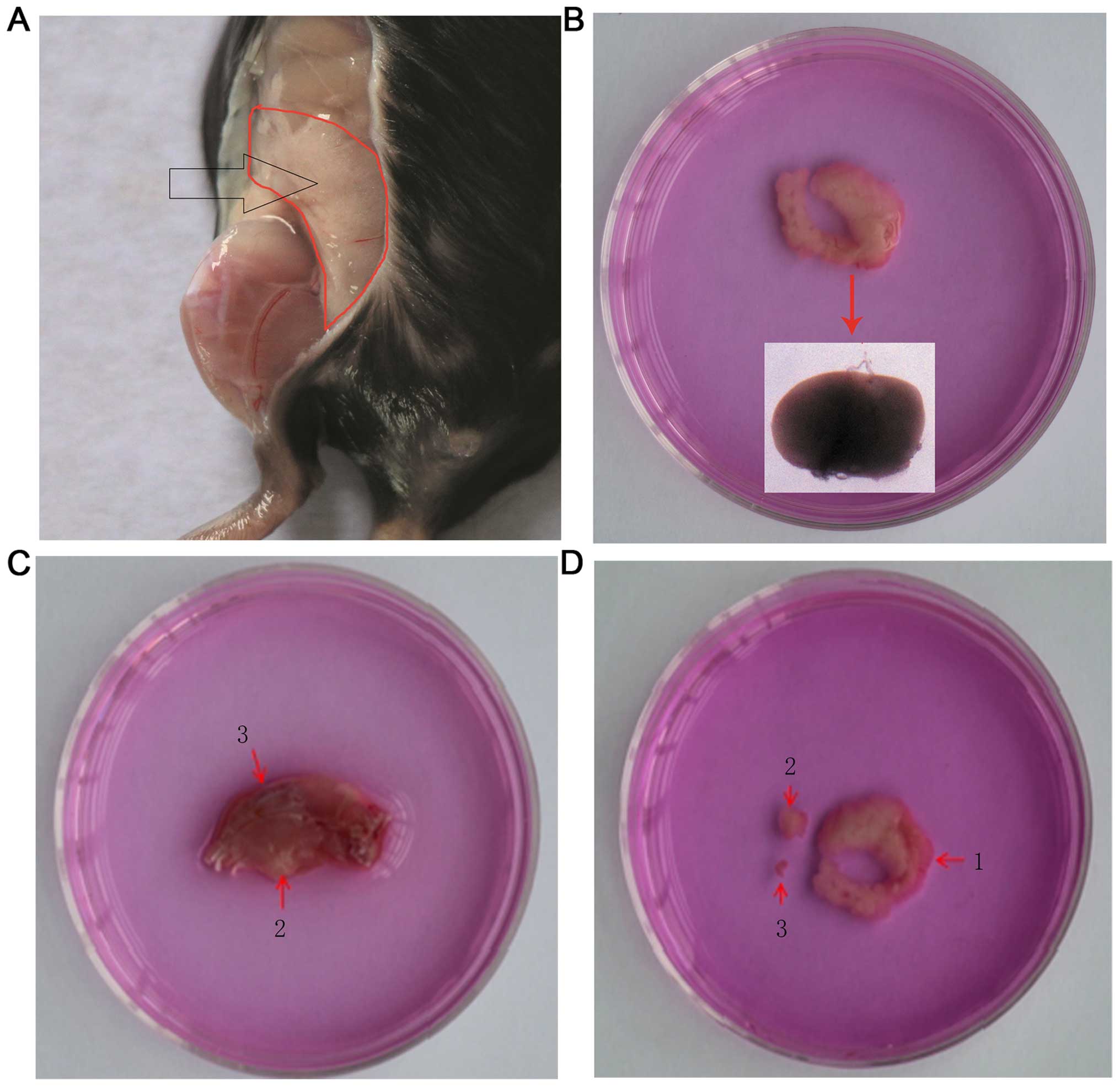 | Figure 1.Isolation of synovial tissues from
C57BL/6 mice. (A) The synovium around the hip joints. Under the
stereomicroscope, the synovium appears white and spongy; however,
the gross observation of the tissues shows a pink colour
neighboring the peritoneum. (B) The dissociated synovium around the
hip joints. At the turning of the specimen, there is a round
substance that is similar to ‘egg-yolk’, as shown in the insert.
The color of the image was distorted under the stereomicroscope;
the actual color is yellow. (C) Dissociated hind limbs. 2, synovium
is hidden in the deep popliteal fossa and was exposed by cutting
open the muscle. The synovium obtained from this area contains a
substance similar to ‘egg-yolk’ that is also found in the synovium
around the hip joints. 3, synovial tissues of the knee joints,
which were exposed by cutting the articular cavity of the knee open
along the two sides of the patella. (D) Dissociated synovium. 1,
synovium around the hip joints; 2, synovium in the popliteal fossa;
3, synovium within the knee joints. The size of the synovium
differs in the different location. |
To obtain greater amounts of synovial tissues, the
following procedures were implemented: Isolated the hind limbs
(preserved all the muscle tissues and discarded foot and ankles)
and placed in a 60-mm Petri dish containing 2 ml of DMEM. Under a
stereomicroscope, the muscle inside the popliteal fossa was cut
open with microsurgery scissors and forceps to harvest the synovium
(insert 2 in Fig. 1C). During this
procedure, attention was focused on eliminating the ‘egg-yolk’-like
yellow oval substance (insert in Fig.
1D) in the middle of the synovium. Subsequently, the synovium
was transferred to another 60-mm Petri dish containing 2 ml of
DMEM. To harvest the intra-articular synovium, the articular cavity
of the knee was cut open along both sides of the patella under a
stereomicroscope (insert 3 in Fig.
1C), and isolated the intra-articular synovium carefully. Of
note, the connective tissues around the synovium were carefully
eliminated under a stereomicroscope.
Digestion of the synovium and culture
of SFs
The synovium was transferred into a 1.5-ml Eppendorf
tube containing 0.5 ml DMEM and 0.5 ml 1% type IV collagenase and
the tissues were separated into 1-mm3 blocks with
microsurgery scissors. Subsequently, the Eppendorf tube was
incubated at a constant temperature of 37°C in an orbital shaker
incubator (200 rpm) for 60 min. Following completion of the
incubation time, the sample was vortexed vigorously for 1.5 min to
release the cells. The sample was centrifuged for 5 min at 300 × g
and resuspended with DMEM supplemented with 10% FBS and 1%
penicillin-streptomycin. The cells were seeded cells into a
75-cm2 flask and placed in a humidified tissue culture
incubator (37°C, 5% CO2). This procedure was performed
on a clean bench.
Immunofluorescence staining of
SFs
Immunofluorescence cytochemical staining was
performed of passage 3 SFs cultured on a 12-well plate with
anti-vimentin antibody. The simplified process is as follows: The
medium was discarded and the cells were washed twice with PBS.
Subsequently, the cells were fixed in 4% paraformaldehyde for 10
min and soaked with 1.5 ml of 0.2% Triton X-100 solution for 5 min.
Following this the samples were washed twice with PBST.
Subsequently, 200–300 µl goat serum blocking reagent was added at
room temperature for 20–30 min. The cells were incubated with 1 ml
of Alexa fluor 488 vimentin antibody (1:200) overnight at 4°C in
the dark. The cells were washed 3 times for 3 min with PBST.
Following this, 200 µl of PBS was added to the cells and the
samples were protected from light until their observation using a
fluorescence microscope.
Flow cytometric analysis of passage 3
SFs
Four cell markers, vimentin, CD90.2, ICAM-1 (CD54)
and VCAM-1 (CD106) were used for the purity analysis of SFs in
accordance with the instructions. Simplified procedure: After 3
passages, the cells were trypsinized, washed twice with 2% BSA and
resuspended in 1 ml of 2% BSA at a density of 1×107
cells/ml. Subsequently, 100 µl of the cell suspension was added to
a 1.5-ml Eppendorf tube. Fluorescently conjugated antibodies (or
purified or biotin-conjugated antibodies) were added to the samples
and incubated for 40 min at room temperature in the dark. The cells
were washed twice with 1 ml of stain buffer (2% BSA). For indirect
immunofluorescence staining, the cells were incubated with an
appropriately labeled secondary antibody in 100 µl of stain buffer
for 20 min at room temperature and washed twice with 2% BSA. The
cells were resuspended in 0.5 ml of stain buffer and analyzed by
flow cytometry.
Results
Culture of isolated SFs
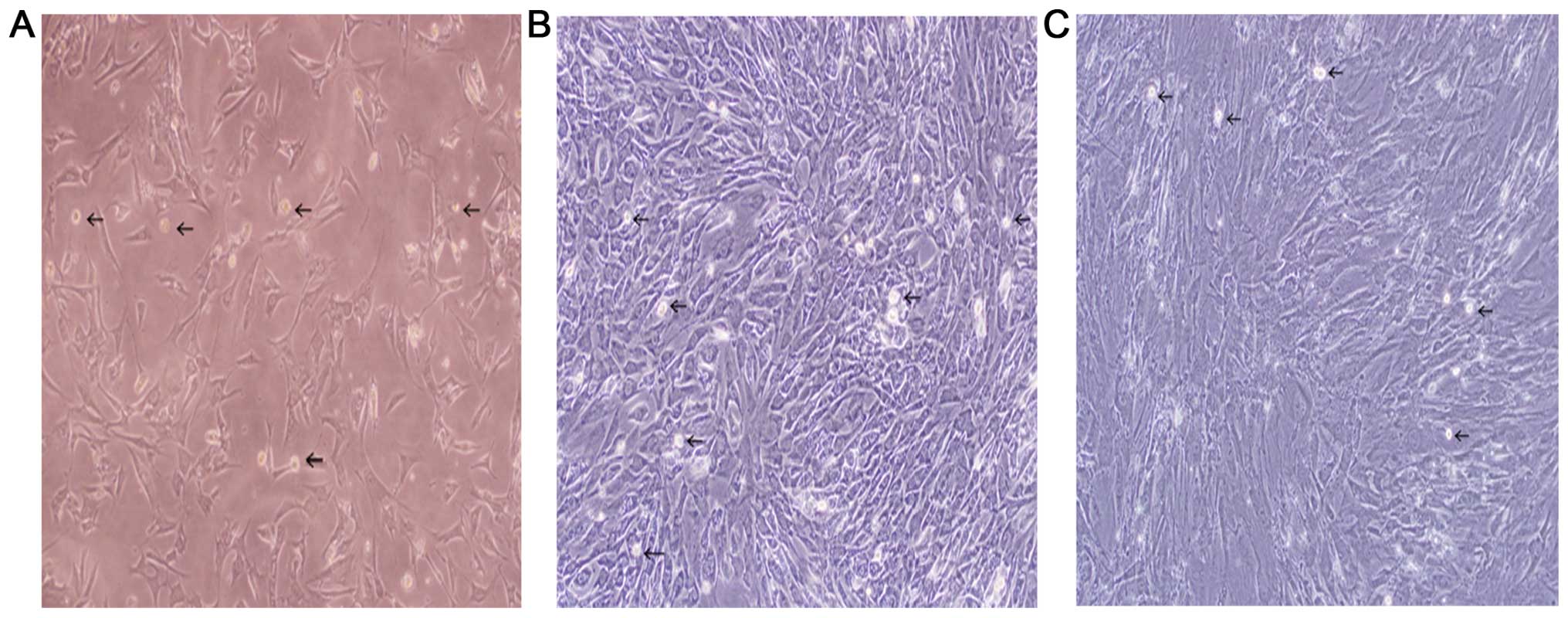
Adhesion of the SFs began on day 2. The medium was
changed initially on day 3 (Fig. 2A)
and following this every 3–4 days. Cells usually reached confluency
6 days after isolation. After the first passage, confluency was
reached 3–4 days later. The first passage was performed by trypsin
digestion avoiding a long incubation with the enzyme (2-min
incubation with pre-warmed trypsin is effective for fibroblast
detachment, whereas macrophages that are highly adhesive remain
attached. Thus, it is highly recommended to change culture flasks
every passage). At this time, the number of synovial macrophages
reached a maximum (Fig. 2B) and
subsequently decreased with the passage number. After 3 passages
(day 10; Fig. 2C), the synovial
macrophages typically disappeared.
Purity analysis of SFs
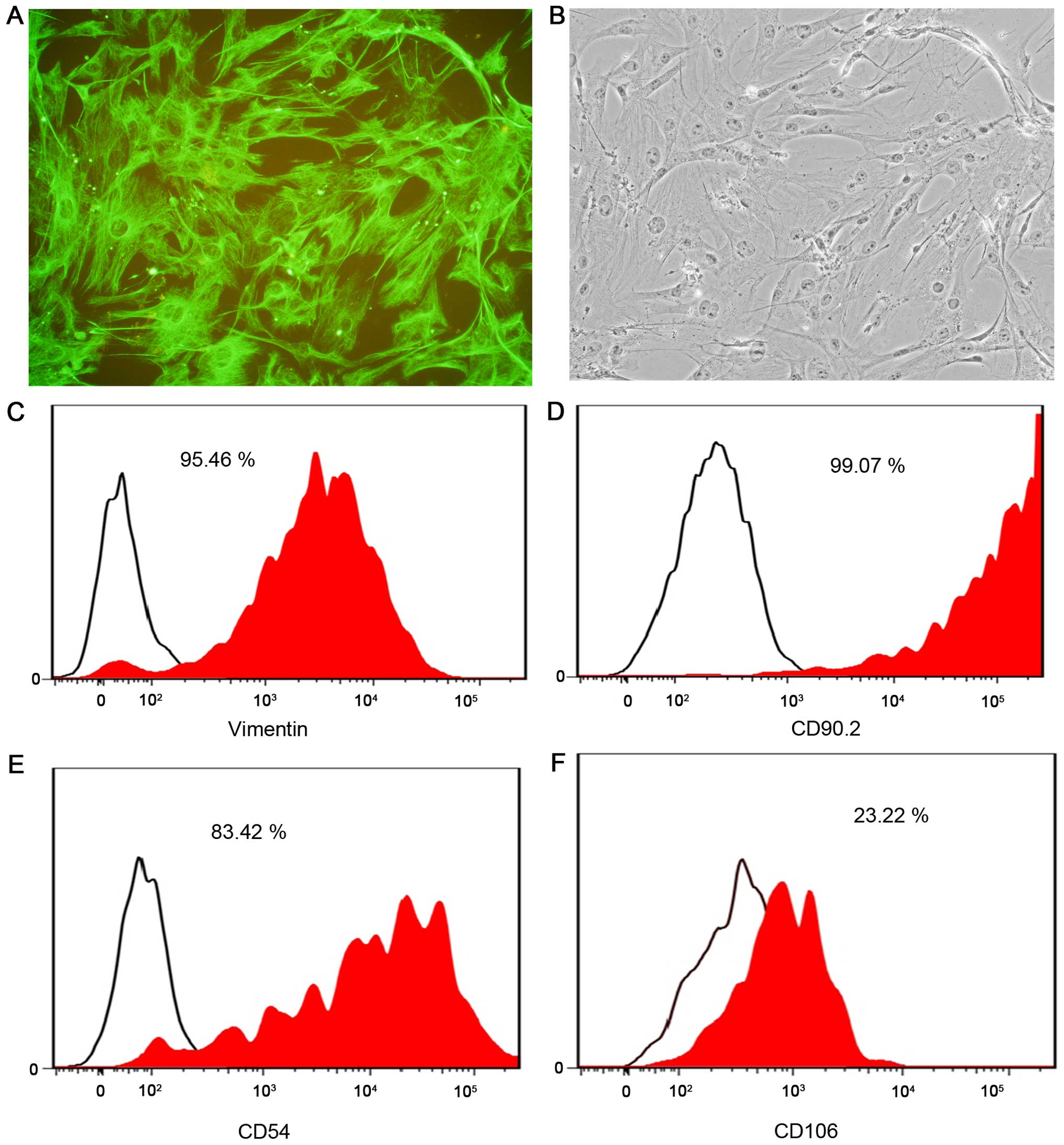
Immunostaining of passage 3 SFs in primary cultures
with an anti-vimentin antibody found that the majority of cells
were positive (Fig. 3A and B). The
flow cytometric analysis indicated that vimentin and CD90.2 labeled
>95% of cells (Fig. 3C and D). The
purity of the CD54-labeled cells was ~80% and the purity of the
CD106-labeled cells was only 23% (Fig. 3E
and F). The cause of the low purity of CD106-labeled cells
remains to be elucidated. The present results demonstrated that the
method described herein was simple and effective and can be used to
obtain a large quantity of SFs (0.75–1×107 cells per
flask).
Discussion
The methods applied for the primary culture of mouse
SFs included tissue block culture and pancreatic enzyme and
collagenase digestion (16–18). All these methods, however, have
apparent shortcomings. The methods of tissue block culture are
relatively easy, but the blocks are small and cannot be evenly
distributed in the culture flasks. The majority of the cells grow
around the blocks. Other challenges associated with the tissue
block culture include blocks adhesive difficulty, a long growth
period of the primary generation, a low survival rate and a small
number of cells. The limitations of the pancreatic enzyme digestion
method are that this technique cannot effectively digest and
separate the fibrous tissues, impeding the dissociation of synovial
cells. During filtering, the cells can block the filter pores.
Therefore, a low cell number is obtained. The type II collagenase
was initially used in combination with pancreatic enzyme digestion,
as reported previously (19). This
method, however, was found to be ineffective. A small quantity of
cells with a low purity was obtained, and 15–20 days were required
for the first passage.
Type IV collagenase can effectively digest and
separate the fibrous tissues and intercellular matrix. The fibrous
tissues are digested into filamentous fibers, and the cells are
completely dissociated. The method only required one type of enzyme
and no filtration process was required, thus, simplifying the
experimental procedure and reducing cell loss. The concentration of
type IV collagenase was increased gradually from 0.1%, and 0.5% was
found to be the most effective concentration. It is possible that a
higher concentration would provide an improved effect. For example,
a concentration of 1% may be superior to a concentration of 0.5%.
However, the concentration of type IV collagenase was not further
increased in this experiment. Following digestion by type IV
collagenase, the samples were vortexed vigorously for 1.5 min to
release cells. A poor effect was observed when the vortex was <1
min. Vortex for 1.5 min was found to be equivalent to vortex for 3
min. Therefore, the present study recommends 1.5 min.
The synovium between the knee joints was also used
for the culture. However, the purity of SFs was found to be low,
potentially due to the small amount of synovial tissue between the
knee joints or possibly due to the presence of cartilage resulting
in a mixture of cartilage cells. In comparison to the synovium
between the knee joints, a large amount of synovium was present
around the hip joints (the greatest amount of dissociated synovium
was present in the hip joints), and it was easy to excise the
connective tissues under the stereomicroscope. Therefore, the
synovium around the hip joints was mainly used for the culture. On
day 3 after isolation, a large quantity of SFs was observed. The
cells were trypsinized when confluence had reached 90–100% of the
plate and they were transferred to 75-cm2 flask (split
1:2) on days 6–8. A maximum number of synovial macrophages was
observed at this time. Due to the firm adhesive of the synovial
macrophages, the cells could not be easily detached by digestion
with 0.25% pancreatic enzyme. Therefore, the cells were purified
during the passage. After 3 passages, the majority of the
macrophages were eliminated.
Vimentin is a type III intermediate filament protein
that is expressed in mesenchymal cells. Due to this, vimentin is
often used as a marker of SFs. CD90.2 is a 25–37 kDa heavily
N-glycosylated, glycophosphatidylinositol-anchored conserved cell
surface protein with a single V-like immunoglobulin domain,
originally discovered as a thymocyte antigen. It is initially
described as SFs-specific, and positive identification of SFs was
attempted using the antibody CD90.2 (20,21). ICAM-1,
also known as CD54, is a transmembrane protein possessing an
amino-terminus extracellular domain, a single transmembrane domain
and a carboxy-terminus cytoplasmic domain. VCAM-1, or CD106, is a
protein that in humans is encoded by the VCAM1 gene. CD106 and CD54
belong to the immunoglobulin family of adhesion molecules, and they
present on the SFs surface. Therefore, these four antibodies were
selected in the present study to identify the purity of SFs.
In conclusion, the protocol described herein for the
primary culture of murine SFs provides millions of primary cells
per experiment, permitting experimentation in biochemistry and
cellular biology investigations on the characteristics of SFs.
Therefore, this method is a simple and effective way to obtain
large quantities of murine SFs.
Acknowledgements
The authors would like to acknowledge the National
Natural Science Fund of China (grant no. 81172875).
References
|
1
|
Emery P, Breedveld FC, Dougados M, Kalden
JR, Schiff MH and Smolen JS: Early referral recommendation for
newly diagnosed rheumatoid arthritis: Evidence based development of
a clinical guide. Ann Rheum Dis. 61:290–297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dougados M, Devauchelle-Pensec V, Ferlet
JF, Jousse-Joulin S, D'Agostino MA, Backhaus M, Bentin J, Chalès G,
Chary-Valckenaere I, Conaghan P, et al: The ability of synovitis to
predict structural damage in rheumatoid arthritis: a comparative
study between clinical examination and ultrasound. Ann Rheum Dis.
72:665–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neumann E, Lefèvre S, Zimmermann B, Gay S
and Müller-Ladner U: Rheumatoid arthritis progression mediated by
activated synovial fibroblasts. Trends Mol Med. 16:458–468. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lefèvre S, Knedla A, Tennie C, Kampmann A,
Wunrau C, Dinser R, Korb A, Schnäker EM, Tarner IH, Robbins PD, et
al: Synovial fibroblasts spread rheumatoid arthritis to unaffected
joints. Nat Med. 15:1414–1420. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karouzakis E, Gay RE, Gay S and Neidhart
M: Epigenetic control in rheumatoid arthritis synovial fibroblasts.
Nat Rev Rheumatol. 5:266–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noss EH and Brenner MB: The role and
therapeutic implications of fibroblast-like synoviocytes in
inflammation and cartilage erosion in rheumatoid arthritis. Immunol
Rev. 223:252–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perlman H and Pope RM: The synovial lining
micromass system: Toward rheumatoid arthritis in a dish? Arthritis
Rheum. 62:643–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Müller-Ladner U, Ospelt C, Gay S, Distler
O and Pap T: Cells of the synovium in rheumatoid arthritis.
Synovial fibroblasts. Arthritis Res Ther. 9:2232007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miao CG, Huang C, Huang Y, Yang YY, He X,
Zhang L, Lv XW, Jin Y and Li J: MeCP2 modulates the canonical Wnt
pathway activation by targeting SFRP4 in rheumatoid arthritis
fibroblast-like synoviocytes in rats. Cell Signal. 25:598–608.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pillinger MH, Dinsell V, Apsel B, Tolani
SN, Marjanovic N, Chan ES, Gomez P, Clancy R, Chang LF and Abramson
SB: Regulation of metalloproteinases and NF-kappaB activation in
rabbit synovial fibroblasts via E prostaglandins and Erk:
Contrasting effects of nabumetone and 6MNA. Br J Pharmacol.
142:973–982. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao K, Zeng H, Xiao DM, Xiong A, Weng J
and Kang B: Influences of IL-6R antibody on PMMA bone
cement-mediated expression of OPG and RANKL in synovial
fibroblasts. J Huazhong Univ Sci Technolog Med Sci. 34:241–246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fiorito S, Magrini L, Adrey J, Mailhé D
and Brouty-Boyé D: Inflammatory status and cartilage regenerative
potential of synovial fibroblasts from patients with osteoarthritis
and chondropathy. Rheumatology (Oxford). 44:164–171. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pierer M, Brentano F, Rethage J, Wagner U,
Hantzschel H, Gay RE, Gay S and Kyburz D: The TNF superfamily
member LIGHT contributes to survival and activation of synovial
fibroblasts in rheumatoid arthritis. Rheumatology (Oxford).
46:1063–1070. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scian R, Barrionuevo P, Rodriguez AM,
Arriola Benitez PC, García Samartino C, Fossati CA, Giambartolomei
GH and Delpino MV: Brucella abortus invasion of synoviocytes
inhibits apoptosis and induces bone resorption through RANKL
expression. Infect Immun. 81:1940–1951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nikitopoulou I, Oikonomou N, Karouzakis E,
Sevastou I, Nikolaidou-Katsaridou N, Zhao Z, Mersinias V, Armaka M,
Xu Y, Masu M, et al: Autotaxin expression from synovial fibroblasts
is essential for the pathogenesis of modeled arthritis. J Exp Med.
209:925–933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohki E, Suzuki M, Aoe T, Ikawa Y, Negishi
E and Ueno K: Expression of histamine H4 receptor in synovial cells
from rheumatoid arthritic patients. Biol Pharm Bull. 30:2217–2220.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brühl H, Mack M, Niedermeier M, Lochbaum
D, Schölmerich J and Straub RH: Functional expression of the
chemokine receptor CCR7 on fibroblast-like synoviocytes.
Rheumatology (Oxford). 47:1771–1774. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann T, Kunisch E, Pfeiffer R, Hirth
A, Stahl HD, Sack U, Laube A, Liesaus E, Roth A, Palombo-Kinne E,
et al: Isolation and characterization of rheumatoid arthritis
synovial fibroblasts from primary culture–primary culture cells
markedly differ from fourth-passage cells. Arthritis Res. 3:72–76.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saalbach A, Aneregg U, Bruns M, Schnabel
E, Herrmann K and Haustein UF: Novel fibroblast-specific monoclonal
antibodies: Properties and specificities. J Invest Dermatol.
106:1314–1319. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosengren S, Boyle DL and Firestein GS:
Acquisition, culture, and phenotyping of synovial fibroblasts.
Methods Mol Med. 135:365–375. 2007. View Article : Google Scholar : PubMed/NCBI
|