Introduction
Liver cancer was the fourth most common cause of
mortality worldwide, and China accounted for ~53% of all liver
cancer-associated mortalities (1). The
incidence of liver cancer gradually increased in developing
(2) and developed (3) countries (4,5). In China,
>90% of patients with primary liver cancer presented with
hepatocellular carcinoma (HCC), which was the second-leading cause
of cancer-associated mortality influencing individuals of all ages
(6,7).
Although treatment and prevention strategies have been clinically
developed, the global, overall survival of HCC patients remained
particularly poor (8,9). The majority of the poor prognoses were
associated with recurrence and metastasis following treatment,
including curative resection (10,11).
Therefore, the mechanisms of liver cancer recurrence and
intervention strategies for liver cancer recurrence and metastasis
are required, thus future investigations with larger sample sizes
are required.
Blueberry is a member of the Vacciniaceae family
(genus, Vaccinium). In the Korean Peninsula and the
Northeast of China, blueberries are grown widely and are commonly
administered as a traditional Chinese therapeutic agent for
treating inflammatory diseases. Blueberry anthocyanins (BAs) were
main medicinal active ingredient. Previous studies indicated that
BA inhibited tumor growth and induced apoptosis of tumor cells in
breast (12), lung (13), and colorectal (14) cancer, amongst others. In addition, BA
was reported to be involved in the control of obesity (15) and diabetes mellitus (16), prevention of cardiovascular disease
(17), vision augmentation (18) and cerebral function (19).
In the present study, different concentrations of
blueberry juice were administered to rats by gastric gavage and,
after 7 days, blood serum was obtained for co-culture with HEPG2
cells. Proliferation, migration, invasion, cell cycle and apoptosis
were detected in HEPG2 cells to investigate the effects of
blueberry on the proliferation, apoptosis and histone acetylation
in HEPG2 cells. The aim of the present study was to establish an
anticancer therapeutic agent for the clinical treatment and
prevention of liver cancer.
Materials and methods
Fresh blueberry juice
Rabbiteye blueberries were obtained from the
Blueberry Production Field (Ma-Jiang, China) of the Guizhou Academy
of Sciences (Guiyang, China) and stored at −20°C. The fresh juice
was prepared from the crude blueberries by homogenization, and
diluted in physiological saline to a final volume of 1 ml [original
blueberry juice (100%) contained 100 g blueberry, which was further
diluted to 25 and 50% in physiological saline].
Animals
Ethical approval was obtained for the animal
experiments from Guizhou Medical University (Guiyang, China), and
animal treatment was in accordance with the Guidelines for Animal
Care and Use.
Twelve, male, specific pathogen-free (SPF) Wistar
rats (weight, 200±20 g) were obtained from the animal center of
Guizhou Medical University (Guiyang, China) and maintained in an
SPF room, at 25°C with a 12-h of light/dark cycle. The rats had
free access to food and water, and were housed separately. The 12
rats were randomly divided into four equal groups as follows:
Low-dose group, fed 25% blueberry juice, which was from the
original blueberry juice (100%) diluted in saline; moderate-dose
group, fed 50% blueberry juice; high-dose group, fed 100% blueberry
juice; the control group, fed 1 ml physiological saline. Gastric
perfusion was performed twice per day at 9:00 a.m. and 9:00 p.m.
for 7 days in total. One additional gastric perfusion was performed
2 h after the final gastric perfusion, in order to prevent vomiting
and maintain the blueberry juice concentration. One hour after the
repeated gastric perfusion, 10-ml blood samples were collected from
the femoral artery of the rats and centrifuged at 1,500 × g for 15
min at 4°C. The supernatants obtained from blood samples of rats in
the same group were combined. The complement in the blood serum was
inactivated at 56°C for 30 min using a water bath and a 0.22-µm
filter (Merck Millipore, Darmstadt, Germany) was used for removing
bacteria. The samples of blood serum from rats that were fed
different dosages of blueberry juice were stored at −80°C. The rats
were sacrificed in saturated CO2.
Serum preparation
Serum from the four groups was diluted with 10%
Dulbecco's modified Eagle's medium (DMEM) containing Gibco 10%
fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Shanghai,
China) for co-culture with the HEPG2 cells.
HEPG2 cell culture
HEPG2 cells were obtained from American Type Culture
Collection (ATCC) and cultured in DMEM containing 10% FBS in 37°C,
5% CO2 and saturated humidity. When the cell confluence
reached 90%, the HEPG2 cells were digested with 0.25% (w/v) trypsin
containing 0.53 mM EDTA (Thermo Fisher Scientific, Inc.) and
subcultured as described above.
MTT detection of HEPG2 cell
proliferation
HEPG2 cells in the logarithmic growth phase were
digested with trypsin as a single cell suspension (density,
1×105/ml) and resuspended with DMEM containing 10% FBS.
Cell suspension (100 µl) was added to each well of a 96-well plate
for 24 h until adhesion. The medium was removed from the 96-well
plate, and serum was added to each well, with 8 parallel
wells/group. The cells were then cultured for 48 and 72 h, and the
cultured supernatant was collected and stored at −20°C. The cells
were washed once with 3 ml DMEM and centrifuged to discard the
DMEM. Subsequently, 100 µl DMEM and 20 µl MTT (5 mg/ml;
Sigma-Aldrich China, Inc., Shanghai, China) were added and
incubated at 37°C for 4 h. Dimethyl sulphoxide (150 µl;
Sigma-Aldrich China, Inc.) was added and agitated for 15 sec in the
dark for dissolution. An enzyme labeling instrument was used to
detect the absorption (A) values at 492 nm. The MTT average value
of each group was obtained and the proliferation rate (%) was
calculated as follows:
Control group - treatment group/control group ×
100%
Transwell assay detection of migration
and invasion in HEPG2 cells
For detecting invasion, 4 µl Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) was placed in a Transwell
chamber (Corning Life Sciences, Shanghai, China) in each well.
HEPG2 cells were co-cultured at 37°C with DMEM containing 10% FBS
and 10% serum from the four groups. After 48 and 72 h, the medium
was replaced with DMEM without FBS or serum, and the HEPG2 cells
were cultured for a further 6 h at 37°C. The HEPG2 cells in the
logarithmic growth phase were digested with trypsin and the cell
density was adjusted to 1×105/ml by resuspension with
DMEM without FBS. The cell suspension (0.4 ml) was added to the
upper part of the chamber, and 0.6 ml DMEM containing 20% FBS
(Thermo Fisher Scientific, Inc.) was added to the lower part of the
chamber in a 24-well plate, with three parallel holes per group.
Subsequently (24 h), the Matrigel and the non-migrating cells in
the chambers were carefully cleaned. Phosphate-buffered saline
(PBS; Wuhan Boster Biological Technology, Ltd., Wuhan, China; 1 ml)
was added to the chambers for washing and was discarded. The cells
were fixed with 4% paraformaldehyde (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) for 15 min, then stained with
0.1% crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd.) solution at 25°C for 20–45 min. The cells were subsequently
washed twice with PBS and the stained cells were observed under a
microscope (BX51/BX51M; Olympus Corporation, Tokyo, Japan).
For detecting cell migration, the steps were the
same as for the detection of invasion, however, Matrigel was not
used.
Flow cytometry detection of the HEPG2
cell cycle and apoptosis
Following co-culture with serum from the four groups
for 48 and 72 h, the HEPG2 cells were digested with trypsin and
centrifuged at 1,000 × g for 5 min at 25°C. The supernatant was
discarded and the cells were washed twice with PBS. A further
centrifugation was performed (at 1,000 × g for 5 min at 25°C) to
remove the PBS. Precooled ethanol (0.6 ml) was added and the HEPG2
cells were placed on ice for 30 min. The cells were then
centrifuged at 1,000 × g for 5 min at 25°C. Subsequently, 0.05 ml
RNase A (10 mg/ml; Sigma-Aldrich China, Inc.) was added and
maintained at 25°C for 1 h. PBS (0.15 ml) and 0.2 ml propidium
iodide (Sigma-Aldrich China, Inc.) were then added to stain the
cells. The cell cycle and apoptosis were then analyzed by flow
cytometry (BD FACSCalibur; BD Biosciences).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed by SPSS 11.0 software (SPSS, Inc., Chicago, IL,
USA). Comparisons between groups were analyzed by Student's t-test
and P<0.05 was considered to indicate a statistically
significant difference.
Results
HEPG2 proliferation
Table I and Fig. 1 demonstrate the A-value and inhibition
rate of HEPG2 cells after co-culturing with the serums from the
different treatment groups for 48 and 72 h. Compared with the
control group at 48 h, the inhibition rates of HEPG2 cells in the
low- (21.97±8.54%), moderate- (20.33±10.60%) and high-
(20.05±12.85%) dosage groups were significantly lower (P<0.05).
However, there were no significant differences in HEPG2 cell
proliferation between the three blueberry treatment groups at 48 h.
Furthermore, the inhibition rate decreased as the concentration of
blueberry juice intake increased.
 | Table I.A-value and inhibition rate of HEPG2
cells following 48- and 72-h co-culturing with serum from rats fed
with different concentrations of blueberry juice. Data are
presented as means ± standard deviations. |
Table I.
A-value and inhibition rate of HEPG2
cells following 48- and 72-h co-culturing with serum from rats fed
with different concentrations of blueberry juice. Data are
presented as means ± standard deviations.
|
|
|
| Blueberry treatment
group |
|---|
|
|
|
|
|
|---|
| Variable | Co-culturing time
(h) | Control group | Low-dosage, 25% | Moderate-dosage,
50% | High-dosage,
100% |
|---|
| A-value | 48 | 0.58±0.19 |
0.46±0.07a |
0.41±0.12a |
0.39±0.05a |
|
| 72 | 0.63±1.77 |
0.43±0.10a |
0.40±0.22a |
0.38±0.20a |
| Inhibition rate
(%) | 48 | 100.00±0.00 |
21.97±8.54a |
21.33±10.60a |
20.05±12.85a |
|
| 72 | 100.00±0.00 |
20.58±9.67a |
22.03±8.14a |
21.90±10.03a |
Compared with the control group at 72 h, the
inhibition rates of HEPG2 cells in the low- (20.58±9.67%),
moderate- (21.03±8.14%) and high- (21.90±10.3%) dosage groups were
significantly lower (P<0.05). However, there were no significant
differences in HEPG2 cell proliferation between the three blueberry
treatment groups at 72 h. Furthermore, the inhibition rate
decreased as the concentration of blueberry juice intake
increased.
In addition, the inhibition rates between the
different groups at 48 and 72 h were not statistically
different.
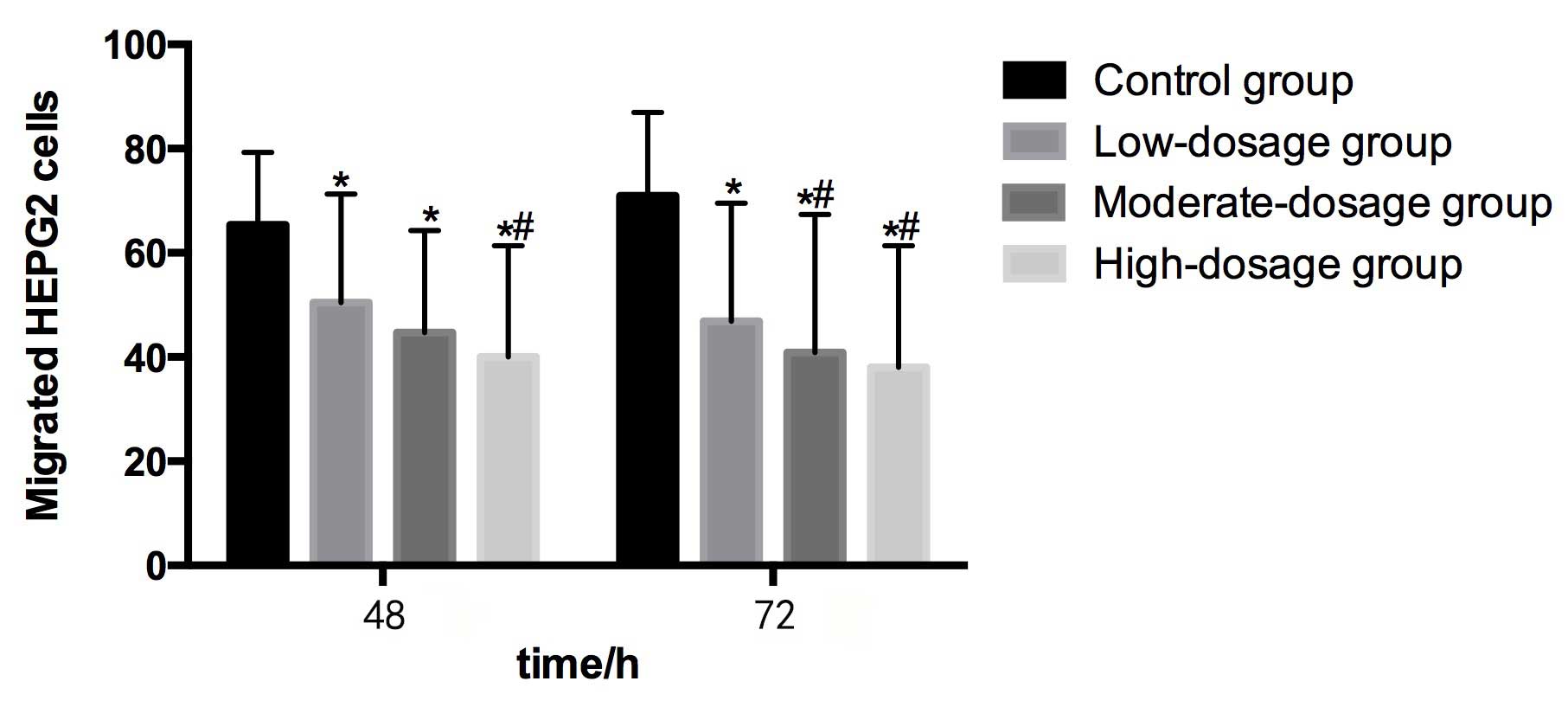
Migration and invasion in HEPG2
cells
Table II demonstrates
the number of migrated or transmembrane HEPG2 cells following
co-culturing with serums for 48 and 72 h, which were obtained from
rats that were fed different concentrations of blueberry juice.
 | Table II.Number of migrated or transmembrane
HEPG2 cells following 48- and 72-h co-culturing with serum from
rats fed with different concentrations of blueberry juice. Data are
presented as means ± standard deviations. |
Table II.
Number of migrated or transmembrane
HEPG2 cells following 48- and 72-h co-culturing with serum from
rats fed with different concentrations of blueberry juice. Data are
presented as means ± standard deviations.
|
|
|
| Blueberry treatment
group |
|---|
|
|
|
|
|
|---|
| Process | Co-culturing time
(h) | Control group | Low-dosage, 25% | Moderate-dosage,
50% | High-dosage,
100% |
|---|
| Migration | 48 | 65.38±13.94 |
50.42±20.90a |
44.67±19.58a |
40.03±21.36a,b |
|
| 72 | 70.93±16.03 |
46.89±22.64a |
40.91±26.50a,b |
38.05±23.36a,b |
| Invasion | 48 | 110.82±25.54 |
91.44±31.26a |
88.47±28.95a |
83.39±34.16a,b |
|
| 72 | 116.85±30.62 |
86.59±36.80a |
82.54±29.47a |
80.10±31.34a |
Compared with the control group (65.38±13.94) at 48
h, the numbers of migrated HEPG2 cells in the low- (50.42±20.90),
moderate- (44.67±19.58) and high- (40.03±21.36) dosage groups were
significantly lower (P<0.05). The number of metastatic HEPG2
cells in the high-dosage group was significantly lower than that in
the low-dosage group (P<0.05). Furthermore, the number of
metastatic HEPG2 cells decreased as the concentration of blueberry
juice intake increased (Table II and
Fig. 2).
Compared with the control group (70.93±16.03) at 72
h, the numbers of migrated HEPG2 cells in the low- (46.89±22.64),
moderate- (40.91±26.50) and high- (38.05±23.36) dosage groups were
significantly lower (P<0.05). The numbers of metastatic HEPG2
cells in the moderate- and high-dosage groups were significantly
lower than in the control group (P<0.05). The number of
metastatic HEPG2 cells decreased as the concentration of blueberry
juice intake increased (Table II and
Fig. 2).
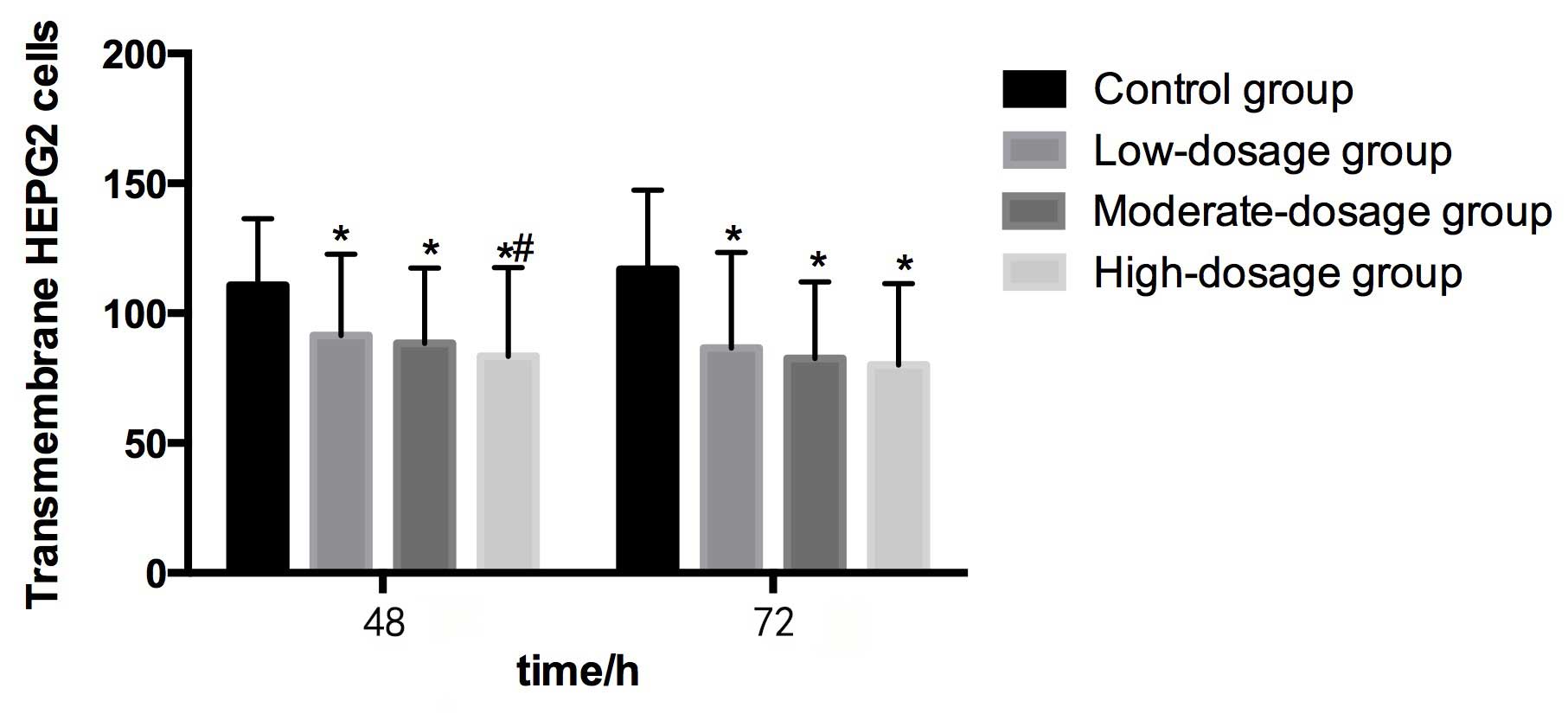
Compared with the control group (110.82±25.54) at 48
h, the numbers of transmembrane HEPG2 cells in the low-
(91.44±31.26), moderate- (88.47±28.95) and high- (83.39±34.16)
dosage groups were significantly lower (P<0.05). The number of
transmembrane HEPG2 cells in the high-dosage group was
significantly lower than in the control group (P<0.05). The
transmembrane HEPG2 cell number tended to decrease as the
concentration of blueberry juice intake increased (Table II and Fig.
3).
Compared with the control group (116.85±30.62) at 72
h, the numbers of transmembrane HEPG2 cells in the low-
(86.59±36.80), moderate- (82.54±29.47) and high- (80.10±31.34)
dosage groups were significantly lower (P<0.05). However, no
significant differences were identified in transmembrane HEPG2 cell
numbers among the three groups that were administered blueberry
juice. The transmembrane HEPG2 cell number tended to decrease as
the concentration of blueberry juice intake increased (Table II and Fig.
3).
Flow cytometric detection of the HEPG2
cell cycle and apoptosis
Table III and
Fig. 4 demonstrate the HEPG2 cell
cycle (in G2/M) subsequent to co-culturing for 48 and 72
h with serum from rats that were administered different
concentrations of blueberry juice.
 | Table III.HEPG2 cell cycle analysis following
48- and 72-h co-culturing with serum from rats fed with different
concentrations of blueberry juice. Data are presented as means ±
standard deviations. |
Table III.
HEPG2 cell cycle analysis following
48- and 72-h co-culturing with serum from rats fed with different
concentrations of blueberry juice. Data are presented as means ±
standard deviations.
|
|
|
| Blueberry treatment
group |
|---|
|
|
|
|
|
|---|
| Stage | Co-culturing time
(h) | Control group | Low-dosage,
25% | Moderate-dosage,
50% | High-dosage,
100% |
|---|
|
G0/G1 (%) | 48 | 65.58±2.68 | 61.40±2.80 | 73.02±1.48 | 76.21±2.53 |
|
| 72 | 59.37±3.90 | 68.14±2.30 | 74.95±3.13 | 69.62±2.98 |
| S (%) | 48 | 20.96±2.05 | 31.09±2.46 | 23.17±1.55 | 22.89±2.60 |
|
| 72 | 25.24±3.07 | 18.79±2.33 | 17.20±1.98 | 23.95±2.65 |
| G2/M
(%) | 48 | 13.96±2.73 |
7.53±1.12a |
4.60±1.08a |
1.52±0.97a,b |
|
| 72 | 17.53±3.54 |
13.82±2.04a |
7.48±1.40a |
6.30±1.52a,b |
Compared with the control group (13.96±2.73%) at 48
h, the numbers of HEPG2 cells at the G2/M stage in the
low- (7.53±1.12%), moderate- (4.60±1.08)% and high- (1.52±0.97%)
dosage groups were significantly lower (P<0.05). The number of
HEPG2 cells at the G2/M stage in the high-dosage group
was significantly lower than in the low-dosage group (P<0.05).
The number of HEPG2 cells at the G2/M stage decreased as
the concentration of blueberry juice intake increased.
Compared with the control group (17.53±3.54%) at 72
h, the numbers of HEPG2 cells at the G2/M stage in the
low- (13.82±2.04%), moderate- (7.48±1.40%) and high- (6.30±1.52%)
dosage groups were significantly lower (P<0.05). The number of
HEPG2 cells at the G2/M stage in the high-dosage group
was significantly lower than in the low-dosage group (P<0.05).
The number of HEPG2 cells at the G2/M stage decreased as
the concentration of blueberry juice intake increased.
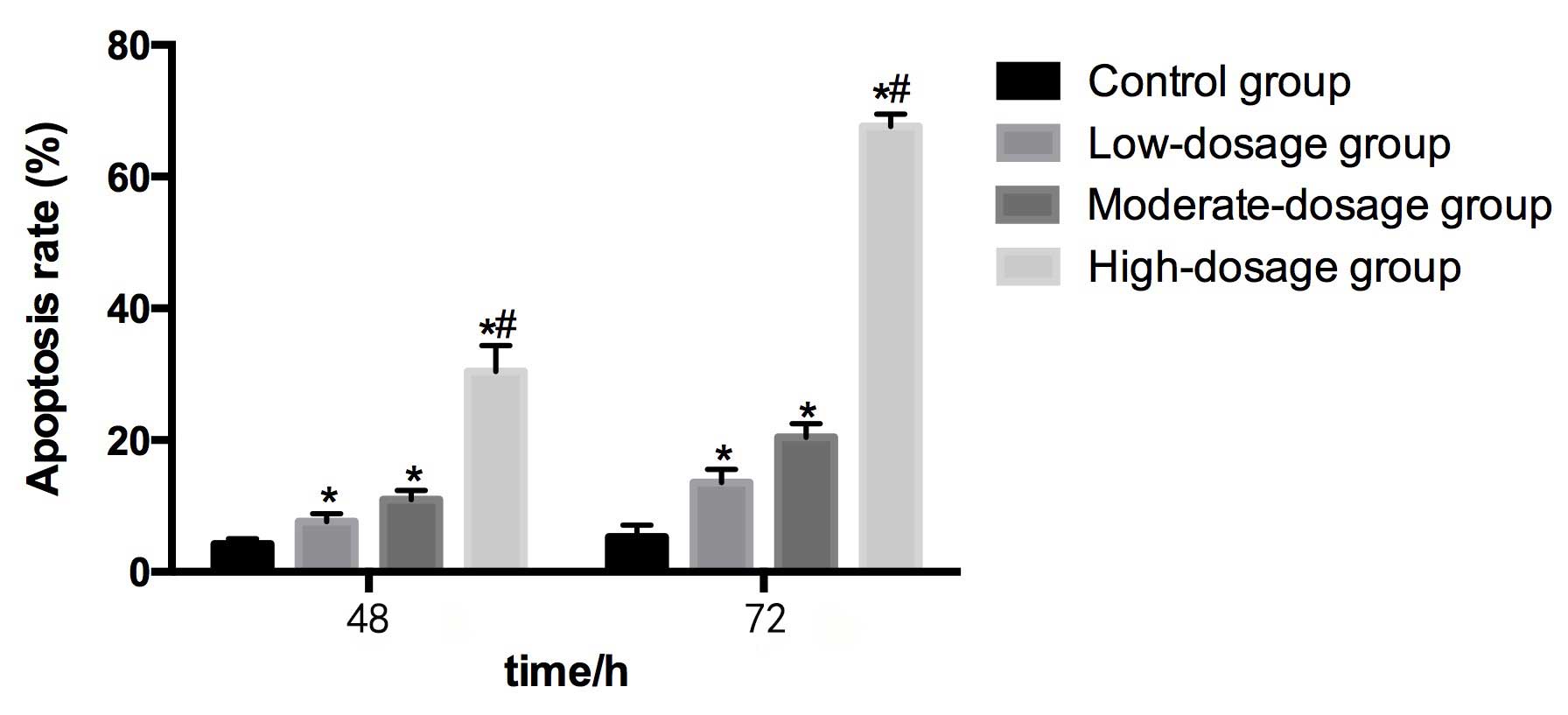
Table IV and Fig. 5 demonstrate the apoptosis rate of HEPG2
cells subsequent to co-culturing for 48 and 72 h with serum from
rats that were administered different concentrations of blueberry
juice.
 | Table IV.Apoptosis rate of HEPG2 cell
following 48- and 72-h co-culturing with serum from rats fed with
different concentrations of blueberry juice. Data are presented as
means ± standard deviations. |
Table IV.
Apoptosis rate of HEPG2 cell
following 48- and 72-h co-culturing with serum from rats fed with
different concentrations of blueberry juice. Data are presented as
means ± standard deviations.
|
|
|
| Blueberry treatment
group |
|---|
|
|
|
|
|
|---|
| Co-culturing time
(h) | Control group | Low-dosage,
25% | Moderate-dosage,
50% | High-dosage,
100% |
|---|
| 48 | 4.25±0.78 |
7.66±1.14a |
10.96±1.38a |
30.42±3.95a,b |
| 72 | 5.32±1.79 |
13.57±1.97a |
20.42±2.06a,b |
67.64±1.85a,b |
Compared with the control group (4.25±0.78%) at 48
h, the apoptosis rates in the low- (7.66±1.14%), moderate-
(10.96±1.38%) and high- (30.42±3.95%) dosage groups were
significantly higher (P<0.05). The apoptosis rate in the
high-dosage group was significantly greater than in the low-dosage
group. The apoptosis rate increased as the concentration of
blueberry juice intake increased.
Compared with the control group (5.32±1.79%) at 72
h, the apoptosis rates in the low- (13.57±1.97%), moderate-
(20.42±2.06%) and high- (67.64±1.85%) dosage groups were
significantly higher (P<0.05). The apoptosis rate in the
high-dosage group was significantly higher than in the low-dosage
group. The apoptosis rate increased as the concentration of
blueberry juice intake increased.
Discussion
Blueberries contain numerous components, some of
which have been reported to facilitate with the prevention of
different diseases. For example, anthocyanin is a main component of
blueberry, and the high anthocyanin content of blueberries was
found to be chemopreventive and exerted therapeutic effects against
breast cancer (19). Pterostilbene,
primarily found in blueberries, is an antioxidant that acts as an
effective anticancer agent in various common malignancies,
including breast and colon cancer (20). In addition, ellagic acid has been
associated with the prevention of oxidative DNA damage and
modulation of DNA repair gene expression (21). These components interact and influence
biological processes in the human body, resulting in the
potentially protective and preventive actions of blueberries.
In the present study, the blueberry components were
not detected and the important components were not extracted from
the fresh blueberries. However, fresh blueberry juice was fed to
the rats and the serum was collected. The serum from the
blueberry-fed rats was used for co-culturing with HEPG2 cells, and
the proliferation, invasion, migration, cell cycle and apoptosis in
HEPG2 cells was detected following culture with serums taken from
rats fed with different concentrations of blueberry juice. The
results indicated that the blueberry juice exerted significant
antitumor and therapeutic effects on HEPG2 cells. The different
concentrations of blueberry juice and varying treatment times
resulted in distinct influences on the invasion, migration,
proliferation, cell cycle and apoptosis in the HEPG2 cells.
Following co-culture with serum obtained from
blueberry-fed rats, the inhibition rates of HEPG2 cells in the
low-, moderate- and high-dosage groups were significantly lower
than in the control group, at 48 and 72 h (P<0.05). The migrated
and transmembrane HEPG2 cells in the three treatment groups were
significantly lower than in the control group, at 48 and 72 h
(P<0.05). The number of migrated HEPG2 cells in the high-dosage
group was lower than in the low-dosage group at 48 h, and that of
the high- and moderate-dosage groups were significantly lower than
in the low-dosage groups at 72 h (P<0.05). The number of
transmembrane HEPG2 cells in the high-dosage group was
significantly lower than in the low-dosage group at 48 h
(P<0.05). Furthermore, the numbers of HEPG2 cells at the
G2/M stage in the treatment groups were significantly
lower than in the control group, and the number of HEPG2 cells at
the G2/M stage in the high-dosage group was
significantly lower than in the low-dosage group at 48 and 72 h
(P<0.05). The apoptosis rates of the treatment groups were
significantly higher than those in the control group, and the
apoptosis rate in the high-dosage group was significantly higher
than that in the low-dosage group at 48 and 72 h (P<0.05).
In the study by Faria et al (12) the anthocyanins and anthocyanin-pyruvic
acid adducts were extracted from blueberries, and demonstrated
anticancer properties in breast cancer cell lines. Similar results
were observed by Li et al (22)
and Lu et al (23). The
majority of previous studies were based on the extraction of key
components of blueberries; however, there are fewer studies with
the comprehensive detection of their (blueberry components or the
original blueberry juice) influence on HEPG2 cells. There were,
however, certain limitations of the present study as follows: No
specific components were extracted from the blueberries. The
blueberry juice was diluted to different concentrations to feed the
rats; however, it was the serum of the rats, and not the blueberry
juice directly, that was co-cultured with the HEPG2 cells.
Furthermore, the treatment times were short and, therefore, did not
provide data on the long-term therapeutic and protective effects of
blueberries on HCC or HEPG2 cells. Thus, the detailed effects of
blueberry on HCC or other types of cancer require further clinical
investigation.
In conclusion, the present study evaluated common
processes that are influenced by blueberry components, including
migration, invasion, proliferation, the cell cycle and apoptosis.
These influences indicate the protective and therapeutic effects of
blueberries on HCC, and their antitumor effects on HEPG2 cells.
References
|
1
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Estimates of the worldwide mortality from 25 cancers in 1990. Int J
Cancer. 83:18–29. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang ZY: Hepatocellular carcinoma - cause,
treatment and metastasis. World J Gastroenterol. 7:445–454. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB and Mason AC: Rising incidence
of hepatocellular carcinoma in the United States. N Engl J Med.
340:745–750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taylor-Robinson SD, Foster GR, Arora S,
Hargreaves S and Thomas HC: Increase in primary liver cancer in the
UK, 1979-94. Lancet. 350:1142–1143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tabor E: Hepatocellular carcinoma: global
epidemiology. Dig Liver Dis. 33:115–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Li L and Lu F: Mortality of
primary liver cancer in China from 1990 through 1992. Zhonghua
Zhong Liu Za Zhi. 21:245–249. 1999.(In Chinese). PubMed/NCBI
|
|
7
|
Chen JG and Zhang SW: Liver cancer
epidemic in China: Past, present and future. Semin Cancer Biol.
21:59–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang ZY, Yu YQ, Zhou XD, Ma ZC and Wu ZQ:
Progress and prospects in hepatocellular carcinoma surgery. Ann
Chir. 52:558–563. 1998.PubMed/NCBI
|
|
9
|
Jiang E, Shangguan AJ, Chen S, Tang L,
Zhao S and Yu Z: The progress and prospects of routine prophylactic
antiviral treatment in hepatitis B-related hepatocellular
carcinoma. Cancer Lett. 379:262–267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Samiei M and Waghorne CG: Clonal selection
within metastatic SP1 mouse mammary tumors is independent of
metastatic potential. Int J Cancer. 47:771–775. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang Z, Zhou X, Lin Z, Yang B, Ma Z, Ye S,
Wu Z, Fan J, Liu Y, Liu K, et al: Surgical treatment of
hepatocellular carcinoma and related basic research with special
reference to recurrence and metastasis. Chin Med J (Engl).
112:887–891. 1999.PubMed/NCBI
|
|
12
|
Faria A, Pestana D, Teixeira D, de Freitas
V, Mateus N and Calhau C: Blueberry anthocyanins and pyruvic acid
adducts: anticancer properties in breast cancer cell lines.
Phytother Res. 24:1862–1869. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang
CL and Hsieh YS: Mulberry anthocyanins, cyanidin 3-rutinoside and
cyanidin 3-glucoside, exhibited an inhibitory effect on the
migration and invasion of a human lung cancer cell line. Cancer
Lett. 235:248–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang SY, Seeram NP, Nair MG and Bourquin
LD: Tart cherry anthocyanins inhibit tumor development in Apc(Min)
mice and reduce proliferation of human colon cancer cells. Cancer
Lett. 194:13–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu T, Qi X, Liu Y, Guo J, Zhu R, Chen W,
Zheng X and Yu T: Dietary supplementation with purified mulberry
(Morus australis Poir) anthocyanins suppresses body weight gain in
high-fat diet fed C57BL/6 mice. Food Chem. 141:482–487. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghosh D and Konishi T: Anthocyanins and
anthocyanin-rich extracts: role in diabetes and eye function. Asia
Pac J Clin Nutr. 16:200–208. 2007.PubMed/NCBI
|
|
17
|
Toufektsian MC, de Lorgeril M, Nagy N,
Salen P, Donati MB, Giordano L, Mock HP, Peterek S, Matros A,
Petroni K, et al: Chronic dietary intake of plant-derived
anthocyanins protects the rat heart against ischemia-reperfusion
injury. J Nutr. 138:747–752. 2008.PubMed/NCBI
|
|
18
|
Tsuda T: Dietary anthocyanin-rich plants:
biochemical basis and recent progress in health benefits studies.
Mol Nutr Food Res. 56:159–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeyabalan J, Aqil F, Munagala R and Gupta
R: Chemopreventive and therapeutic activity of high
anthocyanin-content blueberry against estrogen-mediated breast
cancer. Cancer Res. 73:37052013. View Article : Google Scholar
|
|
20
|
McCormack D and McFadden D: Pterostilbene
and cancer: current review. J Surg Res. 173:e53–e61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aiyer HS, Vadhanam MV, Stoyanova R, Caprio
GD, Clapper ML and Gupta RC: Dietary berries and ellagic acid
prevent oxidative DNA damage and modulate expression of DNA repair
genes. Int J Mol Sci. 9:327–341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li YW, Wang D, Li XG and Jin Y:
Anthocyanins extracted from chinese blueberry and its anticancer
effects on HepG2 cells. Adv Mat Res 887–888. 592–595. 2014.
|
|
23
|
Lu YC, Liu YX, Wu W, Zhou F, Ji BP and Su
CY: Preventive effect of blueberry polyphenols on oleic
acid-induced fat accumulation in HepG2 cells. Food Science.
32:308–312. 2011.(In Chinese).
|