Introduction
Radiation-induced pulmonary toxicity (RILT) is the
most common pulmonary complication in lung cancer patients
receiving external beam radiotherapy. Clinical signs and symptoms
of RILT occur in 5–20% of irradiated patients (1). However, the prevalence may be
underestimated as, following chest radiotherapy (RTx), ≤40% of
patients demonstrate changes on diagnostic imaging, while certain
patients are asymptomatic (2,3). The introduction of standardized lung dose
volume constraints has led to a reduced incidence of
radiation-induced pulmonary injuries, such as fibrosis and
pulmonary insufficiency (4,5); however, the two complications have not
been completely eliminated (6,7).
Irradiated lung tissues manifesting acute adverse
reactions, such as RILT demonstrate enhanced production of
inflammatory mediators (8). Therefore,
the changes in cytokine concentrations during RTx may be predictive
of lung toxicity and may, ultimately, lead to safer radiotherapy.
Studies on biomarkers in lung cancer have been focused on the risk
of developing lung cancer (9).
Previous studies have demonstrated that assessments of cytokines,
such as tumor growth factor-β (TGF-β1), interleukin-6 (IL-6)
(3,10),
eotaxin, and monocyte chemoattractant protein-1 (8) have considerable clinical utility for
predicting RILT and/or survival (11,12). The
majority of studies focused on biomarker levels prior to or
following completion of RTx. Recent results suggest that serum
concentrations of biomarkers from samples taken during the course
of RTx may increase and correlate with exacerbation of RILT, thus
enabling real-time risk assessment, prediction of fibrosis, and
faster implementation of therapeutic interventions (13,14).
Materials and methods
Patient recruitment
The current study was approved by the Bioethics
Committee of the Medical University of Łódź (Łódź, Poland). All
participants provided written informed consent for participation in
the study. A total number of 26 patients (16 males and 10 females)
who were treated between January, 2014 and July, 2015 for non-small
cell lung cancer (NSCLC) clinical stages IIA-IIIB, according to the
American Joint Committee on Cancer Staging Manual, 7th Edition
(15) by sequential chemoradiotherapy
or radiotherapy in a postsurgical setting following adjuvant
chemotherapy were recruited for participation in this study. Blood
samples (5 ml) were taken from the participants at three time
points during treatment for biomarker measurement, as follows: At
baseline, prior to commencing radiotherapy; after a total of 20 Gy;
after a total of 40 Gy. The samples after 20 and 40 Gy were
collected just before the patient received the planned fraction.
All blood samples were collected, stored in 4°C for clotting, then
centrifuged at 1,500 × g (MPW-56; MPW Med Instruments, Warsaw,
Poland) and stored at −80°C until analysis.
Exclusion criteria
Patients were excluded according to the following
criteria: Chronic cardiac insufficiency (class III/IV, according to
the New York Heart Association classification system); severe renal
insufficiency (stage IV, according to The Renal Association;
glomerular filtration rate, <30 ml/1.73m2/min);
advanced stage of other pulmonary disease (chronic obstructive
pulmonary disease stage IV according to the Global Initiative for
Chronic Obstructive Lung Disease (16)
classification or Asthma IV according to the Global Initiative for
Asthma 2011 guidelines (17); or
advanced liver insufficiency (Child-Pugh classification C and D).
Patients that had been treated by stereotactic RTx and those with
superior sulcus tumors or giant cell tumors were not included in
the current study.
Recruitment scheme
Patients enrolled in the study underwent standard
treatment according to National Comprehensive Cancer Network (NCCN)
guidelines (18). Target volumes were
delineated according to the International Commission of Radiation
Units and Measurements Reports 62 and 83 (19). The total radiation doses delivered to
clinical target volumes ranged between 54 and 74 Gy, at photon
energies of 6 MV, or a mix of 6 and 15 MV, using linear
accelerators (Clinac®iX; Varian Medical Systems, Inc., Palo Alto,
CA, USA). Treatment volume planning was performed based on
three-dimensional conformal techniques, using computed tomography
to delineate targets and organs at risk, and to plan the dose
delivery. Dose-volume constraints for organs at risk were based on
NCCN limitations. Every study patient was examined once weekly for
pulmonary signs (a dry cough, fever and dyspnea) and symptoms
suggestive of RILT. Healthy, age- and gender-matched participants
were recruited (n=26) for the control group.
Blood sample collection
Blood samples from the controls were assayed for
cytokine levels and the results were compared with the baseline
cytokine levels of the study patients. The following enzyme-linked
immunosorbent assays were used to determine protein concentrations:
Tumor necrosis factor-α (TNF-α; DTA00C kit; R&D Systems, Inc.,
Minneapolis, MN, USA), IL-6 (D6050 kit; R&D Systems, Inc.),
lipopolysaccharide-binding protein (LBP; KA0448 kit; Abnova
(Taiwan) Corporation, Taipei, Taiwan), and C-reactive protein (CRP;
RD191006200R kit; BioVendor R&D, Brno, Czech Republic),
following the manufacturers' instructions.
Statistical analysis
Student's t-test was used to perform univariate
comparisons of log10-transformed values. The Pearson
product-moment correlation coefficients were determined to assess
the strength of the linear relationship between two variables.
Repeated-measures analysis of variance was used to evaluate
temporal changes in cytokine levels. The sample size of the study
was determined based on 80% statistical power for detecting effects
equal to ≥66% of 1 standard deviation, with a predetermined type 1
error probability of 0.05. An additional 10% of the calculated
sample size of patients (n=22) was recruited to account for missing
data or technical errors, and an additional 10% for dropouts due to
the increased risk of mortality during radiotherapy. P<0.05 was
considered to indicate a statistically significant difference.
Results
Basic statistics and matched-pair
analysis
A total 26 patients (16 males and 10 females), with
a mean age of 64.7±6.6 years were included in the study cohort. The
control group consisted of 17 males and 9 females, with a mean age
of 61.3±5.3 years. There were no differences in gender (P=1.00) and
age (P=0.1031) between the patients with NSCLC and the control
subjects. Table I demonstrates that at
baseline, there were significant differences in three of the four
cytokines between the patients with cancer and the control
subjects. The only difference that was not significant was in the
LBP values. This result indicates that LBP is not upregulated by
the ongoing generalized inflammatory reaction against the tumor.
During radiotherapy, none of the cytokine levels were significantly
associated with the total dose administered at the time of cytokine
assessment (Table I). At all time
points, the IL-6, CRP, and TNF-α levels remained markedly above the
control levels; the LBP levels remained stable and similar to the
control level.
 | Table I.Serum concentrations of inflammatory
markers in the control participants and the patients with NSCLC at
baseline and during radiotherapy.a |
Table I.
Serum concentrations of inflammatory
markers in the control participants and the patients with NSCLC at
baseline and during radiotherapy.a
|
| Baseline serum
concentrations | Radiotherapy serum
concentrations | P-value |
|---|
|
|
|
|
|
|---|
| Marker | Patients with
NSCLC | Controls | P-value | 20 Gy | 40 Gy | Repeated measures
analysis of variance | Linear trend |
|---|
| IL-6 (pg/ml) | 4.34 (2.77–8.67) | 1.51 (0.99–2.59) | <0.0001 | 6.07 (3.15–7.43) | 5.47 (2.90–8.67) | 0.1898 | 0.1270 |
| TNF-α (pg/ml) | 17.20
(15.50–20.81) | 5.66
(4.57–14.81) | <0.0001 | 17.53
(15.50–19.51) | 17.20
(14.14–20.80) | 0.6831 | 0.5156 |
| CRP (µg/ml) | 116.08
(33.3–179.73) | 36.63
(11.95–90.67) |
0.0124 | 121.14
(67.44–239.47) | 118.87
(44.04–266.70) | 0.4442 | 0.4023 |
| LBP (µg/ml) | 36.34
(31.35–39.27) | 36.92
(30.20–44.05) |
0.4219 | 35.96
(33.91–40.53) | 37.62
(34.11–41.03) | 0.2876 | 0.1656 |
Comparisons between dosimetric factors
and serum cytokine levels
Having established that the levels of inflammatory
markers do not change significantly throughout radiotherapy, the
correlation coefficients of their levels in association with the
dosimetric parameters measured at the 40-Gy dose point were
determined (Table II). The LBP level
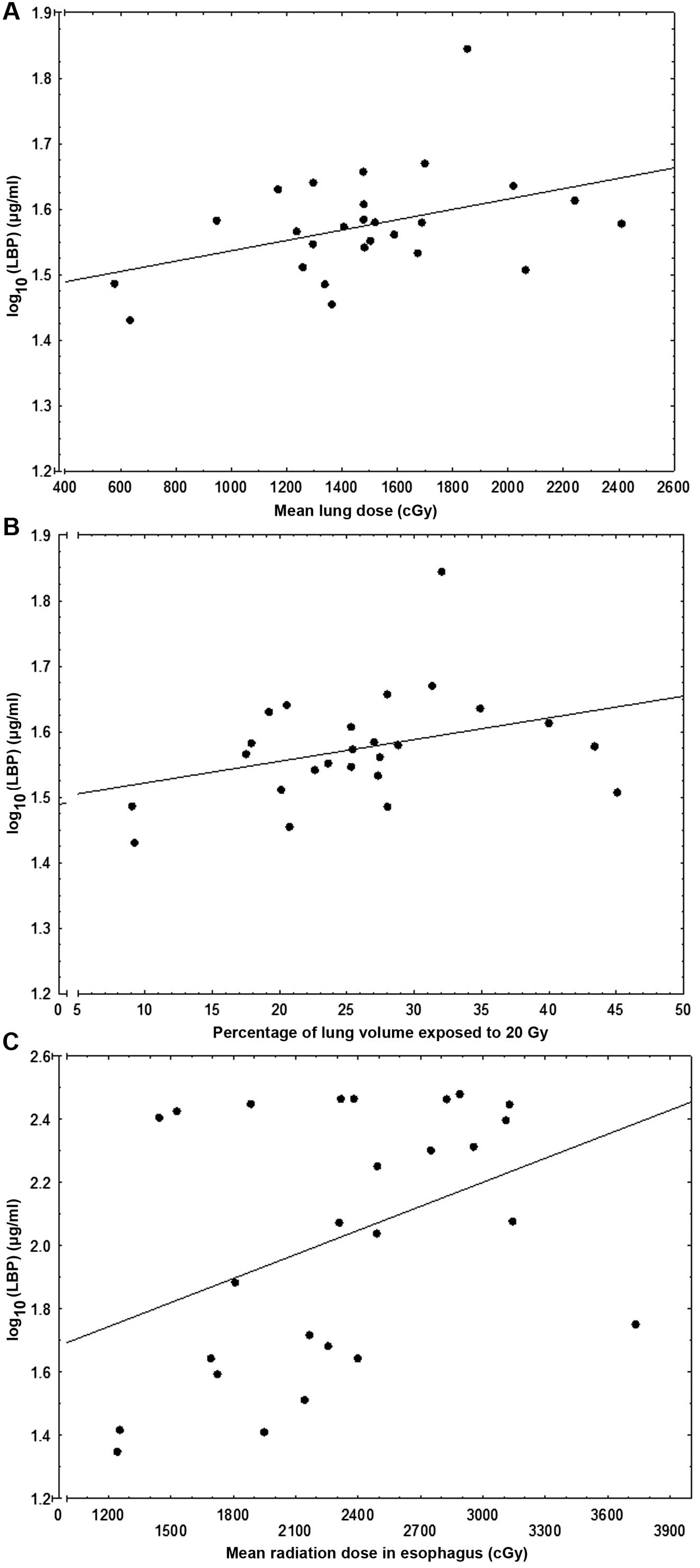
was significantly positively correlated with the mean lung
radiation dose (MLD) (r=0.409; P=0.038; Fig. 1A) and there was borderline, but
statistically insignificant correlation with V20 (r=0.3536;
P=0.076; Fig. 1B). The CRP level
correlated positively with the MLD to the esophagus
(r=0.404; P=0.041; Fig. 1C).
Only three study patients developed RILT more severe than grade 1
on the Radiation Therapy Oncology Group (RTOG) scale (20); therefore, assessments of inflammatory
markers in association with RILT were not possible in the present
study.
 | Table II.Correlation analysis: Dosimetric
parameters of radiotherapy administered to the study patients
correlated with cytokine levels measured at the 40-Gy time
point. |
Table II.
Correlation analysis: Dosimetric
parameters of radiotherapy administered to the study patients
correlated with cytokine levels measured at the 40-Gy time
point.
| Parameter | Lung: V5 (%) | Lung: V20 (%) | Mean lung dose
(Gy) | Mean esophagus dose
(Gy) | GTV
(cm3) | CTV
(cm3) | PTV
(cm3) |
|---|
| Median (25–75%) | 57.55 | 26.21 | 14.88 | 23.13 | 16.46 | 124.35 | 294.00 |
|
| (49.13–60.53) | (20.52–28.80) | (12.95–16.88) | (18.09–28.25) | (0.00–31.42) | (81.90–213.00) | (216.00–464.40) |
| Correlation with
inflammatory marker [P-value] |
| IL-6
(pg/ml) log10 | r=0.082
[P=0.688] | r=0.123
[P=0.546] | r=0.071
[P=0.730] | r=−0.040
[P=0.830] | r=−0.210
[P=0.306] | r=0.045
[P=0.825] | r=0.088
[P=0.668] |
| TNF-α
(pg/ml) log10 | r=0.194
[P=0.342] | r=0.123
[P=0.549] | r=0.044
[P=0.830] | r=0.087
[P=0.671] | r=−0.200
[P=0.320] | r=0.017
[P=0.933] | r=0.093
[P=0.651] |
| LBP
(µg/ml) log10 | r=0.285
[P=0.158] | r=0.353
[P=0.076] | r=0.409
[P=0.038] | r=−0.02
[P=0.892] | r=−0.010
[P=0.953] | r=0.141
[P=0.491] | r=0.134
[P=0.511] |
| CRP
(µg/ml) log10 | r=0.073
[P=0.722] | r=0.225
[P=0.267] | r=0.206
[P=0.311] | r=0.404
[P=0.041] | r=0.211
[P=0.309] | r=0.300
[P=0.136] | r=0.263
[P=0.193] |
Discussion
The median LBP level was identified to be positively
correlated with the radiation dose to the pulmonary tissue, which
suggests that LBP assessments may warrant further investigations on
radiotoxicity in NSCLC patients. An increased level of CRP seems to
be associated with radiotoxicity to the esophagus, although cancer
patients with elevated serum CRP levels prior to receiving
radiotherapy hinder its utility for biodosimetry.
LBP belongs to the tubular lipid-binding protein
family. It is produced by lung parenchyma, hepatocytes (21) and salivary glands (22). An increased LBP level was shown to be
associated with worse outcomes of patients with infectious
pneumonitis (23). In patients
undergoing radiotherapy for NSCLC, the pretreatment levels of LBP
were not correlated with outcome, although that study did not
investigate the impact of RTx on LBP levels (12). However, in the current study serum LBP
levels were observed to increase with V20 and MLD dosimetric
parameters, although these parameters were well within the dose
constraints described in the NCCN guidelines (16). This finding may be attributed to a
localized inflammatory process in the lungs that may not be
obvious, with radiation as the trigger. It is hypothesized that the
increase in LBP is regulated via IL-6 secretion. However, as blood
samples were collected just before the patient underwent the daily
RTx fraction, the serum levels of IL-6 may have already dropped,
accounting for measured IL-6 concentrations that were not
significantly increased (24).
The next stage of the current study, which will be
conducted when the study cohort increases to a final size of 52
participants and when there is a 3-year follow-up, whether the
serum level of LBP measured during RTx is correlated with clinical
toxicity and late adverse effects will be evaluated. This analysis
could not be performed in this pilot study as only a few patients
presented with RILT during treatment.
Previous studies reported that the levels of IL-6
and TNF-α cytokines were correlated with RILT. The RTOG 91–03 study
(10) reported finding these
correlations after an acquired total dose of 10 or 20 Gy. IL-6 was
also found to be elevated before RTx and in patients with late
manifestations of RILT (8). The
predominant problem with IL-6 as a biomarker may be that it is a
pleiotropic cytokine, which regulates many inflammatory processes,
including induction of the acute phase reaction to injuries and
infections. Therefore, IL-6 is a sensitive, but non-specific marker
of the inflammatory process and potentially accounted for the
significantly elevated pre-RTx levels of IL-6, as well as those of
TNF-α and CRP in the study patients with NSCLC, compared to the
control participants.
There were certain limitations of the present study.
The patients enrolled in this study were eligible for either
sequential chemoradiotherapy or adjuvant RTx. Although the current
standard of radical treatment for locally advanced NSCLC is
concurrent chemoradiotherapy (25),
~60% of patients are deemed ineligible (26). The selection criteria of the present
study may have led to bias, because the study patients were limited
to those who underwent sequential chemotherapy or adjuvant RTx.
However, as a result of our selection criteria, the effects of
chemotherapy on the early inflammatory response were avoided. The
absence of a temporal trend in LBP levels during RTx (P=0.167)
suggest that the significant correlation of LBP with MLD was not
due to disease progression or the initial disease stage, but rather
was associated with the actual radiation dose delivered to
pulmonary tissue in a specific volume.
In conclusion, the present study found that LBP
levels are associated with the radiation dose delivered to the
pulmonary tissue and may have the potential to be markers of RILT.
A larger study group and longer follow-up time are required to
establish whether the observed differences have clinical
implications.
Acknowledgements
The present study was funded by the National Science
Center (grant no. 2012/05/N/NZ5/02621) and the INTER program of the
FNP (grant no. 127/UD/SKILLS/2015).
References
|
1
|
Garipagaoglu M, Munley MT, Hollis D,
Poulson JM, Bentel GC, Sibley G, Anscher MS, Fan M, Jaszczak RJ,
Coleman RE, et al: The effect of patient-specific factors on
radiation-induced regional lung injury. Int J Radiat Oncol Biol
Phys. 45:331–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Claude L, Pérol D, Ginestet C, Falchero L,
Arpin D, Vincent M, Martel I, Hominal S, Cordier JF and Carrie C: A
prospective study on radiation pneumonitis following conformal
radiation therapy in non-small-cell lung cancer: Clinical and
dosimetric factors analysis. Radiother Oncol. 71:175–181. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong FM, Ao X, Wang L and Lawrence TS: The
use of blood biomarkers to predict radiation lung toxicity: A
potential strategy to individualize thoracic radiation therapy.
Cancer Control. 15:140–150. 2008.PubMed/NCBI
|
|
4
|
Abratt RP and Morgan GW: Lung toxicity
following chest irradiation in patients with lung cancer. Lung
Cancer. 35:103–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marks LB, Bentzen SM, Deasy JO, Kong FM,
Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV,
Timmerman RD, et al: Radiation dose-volume effects in the lung. Int
J Radiat Oncol Biol Phys. 76:(Suppl). S70–S76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubin P, Finkelstein J and Shapiro D:
Molecular biology mechanisms in the radiation induction of
pulmonary injury syndromes: Interrelationship between the alveolar
macrophage and the septal fibroblast. Int J Radiat Oncol Biol Phys.
24:93–101. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morgan GW and Breit SN: Radiation and the
lung: A reevaluation of the mechanisms mediating pulmonary injury.
Int J Radiat Oncol Biol Phys. 31:361–369. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siva S, MacManus M, Kron T, Best N, Smith
J, Lobachevsky P, Ball D and Martin O: A pattern of early
radiation-induced inflammatory cytokine expression is associated
with lung toxicity in patients with non-small cell lung cancer.
PLoS One. 9:e1095602014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaturvedi AK, Caporaso NE, Katki HA, Wong
HL, Chatterjee N, Pine SR, Chanock SJ, Goedert JJ and Engels EA:
C-reactive protein and risk of lung cancer. J Clin Oncol.
28:2719–2726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hartsell WF, Scott CB, Dundas GS,
Mohiuddin M, Meredith RF, Rubin P and Weigensberg IJ: Can serum
markers be used to predict acute and late toxicity in patients with
lung cancer? Analysis of RTOG 91–03. Am J Clin Oncol. 30:368–376.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dehing-Oberije C, Aerts H, Yu S, De
Ruysscher D, Menheere P, Hilvo M, van der Weide H, Rao B and Lambin
P: Development and validation of a prognostic model using blood
biomarker information for prediction of survival of non-small-cell
lung cancer patients treated with combined chemotherapy and
radiation or radiotherapy alone (NCT00181519, NCT00573040, and
NCT00572325). Int J Radiat Oncol Biol Phys. 81:360–368. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walker MJ, Zhou C, Backen A, Pernemalm M,
Williamson AJ, Priest LJ, Koh P, Faivre-Finn C, Blackhall FH, Dive
C, et al: Discovery and validation of predictive biomarkers of
survival for non-small cell lung cancer patients undergoing radical
radiotherapy: Two proteins with predictive value. E Bio Medicine.
2:841–850. 2015.
|
|
13
|
Chen Y, Williams J, Ding I, Hernady E, Liu
W, Smudzin T, Finkelstein JN, Rubin P and Okunieff P: Radiation
pneumonitis and early circulatory cytokine markers. Semin Radiat
Oncol. 12(Suppl 1): 26–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazeron R, Etienne-Mastroianni B, Pérol D,
Arpin D, Vincent M, Falchero L, Martel-Lafay I, Carrie C and Claude
L: Predictive factors of late radiation fibrosis: A prospective
study in non-small cell lung cancer. Int J Radiat Oncol Biol Phys.
77:38–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wrona A and Jassem J: The new TNM
classification in lung cancer. Pneumonol Alergol Pol. 78:407–417.
2010.(In Polish). PubMed/NCBI
|
|
16
|
Soriano JB, Lamprecht B, Ramírez AS,
Martinez-Camblor P, Kaiser B, Alfageme I, Almagro P, Casanova C,
Esteban C, Soler-Cataluña JJ, et al: Mortality prediction in
chronic obstructive pulmonary disease comparing the GOLD 2007 and
2011 staging systems: A pooled analysis of individual patient data.
Lancet Respir Med. 3:443–450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miedinger D, Neukomm E, Chhajed PN,
Schnyder A, Naef M, Ackermann M and Leuppi JD: The use of the
Asthma Control Test in general practice and its correlation with
asthma control according to the GINA guidelines. Curr Med Res Opin.
27:2301–2308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Comprehensive Cancer Network
(NCCN), . NCCN Guidelines®. NCCN; Fort Washington, PA, USA:
2015
|
|
19
|
No authors listed: Prescribing, recording,
and reporting photon-beam intensity-modulated radiation therapy
(IMRT): Contents. J ICRU. 10(NP)2010.
|
|
20
|
Faria SL, Aslani M, Tafazoli FS, Souhami L
and Freeman CR: The challenge of scoring radiation-induced lung
toxicity. Clin Oncol (R Coll Radiol). 21:371–375. 2009. View Article : Google Scholar
|
|
21
|
Wolk K, Witte E, Hoffmann U, Doecke WD,
Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM and Sabat
R: IL-22 induces lipopolysaccharide-binding protein in hepatocytes:
A potential systemic role of IL-22 in Crohn's disease. J Immunol.
178:5973–5981. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abdolhosseini M, Sotsky JB, Shelar AP,
Joyce PB and Gorr SU: Human parotid secretory protein is a
lipopolysaccharide-binding protein: Identification of an
anti-inflammatory peptide domain. Mol Cell Biochem. 359:1–8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Villar J, Pérez-Méndez L, Espinosa E,
Flores C, Blanco J, Muriel A, Basaldúa S, Muros M, Blanch L,
Artigas A, et al: GRECIA and GEN-SEP Groups: Serum
lipopolysaccharide binding protein levels predict severity of lung
injury and mortality in patients with severe sepsis. PLoS One.
4:e68182009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dentener MA, Vreugdenhil AC, Hoet PH,
Vernooy JH, Nieman FH, Heumann D, Janssen YM, Buurman WA and
Wouters EF: Production of the acute-phase protein
lipopolysaccharide-binding protein by respiratory type II
epithelial cells: Implications for local defense to bacterial
endotoxins. Am J Respir Cell Mol Biol. 23:146–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aupérin A, Le Péchoux C, Rolland E, Curran
WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus
R, et al: Meta-analysis of concomitant versus sequential
radiochemotherapy in locally advanced non-small-cell lung cancer. J
Clin Oncol. 28:2181–2190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Ruysscher D, Botterweck A, Dirx M,
Pijls-Johannesma M, Wanders R, Hochstenbag M, Dingemans AM, Bootsma
G, Geraedts W, Simons J, et al: Eligibility for concurrent
chemotherapy and radiotherapy of locally advanced lung cancer
patients: A prospective, population-based study. Ann Oncol.
20:98–102. 2009. View Article : Google Scholar : PubMed/NCBI
|