Introduction
Target-controlled infusion (TCI) is an intravenous
administration system, which provides desired target plasma
concentrations of therapeutic agents and aims to maintain an
appropriate depth of anesthesia (1–5). TCI has
become increasingly popular in clinical practice, due to its
ability to maintain more consistent plasma concentrations with
fewer fluctuations (6), the smooth
process of induction (7) and easily
adjustable depth of anesthesia (8), as
well as more predictable recovery time (9). The Marsh pharmacokinetic parameters
(10) that are incorporated into the
Diprifusor TCI system were derived from a relatively small number
of healthy individuals without organs dysfunction (11). These parameters have been proven to
provide a stable blood-therapeutic agent concentration for propofol
induction and maintenance of anesthesia in patients without organ
dysfunction (10,12–14).
Propofol is widely administered in clinical practice
for induction and maintenance of anesthesia due to its rapid onset
of action, large volume of distribution and high-clearance rate
(15–17). The pharmacokinetics of propofol are
dependent on the liver in multiple ways. Previous studies
demonstrated that propofol could be viewed as an acceptable choice
for patients with liver dysfunction, as it was proven to be safe in
patients with moderate cirrhosis undergoing gastrointestinal
endoscopy (18,19), and displayed a protective,
antioxidant-like effect on liver damage and dysfunction, as well as
ischemic reperfusion injury in liver transplant recipients
(20,21). However, the free fraction of the
therapeutic agent in circulation depends on the liver's synthetic
ability to produce albumin (22) and
its clearance is also dependent on hepatic metabolism (23). Therefore, the actual propofol
concentrations that are administrated via Diprifusor TCI, where the
parameters are derived from healthy individuals, may be higher than
expected due to decreased hepatic function and should not be
overlooked in patients with hepatic insufficiency.
Thus, the metabolism of propofol is predominantly
reliant on the liver; therefore, the reliability of TCI of propofol
in patients with hepatic insufficiency remains largely unknown.
Whether TCI of propofol to 3 µg/ml, which is recommended to
patients without severe liver dysfunction, is suitable for patients
with liver dysfunction during induction and intubation remains
unclear. Thus, the purpose of the current study was to assess the
performance of induction, via hemodynamics and the depth of
anesthesia during TCI of propofol to 3 µg/ml, in patients with
varying degrees of liver dysfunction.
Materials and methods
Ethical approval
Ethical approval for the current study was provided
by the Ethics committee of the Third Affiliated Hospital, Sun
Yat-sen University (Guangzhou, China). Written informed consent was
obtained from all patients prior to commencing the investigations
(Trial registration no. ChiCTR-OCH-12002255).
Selection and description of
participants
Fifty-three (45 males and 7 females) consecutive
patients (aged, 18–65 years), with cirrhosis or hepatic carcinoma,
who were scheduled for elective liver transplantation, partial
hepatectomy or splenectomy from the Third Affiliated Hospital, Sun
Yat-sen University (Guangzhou, China), between June 2014 and June
2015 were recruited for this prospective observational study.
Exclusion criteria included a history of serious impairment in
respiratory, cardiovascular, renal and central nervous systems, and
long-term use of mental or neurological drugs.
Administration of anesthesia
No premedication was provided. Heart rate (HR),
peripheral arterial oxygen saturation (SpO2), invasive
arterial pressure and central venous pressure were continuously
monitored (IntelliVue MP60; Philips Medizin Systeme Boeblingen
GmbH, Boeblingen, Baden-Wurttemberg, Germany).
Prior to induction, patients were intravenously
administered with Plasma-Lyte A in order to maintain a steady state
from induction to the time just prior to commencing surgery.
General anesthesia was induced with TCI propofol [Diprivan (200
mg/20 ml); Corden Pharma S.P.A., Caponago, Milano, Italy] set at a
plasma target concentration of 3 µg/ml. Following loss of
consciousness (LOC), tracheal intubation was facilitated with 0.2
mg/kg cisatracurium [Cisatracurium Besilate (10 mg); Jiangsu
Hengrui Medicine Co., Ltd., Lianyungang, China] and 4.0 µg/kg
fentanyl [Fentanyl Citrate (0.1 mg/2 ml); Yichang Humanwell
Pharmaceutical Co., Ltd., Yichang, China]. Lungs were mechanically
ventilated with 50% oxygen to maintain the partial pressure of
carbon dioxide between 30–35 mmHg. The propofol infusion was
discontinued 30 min after its administration and surgery was then
performed. Thereafter, anesthesia was maintained with sevoflurane
[Sevofrane (250 ml); Maruishi Pharmaceutical Co., Ltd., Chuoku,
Osaka, Japan] inhalation. A bolus dose of cisatracurium (5 mg) and
fentanyl (50 µg) was administered when necessary. All data were
collected just before starting surgeries.
On occasion, bolus doses of either 50 µg
phenylephrine [Phenylephrine Hydrochloride (10 mg/1 ml); Shanghai
Harvest Pharmaceutical Co., Ltd., Shanghai, China] or 5 mg urapidil
[Urapidil Hydrochloride (25 mg/5 ml); Takeda GmbH, Konstanz,
Freiburg, Germany] were administered to maintain the mean arterial
pressure (MAP) within a physiological range. Hypotension was
defined as a 30% decrease in MAP and was treated with an
intravenous bolus of phenylephrine (24,25).
Atropine was administered at doses of 0.25 mg to maintain HR ≥50
bpm and doses were repeated as necessary.
Data collection
HR, MAP and bispectral index (BIS) were monitored
and recorded at the following measurement time points: Before the
study (baseline), and 1, 2, 5, 10, 20 and 30 min after drug
administration. In addition, time to LOC (defined as the interval
between the start of TCI and loss of responsiveness to a verbal
command to open the eyes, which was assessed every 5 sec) and
propofol consumption until LOC were recorded.
The primary outcomes of the study were fluctuation
of intraoperative hemodynamics, defined by changes of HR, MAP, and
the occurrence of hypotension and bradycardia during induction, and
changes of BIS. Additional outcomes were time to LOC and the
administration of vasoactive drugs.
Statistical analysis
Data were expressed as means ± standard deviation,
median (25th percentile, 75th percentile), n (%), or n/total number
(%), and analyzed using SPSS version 12.0 software package (SPSS,
Inc., Chicago, IL, USA). General information was analyzed with
either one-way analysis of variance (ANOVA) or Fisher's exact test.
The time to LOC, dosage of propofol until LOC, dosage of
phenylephrine and atropine were analyzed using the Wilcoxon
signed-rank test. MAP, HR and BIS were analyzed with using measures
ANOVA. The ratios of hypotension and bradycardia were analyzed with
Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Grouping and characteristics of
patients
Fifty-three patients exhibiting hepatic
insufficiency were enrolled in the current study. A previous study
revealed that patients with a model of end stage liver disease
(MELD) score <9 experienced a mortality rate of 1.9% at 3
months, whereas those with a MELD score >10, were associated
with a mortality rate that was increased by more than three times,
and increased exponentially as the MELD score increased (26). Accordingly, the patients were divided
into two groups based on MELD score (min., −1, max., 33) as
follows: 32 patients were enrolled in the group with a MELD score
of ≤9 and 21 were enrolled in the group with a MELD score of ≥10.
Patient characteristics were comparable between the two groups,
except for MELD score (Table I).
Furthermore, the types of disease and surgery are presented in
Table I.
 | Table I.Characteristics of the patients. |
Table I.
Characteristics of the patients.
| Characteristics | MELD, ≤9 [n=32
(60.38%)] | MELD, ≥10 [n=21
(39.62%)] |
|---|
| Age (years) | 46.44±7.26 | 50.50±8.64 |
| Gender
(male/female) | 27/5 | 19/2 |
| Body mass index | 21.75±2.80 | 22.19±3.19 |
| MELD score |
5.19±2.74 | 16.71±8.09 |
| Median
(25th percentile, 75th percentile) | 5.5 (4.0, 7.8) | 11.0 (11.0,
23.5) |
| Types of disease |
|
|
|
Cirrhosis | 19 (59.38%) | 12 (57.14%) |
| Hepatic
carcinoma | 13 (40.63%) | 9 (42.86%) |
| Types of
surgery |
|
|
| Liver
transplantation | 5 (15.63%) | 17 (80.95%) |
| Partial
hepatectomy | 19 (59.68%) | 4 (19.05%) |
|
Splenectomy | 8 (25.00%) | 0 (0.00%) |
| Time to LOC
(sec) | 77 (62, 142) | 84 (58, 129) |
| Dosage of propofol
until LOC (mg/kg) | 0.90 (0.85,
1.10) | 0.91 (0.81,
1.06) |
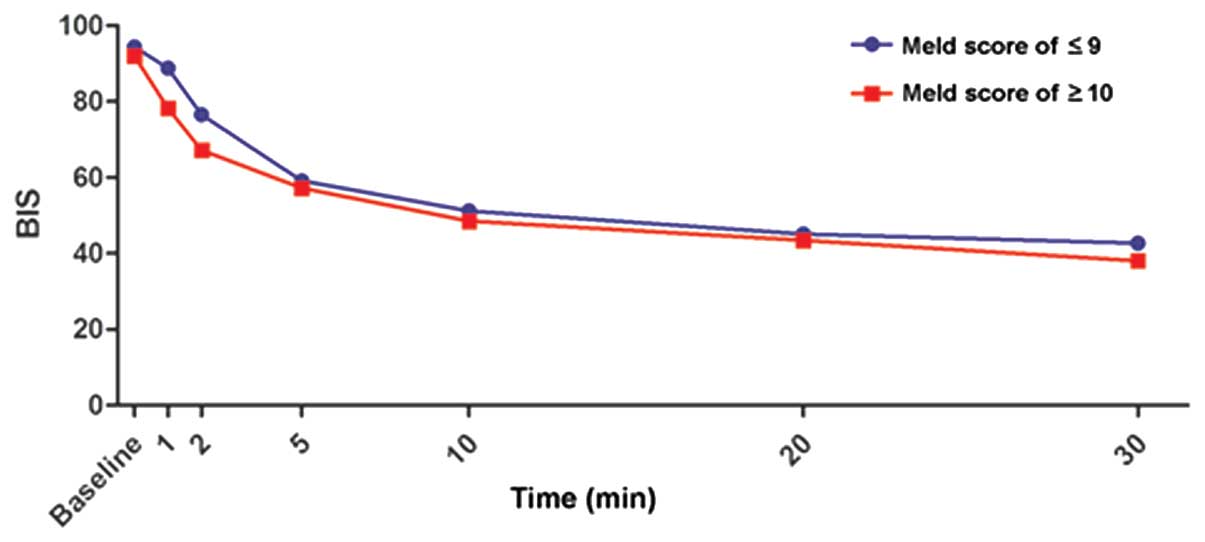
Time to LOC and BIS changes subsequent
to TCI with equal concentrations of propofol
Liver dysfunction affected neither the time nor the
dosage of propofol until LOC (Table
I). In addition, repeated measures ANOVA indicated that BIS was
impacted by time, but not by liver dysfunction (Fig. 1).
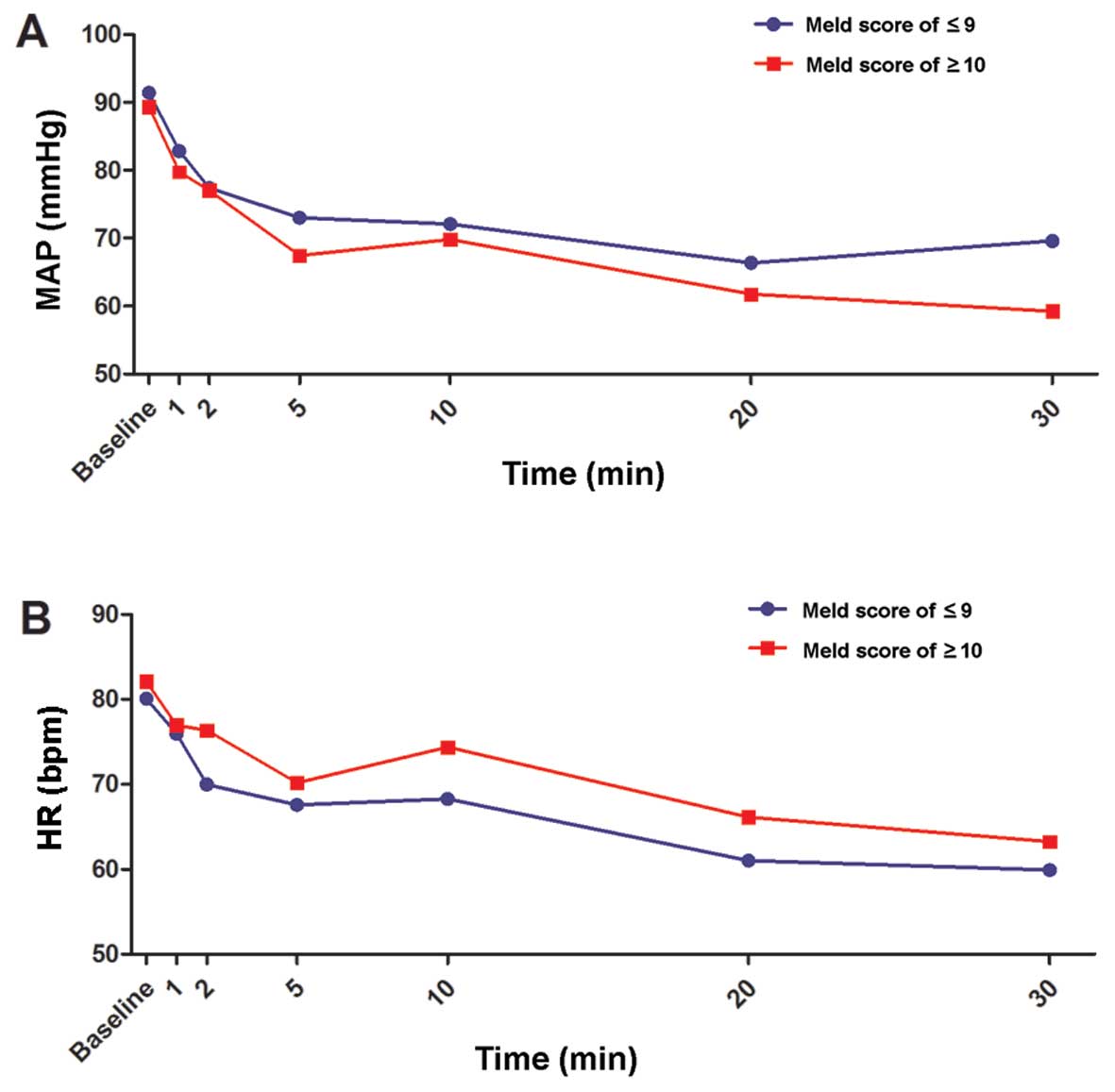
Changes of MAP and HR subsequent to
TCI with equal concentrations of propofol
During TCI, the MAP and HR of all patients
significantly decreased subsequent to induction of anesthesia
(P<0.05; Fig. 2A and B). MAP or HR
were not significantly different between the two MELD score groups
(P>0.05).
Hypotension occurred 5 min after TCI of propofol in
the two groups, although not in all of the patients. Furthermore,
the proportion of hypotension was not significantly different
between patients in the two groups, except for at 30 min (Table II).
 | Table II.Proportion of
hypotension.a |
Table II.
Proportion of
hypotension.a
| Time (min) | MELD, ≤9 | MELD, ≥10 | P-value |
|---|
| 1 | 0/32 | 0/21 | – |
|
| (0) | (0) |
|
| 2 | 0/32 | 0/21 | – |
|
| (0) | (0) |
|
| 5 | 11/32 | 9/21 | 0.573 |
|
| (34.38) | (42.86) |
|
| 10 | 5/32 | 6/21 | 0.310 |
|
| (15.63) | (28.57) |
|
| 20 | 12/32 | 13/21 | 0.099 |
|
| (37.50) | (61.90) |
|
| 30 | 11/32 | 14/21 | 0.027 |
|
| (34.38) | (66.67) |
|
The proportion of bradycardia was not significantly
different between the two groups (P>0.05); however, it should be
emphasized that there was no bradycardia observed in patients with
MELD scores of ≥10 (Table III). In
addition, no differences were observed concerning the quantity of
phenylephrine or atropine administered (Table IV).
 | Table III.Proportion of
bradycardia.a |
Table III.
Proportion of
bradycardia.a
| Time (min) | MELD, ≤9 | MELD, ≥10 | P-value |
|---|
| 1 | 0/32 | 0/21 | – |
|
| (0) | (0) |
|
| 2 | 1/32 | 0/21 | 1.000 |
|
| (3.13) | (0) |
|
| 5 | 2/32 | 0/21 | 0.512 |
|
| (6.25) | (0) |
|
| 10 | 1/32 | 0/21 | 1.000 |
|
| (3.13) | (0) |
|
| 20 | 2/32 | 0/21 | 0.512 |
|
| (6.25) | (0) |
|
| 30 | 3/32 | 0/21 | 0.269 |
|
| (9.38) | (0) |
|
 | Table IV.Quantity of vasoactive therapeutic
agents.a |
Table IV.
Quantity of vasoactive therapeutic
agents.a
| Therapeutic
agent | MELD, ≤9 | MELD, ≥10 | P-value |
|---|
| Phenylephrine
(µg) | 0.0 (0.0, 0.0) | 0.0 (0.0,
37.5) | 0.134 |
|
| 17.19±51.76 | 28.57±56.06 | 0.399 |
| Atropine (mg) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.095 |
|
| 0.04±0.13 | 0.00 | 1.000 |
Discussion
This prospective observational study assessed the
differences of pharmacodynamics of TCI of 3 µg/ml propofol during
induction and intubation in patients with varying degrees of liver
dysfunction. The results of the current study demonstrated that the
proportion of bradycardia and depth of anesthesia was not
significantly different between the different MELD score groups.
However, bradycardia and hypotension were observed, and the
patients with severe liver dysfunction were more likely to develop
into hypotension over time. These results provided novel evidence
and a possible research direction for TCI of propofol in patients
with hepatic insufficiency.
The TCI system is a frequently used device in daily
clinical practice. Marsh parameters incorporated into the
Diprifusor TCI system have been derived from subjects with normal
liver function (27). In previous
studies (28,29), propofol predictive concentrations (Cp)
set at 3 µg/ml provided effective conditions for intubation,
stabilized hemodynamics and appropriate depth of anesthesia,
whether the patients were adults or children, with mild or moderate
liver disease (30). In line with
these studies, the present study demonstrated that with the same
induction program of TCI of propofol, the patients with different
degrees of hepatic dysfunction experienced the same trend of depth
of anesthesia. However, a recent study indicated that, to maintain
similar depths of anesthesia, the propofol requirements
administered by TCI were dependent on the severity of liver
dysfunction (30). It was suggested
that greater central nervous sensitivity to intravenous anesthetics
was affected in certain ways by liver dysfunction, such as by
progressive cognitive dysfunction or slowing of brain activity
(31). Hepatic dysfunction has already
been demonstrated to enhance sensitivity to sedative agents
(32). Therefore, it was suggested
that the exact dose of propofol, administered by TCI for
appropriate depth of anesthesia in patients with severe impaired
liver function, requires further investigation.
MELD has been used as an objective scale of disease
severity for management of patients with end-stage liver disease,
and validated as a predictor of long-term survival or short-term
mortality for patients with decompensated cirrhosis (33,34). Thus,
the present study classified patients according to MELD score.
Furthermore, the MELD score includes renal function, which may be
more suitable for assessing pharmacokinetics and pharmacodynamics
of propofol in patients with hepatic insufficiency that is often
accompanied by renal insufficiency. It has been widely recognized
that the actual concentration of propofol in patients with severe
liver dysfunction is higher (35,36). The
increased concentration of propofol did not lead to significant
changes in the depth of anesthesia in the present study. However,
the incidence of cardiovascular events tended to differ between the
two groups, particularly hypotension. In the present study, the
proportion of hypotension was significantly prominent in the MELD
score of ≥10 group when compared with the MELD score of ≤9 group at
30 min, but not at the other time points. Propofol exhibits
suppressive cardiac effects, and the magnitude of hypotension
depends on the drug concentration in plasma (37). A higher actual plasma concentration of
propofol in more severe hepatic dysfunction was shown to suppress
cardiac function more significantly (38). In the present study, the association of
liver function with blood pressure (BP) became more significant
over time, although the proportion of hypotension did not vary
between the different severities of liver dysfunction during the
first 20 min. With regard to HRs, the proportion of bradycardia was
not significantly different between patients in the two groups. It
was, however, noteworthy that no bradycardia was observed in the
MELD score of ≥10 group. This was consistent with a previous study,
which showed that propofol was often accompanied by a significant
decrease in arterial BP and HR, while bradycardia and hypotension
were not commonly associated (39).
The lack of association between bradycardia and hypotension may be
attributed to the cardiac depressor reflex, although the exact
mechanism remains unclear.
China is one of the most highly endemic areas of the
hepatitis virus infection, with an incidence of HBV infection of
>8% (40,41). Patients that are infected with the
hepatitis virus may develop liver dysfunction to varying degrees.
This may result in abnormal levels of serum albumin, bilirubin and
coagulation, which affects the pharmacokinetics and
pharmacodynamics of certain therapeutic agents. In the present
study, the incidence of cardiovascular events in patients with
liver dysfunction may potentially have resulted from a higher
measured concentration than Cp of propofol. Although the liver
dysfunction was moderate it should be acknowledged. The target
concentration requires further investigation and adjustment,
although a previous study reported that the pharmacokinetics and
protein binding of propofol were not markedly affected by cirrhosis
(42). Thus, the difference in
pharmacokinetics of TCI of propofol in patients with liver
dysfunction may explain the difference of pharmacodynamics;
however, this requires further investigation in our future
research.
There were certain limitations of the present study.
First, the actual measured plasma concentration and
pharmacokinetics were not analyzed at the same time, predominantly
due to a limited observation time and lack of manpower. Secondly,
the study observation time period in this current investigation was
too short, limited to the period between anesthesia induction and
surgery initiation. Third, the small number of patients may have
reduced the power of the present results, which require
clarification in a larger population.
In conclusion, the current study revealed that TCI
of propofol to 3 µg/ml in patients with liver dysfunction did not
result in a varying depth of anesthesia, while bradycardia and
hypotension was observed in patients over time. It was suggested
that TCI of propofol Cp requires investigation and adjustment in
patients with hepatic insufficiency. A lower target concentration
may be more suitable for this type of patients; however, further
verification in future studies is required.
References
|
1
|
Anderson BJ: Pediatric models for adult
target-controlled infusion pumps. Paediatr Anaesth. 20:223–232.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fanti L, Agostoni M, Arcidiacono PG,
Albertin A, Strini G, Carrara S, Guslandi M, Torri G and Testoni
PA: Target-controlled infusion during monitored anesthesia care in
patients undergoing EUS: propofol alone versus midazolam plus
propofol. A prospective double-blind randomised controlled. Dig
Liver Dis. 39:81–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eriksson O, Josephsson R, Långstrom B and
Bergström M: Positron emission tomography and target-controlled
infusion for precise modulation of brain drug concentration. Nucl
Med Biol. 35:299–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin BF, Huang YS, Kuo CP, Ju DT, Lu CH,
Cherng CH and Wu CT: Comparison of A-line autoregressive index and
observer assessment of alertness/sedation scale for monitored
anesthesia care with target-controlled infusion of propofol in
patients undergoing percutaneous vertebroplasty. J Neurosurg
Anesthesiol. 23:6–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo Z, Pang L, Jia X, Wang X, Su X, Li P,
Mi W and Hao J: Intraoperative target-controlled infusion
anesthesia application using remifentanil hydrochloride with
etomidate in patients with severe burn as monitored using
Narcotrend. Burns. 41:100–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Egan TD: Target-controlled drug delivery:
Progress toward an intravenous ‘vaporizer’ and automated anesthetic
administration. Anesthesiology. 99:1214–1219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu LG, Pan JH, Li J, Kang F and Jiang L:
Effects of different doses of sufentanil and remifentanil combined
with propofol in target-controlled infusion on stress reaction in
elderly patients. Exp Ther Med. 5:807–812. 2013.PubMed/NCBI
|
|
8
|
Derrode N, Lebrun F, Levron JC, Chauvin M
and Debaene B: Influence of peroperative opioid on postoperative
pain after major abdominal surgery: Sufentanil TCI versus
remifentanil TCI. A randomized, controlled study. Br J Anaesth.
91:842–849. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kreuer S, Biedler A, Larsen R, Altmann S
and Wilhelm W: Narcotrend monitoring allows faster emergence and a
reduction of drug consumption in propofol-remifentanil anesthesia.
Anesthesiology. 99:34–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marsh B, White M, Morton N and Kenny GN:
Pharmacokinetic model driven infusion of propofol in children. Br J
Anaesth. 67:41–48. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Absalom AR, Mani V, De Smet T and Struys
MM: Pharmacokinetic models for propofol - defining and illuminating
the devil in the detail. Br J Anaesth. 103:26–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coppens M, Van Limmen JG, Schnider T,
Wyler B, Bonte S, Dewaele F, Struys MM and Vereecke HE: Study of
the time course of the clinical effect of propofol compared with
the time course of the predicted effect-site concentration:
Performance of three pharmacokinetic-dynamic models. Br J Anaesth.
104:452–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cortínez LI, De la Fuente N, Eleveld DJ,
Oliveros A, Crovari F, Sepulveda P, Ibacache M and Solari S:
Performance of propofol target-controlled infusion models in the
obese: Pharmacokinetic and pharmacodynamic analysis. Anesth Analg.
119:302–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomson AJ, Morrison G, Thomson E, Beattie
C, Nimmo AF and Glen JB: Induction of general anaesthesia by
effect-site target-controlled infusion of propofol: Influence of
pharmacokinetic model and ke0 value. Anaesthesia. 69:429–435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Cosmo G, Congedo E, Clemente A and
Aceto P: Sedation in PACU: The role of propofol. Curr Drug Targets.
6:741–744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vanlersberghe C and Camu F: Propofol.
Handb Exp Pharmacol. 182:227–252. 2008. View Article : Google Scholar
|
|
17
|
Simoni RF, Esteves LO, Miziara LE,
Cangiani LM, Alves GG, Romano AL, Hansen PÚ and Vianna PT: Clinical
evaluation of two Ke0 in the same pharmacokinetic propofol model:
Study on loss and recovery of consciousness. Rev Bras Anestesiol.
61:397–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma P, Singh S, Sharma BC, Kumar M,
Garg H, Kumar A and Sarin SK: Propofol sedation during endoscopy in
patients with cirrhosis, and utility of psychometric tests and
critical flicker frequency in assessment of recovery from sedation.
Endoscopy. 43:400–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai HC, Lin YC, Ko CL, Lou HY, Chen TL,
Tam KW and Chen CY: Propofol versus midazolam for upper
gastrointestinal endoscopy in cirrhotic patients: A meta-analysis
of randomized controlled trials. PLoS One. 10:e01175852015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laviolle B, Basquin C, Aguillon D,
Compagnon P, Morel I, Turmel V, Seguin P, Boudjema K, Bellissant E
and Mallédant Y: Effect of an anesthesia with propofol compared
with desflurane on free radical production and liver function after
partial hepatectomy. Fundam Clin Pharmacol. 26:735–742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai YF, Lin CC, Lee WC and Yu HP:
Propofol attenuates ischemic reperfusion-induced formation of lipid
peroxides in liver transplant recipients. Transplant Proc.
44:376–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takizawa D, Sato E, Ito N, Ogino Y,
Hiraoka H, Goto F, Cavaliere F, Conti G, Moscato U, Meo F, et al:
Hypoalbuminaemia and propofol pharmacokinetics. Br J Anaesth.
95:559author reply 559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sayama H, Takubo H, Komura H, Kogayu M and
Iwaki M: Application of a physiologically based pharmacokinetic
model informed by a top-down approach for the prediction of
pharmacokinetics in chronic kidney disease patients. AAPS J.
16:1018–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klimscha W, Weinstabl C, Ilias W, Mayer N,
Kashanipour A, Schneider B and Hammerle A: Continuous spinal
anesthesia with a microcatheter and low-dose bupivacaine decreases
the hemodynamic effects of centroneuraxis blocks in elderly
patients. Anesth Analg. 77:275–280. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biboulet P, Jourdan A, Van Haevre V, Morau
D, Bernard N, Bringuier S and Capdevila X: Hemodynamic profile of
target-controlled spinal anesthesia compared with 2
target-controlled general anesthesia techniques in elderly patients
with cardiac comorbidities. Reg Anesth Pain Med. 37:433–440. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wiesner R, Edwards E, Freeman R, Harper A,
Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, et al:
United Network for Organ Sharing Liver Disease Severity Score
Committee: Model for end-stage liver disease (MELD) and allocation
of donor livers. Gastroenterology. 124:91–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eleveld DJ, Proost JH, Cortínez LI,
Absalom AR and Struys MM: A general purpose pharmacokinetic model
for propofol. Anesth Analg. 118:1221–1237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muñoz HR, Cortínez LI, Ibacache ME and
León PJ: Effect site concentrations of propofol producing hypnosis
in children and adults: Comparison using the bispectral index. Acta
Anaesthesiol Scand. 50:882–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu HC, Li J, Yang B, Shangguan WN, Cai MY
and Lian QQ: Effect of pediatric TCI system for propofol plus
remifentanil in pediatric short-duration surgery with laryngeal
mask airway anesthesia. Zhonghua Yi Xue Za Zhi. 91:595–599.
2011.(In Chinese). PubMed/NCBI
|
|
30
|
Wu J, Huang SQ, Chen QL and Zheng SS: The
influence of the severity of chronic virus-related liver disease on
propofol requirements during propofol-remifentanil anesthesia.
Yonsei Med J. 54:231–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Felipo V: Hepatic encephalopathy: Effects
of liver failure on brain function. Nat Rev Neurosci. 14:851–858.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haq MM, Faisal N, Khalil A, Haqqi SA,
Shaikh H and Arain N: Midazolam for sedation during diagnostic or
therapeutic upper gastrointestinal endoscopy in cirrhotic patients.
Eur J Gastroenterol Hepatol. 24:1214–1218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dunn W, Jamil LH, Brown LS, Wiesner RH,
Kim WR, Menon KV, Malinchoc M, Kamath PS and Shah V: MELD
accurately predicts mortality in patients with alcoholic hepatitis.
Hepatology. 41:353–358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
D'Amico G, Garcia-Tsao G and Pagliaro L:
Natural history and prognostic indicators of survival in cirrhosis:
A systematic review of 118 studies. J Hepatol. 44:217–231. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Servin F, Cockshott ID, Farinotti R,
Haberer JP, Winckler C and Desmonts JM: Pharmacokinetics of
propofol infusions in patients with cirrhosis. Br J Anaesth.
65:177–183. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Servin FS, Bougeois B, Gomeni R, Mentré F,
Farinotti R and Desmonts JM: Pharmacokinetics of propofol
administered by target-controlled infusion to alcoholic patients.
Anesthesiology. 99:576–585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Das S, Forrest K and Howell S: General
anaesthesia in elderly patients with cardiovascular disorders:
Choice of anaesthetic agent. Drugs Aging. 27:265–282. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krag A, Bendtsen F, Dahl EK, Kjær A,
Petersen CL and Møller S: Cardiac function in patients with early
cirrhosis during maximal beta-adrenergic drive: A dobutamine stress
study. PLoS One. 9:e1091792014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hug CC Jr, McLeskey CH, Nahrwold ML,
Roizen MF, Stanley TH, Thisted RA, Walawander CA, White PF,
Apfelbaum JL, Grasela TH, et al: Hemodynamic effects of propofol:
Data from over 25,000 patients. Anesth Analg. 77:(Suppl 4).
S21–S29. 1993.PubMed/NCBI
|
|
40
|
Trépo C, Chan HL and Lok A: Hepatitis B
virus infection. Lancet. 384:2053–2063. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao S, Duan ZP and Coffin CS: Clinical
relevance of hepatitis B virus variants. World J Hepatol.
7:1086–1096. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Servin F, Desmonts JM, Haberer JP,
Cockshott ID, Plummer GF and Farinotti R: Pharmacokinetics and
protein binding of propofol in patients with cirrhosis.
Anesthesiology. 69:887–891. 1988. View Article : Google Scholar : PubMed/NCBI
|