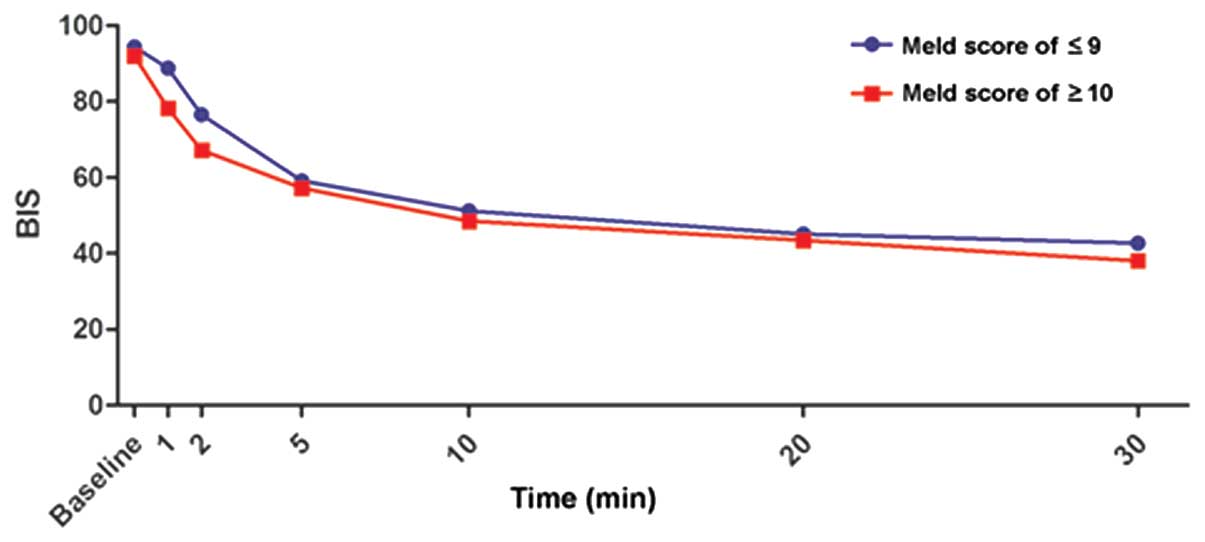
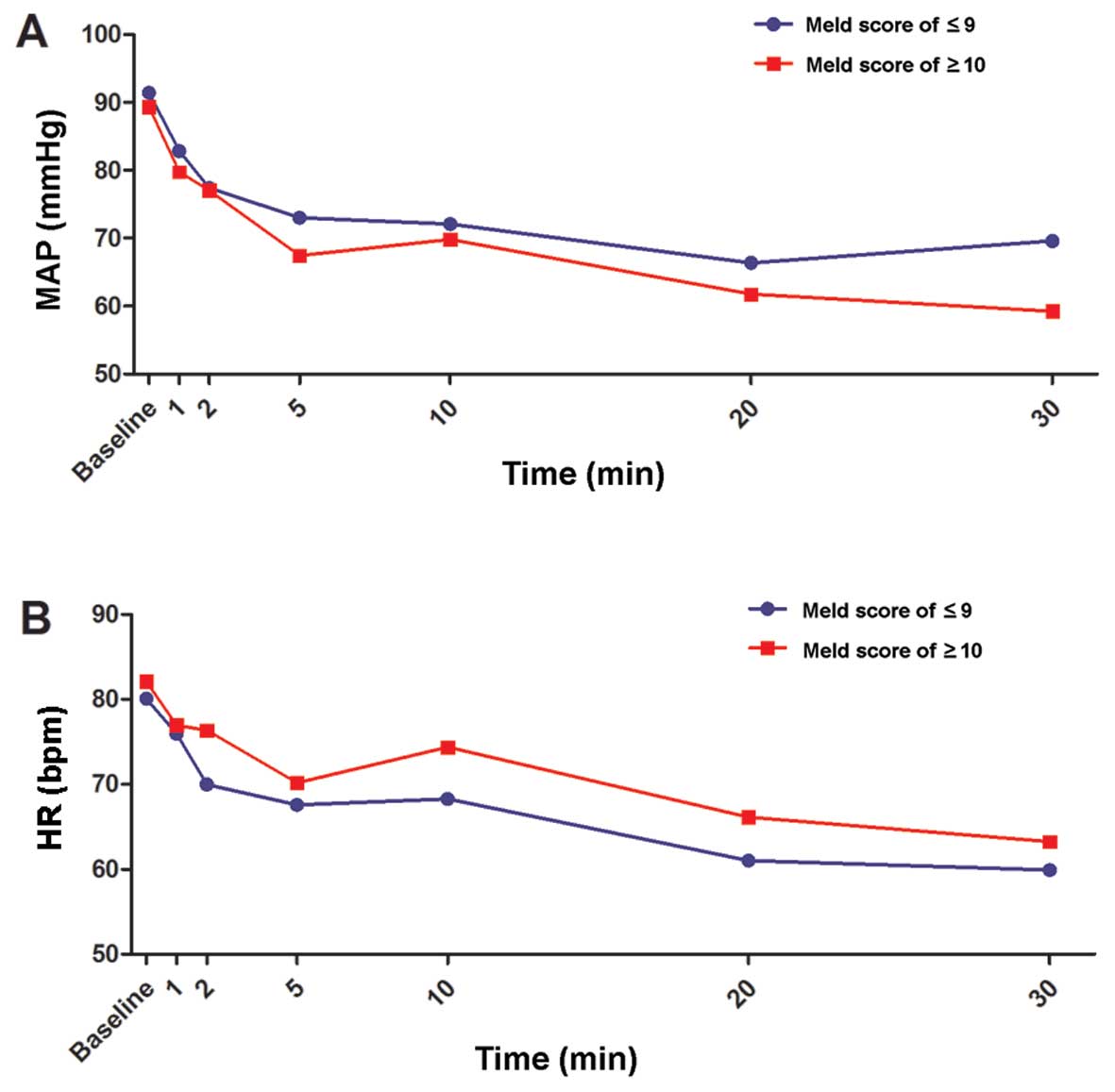
|
1
|
Anderson BJ: Pediatric models for adult
target-controlled infusion pumps. Paediatr Anaesth. 20:223–232.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fanti L, Agostoni M, Arcidiacono PG,
Albertin A, Strini G, Carrara S, Guslandi M, Torri G and Testoni
PA: Target-controlled infusion during monitored anesthesia care in
patients undergoing EUS: propofol alone versus midazolam plus
propofol. A prospective double-blind randomised controlled. Dig
Liver Dis. 39:81–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eriksson O, Josephsson R, Långstrom B and
Bergström M: Positron emission tomography and target-controlled
infusion for precise modulation of brain drug concentration. Nucl
Med Biol. 35:299–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin BF, Huang YS, Kuo CP, Ju DT, Lu CH,
Cherng CH and Wu CT: Comparison of A-line autoregressive index and
observer assessment of alertness/sedation scale for monitored
anesthesia care with target-controlled infusion of propofol in
patients undergoing percutaneous vertebroplasty. J Neurosurg
Anesthesiol. 23:6–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo Z, Pang L, Jia X, Wang X, Su X, Li P,
Mi W and Hao J: Intraoperative target-controlled infusion
anesthesia application using remifentanil hydrochloride with
etomidate in patients with severe burn as monitored using
Narcotrend. Burns. 41:100–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Egan TD: Target-controlled drug delivery:
Progress toward an intravenous ‘vaporizer’ and automated anesthetic
administration. Anesthesiology. 99:1214–1219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu LG, Pan JH, Li J, Kang F and Jiang L:
Effects of different doses of sufentanil and remifentanil combined
with propofol in target-controlled infusion on stress reaction in
elderly patients. Exp Ther Med. 5:807–812. 2013.PubMed/NCBI
|
|
8
|
Derrode N, Lebrun F, Levron JC, Chauvin M
and Debaene B: Influence of peroperative opioid on postoperative
pain after major abdominal surgery: Sufentanil TCI versus
remifentanil TCI. A randomized, controlled study. Br J Anaesth.
91:842–849. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kreuer S, Biedler A, Larsen R, Altmann S
and Wilhelm W: Narcotrend monitoring allows faster emergence and a
reduction of drug consumption in propofol-remifentanil anesthesia.
Anesthesiology. 99:34–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marsh B, White M, Morton N and Kenny GN:
Pharmacokinetic model driven infusion of propofol in children. Br J
Anaesth. 67:41–48. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Absalom AR, Mani V, De Smet T and Struys
MM: Pharmacokinetic models for propofol - defining and illuminating
the devil in the detail. Br J Anaesth. 103:26–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coppens M, Van Limmen JG, Schnider T,
Wyler B, Bonte S, Dewaele F, Struys MM and Vereecke HE: Study of
the time course of the clinical effect of propofol compared with
the time course of the predicted effect-site concentration:
Performance of three pharmacokinetic-dynamic models. Br J Anaesth.
104:452–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cortínez LI, De la Fuente N, Eleveld DJ,
Oliveros A, Crovari F, Sepulveda P, Ibacache M and Solari S:
Performance of propofol target-controlled infusion models in the
obese: Pharmacokinetic and pharmacodynamic analysis. Anesth Analg.
119:302–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomson AJ, Morrison G, Thomson E, Beattie
C, Nimmo AF and Glen JB: Induction of general anaesthesia by
effect-site target-controlled infusion of propofol: Influence of
pharmacokinetic model and ke0 value. Anaesthesia. 69:429–435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Cosmo G, Congedo E, Clemente A and
Aceto P: Sedation in PACU: The role of propofol. Curr Drug Targets.
6:741–744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vanlersberghe C and Camu F: Propofol.
Handb Exp Pharmacol. 182:227–252. 2008. View Article : Google Scholar
|
|
17
|
Simoni RF, Esteves LO, Miziara LE,
Cangiani LM, Alves GG, Romano AL, Hansen PÚ and Vianna PT: Clinical
evaluation of two Ke0 in the same pharmacokinetic propofol model:
Study on loss and recovery of consciousness. Rev Bras Anestesiol.
61:397–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma P, Singh S, Sharma BC, Kumar M,
Garg H, Kumar A and Sarin SK: Propofol sedation during endoscopy in
patients with cirrhosis, and utility of psychometric tests and
critical flicker frequency in assessment of recovery from sedation.
Endoscopy. 43:400–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai HC, Lin YC, Ko CL, Lou HY, Chen TL,
Tam KW and Chen CY: Propofol versus midazolam for upper
gastrointestinal endoscopy in cirrhotic patients: A meta-analysis
of randomized controlled trials. PLoS One. 10:e01175852015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laviolle B, Basquin C, Aguillon D,
Compagnon P, Morel I, Turmel V, Seguin P, Boudjema K, Bellissant E
and Mallédant Y: Effect of an anesthesia with propofol compared
with desflurane on free radical production and liver function after
partial hepatectomy. Fundam Clin Pharmacol. 26:735–742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai YF, Lin CC, Lee WC and Yu HP:
Propofol attenuates ischemic reperfusion-induced formation of lipid
peroxides in liver transplant recipients. Transplant Proc.
44:376–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takizawa D, Sato E, Ito N, Ogino Y,
Hiraoka H, Goto F, Cavaliere F, Conti G, Moscato U, Meo F, et al:
Hypoalbuminaemia and propofol pharmacokinetics. Br J Anaesth.
95:559author reply 559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sayama H, Takubo H, Komura H, Kogayu M and
Iwaki M: Application of a physiologically based pharmacokinetic
model informed by a top-down approach for the prediction of
pharmacokinetics in chronic kidney disease patients. AAPS J.
16:1018–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klimscha W, Weinstabl C, Ilias W, Mayer N,
Kashanipour A, Schneider B and Hammerle A: Continuous spinal
anesthesia with a microcatheter and low-dose bupivacaine decreases
the hemodynamic effects of centroneuraxis blocks in elderly
patients. Anesth Analg. 77:275–280. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biboulet P, Jourdan A, Van Haevre V, Morau
D, Bernard N, Bringuier S and Capdevila X: Hemodynamic profile of
target-controlled spinal anesthesia compared with 2
target-controlled general anesthesia techniques in elderly patients
with cardiac comorbidities. Reg Anesth Pain Med. 37:433–440. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wiesner R, Edwards E, Freeman R, Harper A,
Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, et al:
United Network for Organ Sharing Liver Disease Severity Score
Committee: Model for end-stage liver disease (MELD) and allocation
of donor livers. Gastroenterology. 124:91–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eleveld DJ, Proost JH, Cortínez LI,
Absalom AR and Struys MM: A general purpose pharmacokinetic model
for propofol. Anesth Analg. 118:1221–1237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muñoz HR, Cortínez LI, Ibacache ME and
León PJ: Effect site concentrations of propofol producing hypnosis
in children and adults: Comparison using the bispectral index. Acta
Anaesthesiol Scand. 50:882–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu HC, Li J, Yang B, Shangguan WN, Cai MY
and Lian QQ: Effect of pediatric TCI system for propofol plus
remifentanil in pediatric short-duration surgery with laryngeal
mask airway anesthesia. Zhonghua Yi Xue Za Zhi. 91:595–599.
2011.(In Chinese). PubMed/NCBI
|
|
30
|
Wu J, Huang SQ, Chen QL and Zheng SS: The
influence of the severity of chronic virus-related liver disease on
propofol requirements during propofol-remifentanil anesthesia.
Yonsei Med J. 54:231–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Felipo V: Hepatic encephalopathy: Effects
of liver failure on brain function. Nat Rev Neurosci. 14:851–858.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haq MM, Faisal N, Khalil A, Haqqi SA,
Shaikh H and Arain N: Midazolam for sedation during diagnostic or
therapeutic upper gastrointestinal endoscopy in cirrhotic patients.
Eur J Gastroenterol Hepatol. 24:1214–1218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dunn W, Jamil LH, Brown LS, Wiesner RH,
Kim WR, Menon KV, Malinchoc M, Kamath PS and Shah V: MELD
accurately predicts mortality in patients with alcoholic hepatitis.
Hepatology. 41:353–358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
D'Amico G, Garcia-Tsao G and Pagliaro L:
Natural history and prognostic indicators of survival in cirrhosis:
A systematic review of 118 studies. J Hepatol. 44:217–231. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Servin F, Cockshott ID, Farinotti R,
Haberer JP, Winckler C and Desmonts JM: Pharmacokinetics of
propofol infusions in patients with cirrhosis. Br J Anaesth.
65:177–183. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Servin FS, Bougeois B, Gomeni R, Mentré F,
Farinotti R and Desmonts JM: Pharmacokinetics of propofol
administered by target-controlled infusion to alcoholic patients.
Anesthesiology. 99:576–585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Das S, Forrest K and Howell S: General
anaesthesia in elderly patients with cardiovascular disorders:
Choice of anaesthetic agent. Drugs Aging. 27:265–282. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krag A, Bendtsen F, Dahl EK, Kjær A,
Petersen CL and Møller S: Cardiac function in patients with early
cirrhosis during maximal beta-adrenergic drive: A dobutamine stress
study. PLoS One. 9:e1091792014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hug CC Jr, McLeskey CH, Nahrwold ML,
Roizen MF, Stanley TH, Thisted RA, Walawander CA, White PF,
Apfelbaum JL, Grasela TH, et al: Hemodynamic effects of propofol:
Data from over 25,000 patients. Anesth Analg. 77:(Suppl 4).
S21–S29. 1993.PubMed/NCBI
|
|
40
|
Trépo C, Chan HL and Lok A: Hepatitis B
virus infection. Lancet. 384:2053–2063. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao S, Duan ZP and Coffin CS: Clinical
relevance of hepatitis B virus variants. World J Hepatol.
7:1086–1096. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Servin F, Desmonts JM, Haberer JP,
Cockshott ID, Plummer GF and Farinotti R: Pharmacokinetics and
protein binding of propofol in patients with cirrhosis.
Anesthesiology. 69:887–891. 1988. View Article : Google Scholar : PubMed/NCBI
|