Introduction
Bone metabolism is strictly regulated by
osteoblastic bone formation and osteoclastic bone resorption
(1). Bone tissue is continuously
regenerated through a process known as bone remodeling. This bone
remodeling process begins with osteoclastic bone resorption,
followed by osteoblastic bone formation (1). It is generally established that
osteoblasts play a crucial role in the regulation of bone
resorption through the expression of the receptor activator of
nuclear factor κB ligand (RANKL) in response to a variety of bone
resorptive stimuli (2). Osteoblasts
express various cell type-specific markers during their
differentiation process. Osteocalcin, which is synthesized by
osteoblasts and recognized as a marker of mature osteoblast
phenotype, is the most abundant non-collagenous protein (3). It is generally known that osteocalcin is
post-translationally modified by vitamin K-dependent
γ-carboxylation as bone Gla-protein (3). It has been reported that
osteocalcin-deficient mice develop an increase of bone formation
without impairing bone resorption, suggesting that osteocalcin is a
determinant of bone formation (4). In
addition, uncarboxylated osteocalcin functions as a potent hormone,
which regulates energy metabolism by stimulating the insulin
secretion from β-cells of pancreatic islets and upregulating the
insulin sensitivity of peripheral organs such as muscle and adipose
(5). Thus, bone is currently
recognized to act as an endocrine organ through the release of
osteocalcin.
The thyroid hormone acts as an important regulator
in the skeletal function as well as whole-body metabolism. An
excess of thyroid hormone, known as hyperthyroidism, upregulates
the bone metabolic turnover and increases the ratio of bone
resorption to bone formation, resulting in osteoporosis associated
with an increased risk of bone fracture (6). It has been reported that bone mineral
density is markedly decreased in untreated patients of
hyperthyroidism (7). The receptor of
thyroid hormone belongs to the nuclear receptor superfamily
(8). The biological functions of the
thyroid hormone are mainly mediated by binding to specific
receptors in the nucleus, and that the receptor-hormone complex
activates the transcription of related genes (9). In osteoblasts, the thyroid hormone
stimulates alkaline phosphatase activity and modulates the
proliferation of osteoblasts (10).
Previously, we showed that triiodothyronine (T3)
stimulates osteocalcin synthesis at least in part via p38
mitogen-activated protein (MAP) kinase in osteoblast-like MC3T3-E1
cells and that the adenylyl cyclase-cAMP system regulates the
osteocalcin synthesis via the suppression of p38 MAP kinase
activation (11,12). However, the exact mechanism underlying
the thyroid hormone-induced synthesis of osteocalcin remains to be
elucidated.
Incretins, endogenous polypeptide hormones released
from the small intestine in response to oral food intake, stimulate
insulin secretion from pancreatic β-cells (13). Glucose-dependent insulinotropic
polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), which exert
their effects by the specific guanine nucleotide-binding protein
(G-protein)-coupled receptors highly expressed on pancreatic islet
β-cells, are currently recognized as incretins (14). Regarding the effects of incretins in
bone, it has been shown that GIP stimulates the expression of
collagen type I mRNA and alkaline phosphatase activity in
osteoblasts (15), and ameliorates the
bone mineral density in ovariectomized rat established as a model
of postmenopausal osteoporosis (16).
In addition, GLP-1 reportedly induces osteoblast differentiation
(17). However, the roles of incretins
in bone metabolism have not yet been fully clarified.
In the present study, we investigated the effects of
incretins, GIP and GLP-1, on T3-stimulated osteocalcin
synthesis in osteoblast-like MC3T3-E1 cells. The results
demonstrated that incretins suppressed the T3-stimulated
osteocalcin synthesis in MC3T3-E1 cells, and the suppressive effect
of incretins was mediated through transcriptional levels.
Materials and methods
Materials
T3 was obtained from Sigma-Aldrich (cat.
no. T2752; St. Louis, MO, USA). GIP (cat. no. 4178-v) and GLP-1
(cat. no. 4344-v) were purchased from Peptide Institute, Inc.
(Osaka, Japan). The mouse osteocalcin enzyme-linked immunosorbent
assay (ELISA) kit (cat. no. J64239) was obtained from Alfa Aesar;
Thermo Fisher Scientific (Lancashire, UK). Other materials and
chemicals were obtained from commercial sources. T3 was
dissolved in 0.1 M NaOH. The concentration of NaOH was 10 µM, which
did not affect the assay for osteocalcin.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from
newborn mouse calvaria were maintained as previously described
(18,19). Briefly, the cells were cultured in
α-minimum essential medium (α-MEM) containing 10% fetal bovine
serum (FBS) at 37°C in a humidified atmosphere of 5%
CO2/95% air. The cells were seeded in 35-mm diameter
dishes (5×104 cells/dish) or 90-mm diameter dishes
(2×105 cells/dish) in α-MEM containing 10% FBS. After 5
days, the medium was exchanged for α-MEM containing 0.3% FBS. The
cells were used for experiments following a 48-h incubation period
at 37°C.
Assay for osteocalcin
The cultured cells were stimulated by 10 nM of
T3 or vehicle in 1 ml of α-MEM containing 0.3% FBS for
the indicated periods. When indicated, the cells were pretreated
with various doses of GIP or GLP-1 for 60 min. The conditioned
medium was collected at the end of incubation, and the osteocalcin
concentration in the medium was then measured using the mouse
osteocalcin ELISA kit according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cultured cells were pretreated with 100 nM of
GIP, 100 nM of GLP-1 or vehicle for 60 min, and then stimulated
with 10 nM of T3 or vehicle in α-MEM containing 0.3% FBS
for 48 h. Total RNA was isolated and reverse transcribed into
complementary DNA using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Beijing, China) and Omniscript Reverse
Transcriptase kit (Qiagen Inc., Valencia, CA, USA), respectively.
RT-qPCR was performed in capillaries using a LightCycler system
with the LightCycler FastStart DNA Master SYBR-Green I (Roche
Diagnostics, Basel, Switzerland). Sense and antisense primers were
synthesized based on the reports of Zhang et al for mouse
osteocalcin and Simpson et al for mouse GAPDH (20,21). The
amplified products were determined using a melting curve analysis
according to the system protocol. The osteocalcin mRNA levels were
normalized to those of GAPDH mRNA.
Luciferase reporter assay
A reporter plasmid, pDR4 (thyroid hormone response
element)-Luc was purchased from Stratagene (Santa Clara, CA, USA).
The cultured cells were pretreated with 100 nM of GIP, 100 nM of
GLP-1 or vehicle at 6 h after transfection with the pDR4-Luc
reporter plasmid (1 µg/dish) using UniFector transfection reagent
(B-Bridge International, Inc., Santa Clara, CA, USA). After the
pretreatment (60 min) with GIP or GLP-1, the cells were stimulated
by 10 nM of T3 or vehicle for 48 h. The samples were
lysed by passive lysis buffer (Promega Corp., Madison, WI, USA),
and obtained using a cell scraper. The measurement of the
luciferase activity of the cell lysates were performed using a dual
luciferase reporter assay system (Promega Corp.) according to the
manufacture's protocol. The cells were cotransfected with pRL-CMV
(Renilla luciferase; 0.1 µg/dish) as an internal standard to
normalize transfection efficiency.
Statistical analysis
The data were analyzed by analysis of variance
followed by Bonferroni method for multiple comparisons between
pairs. P<0.05 was considered to indicate a statistically
significant difference. The data are presented as the mean ± SEM of
triplicate determinations from three independent cell
preparations.
Results
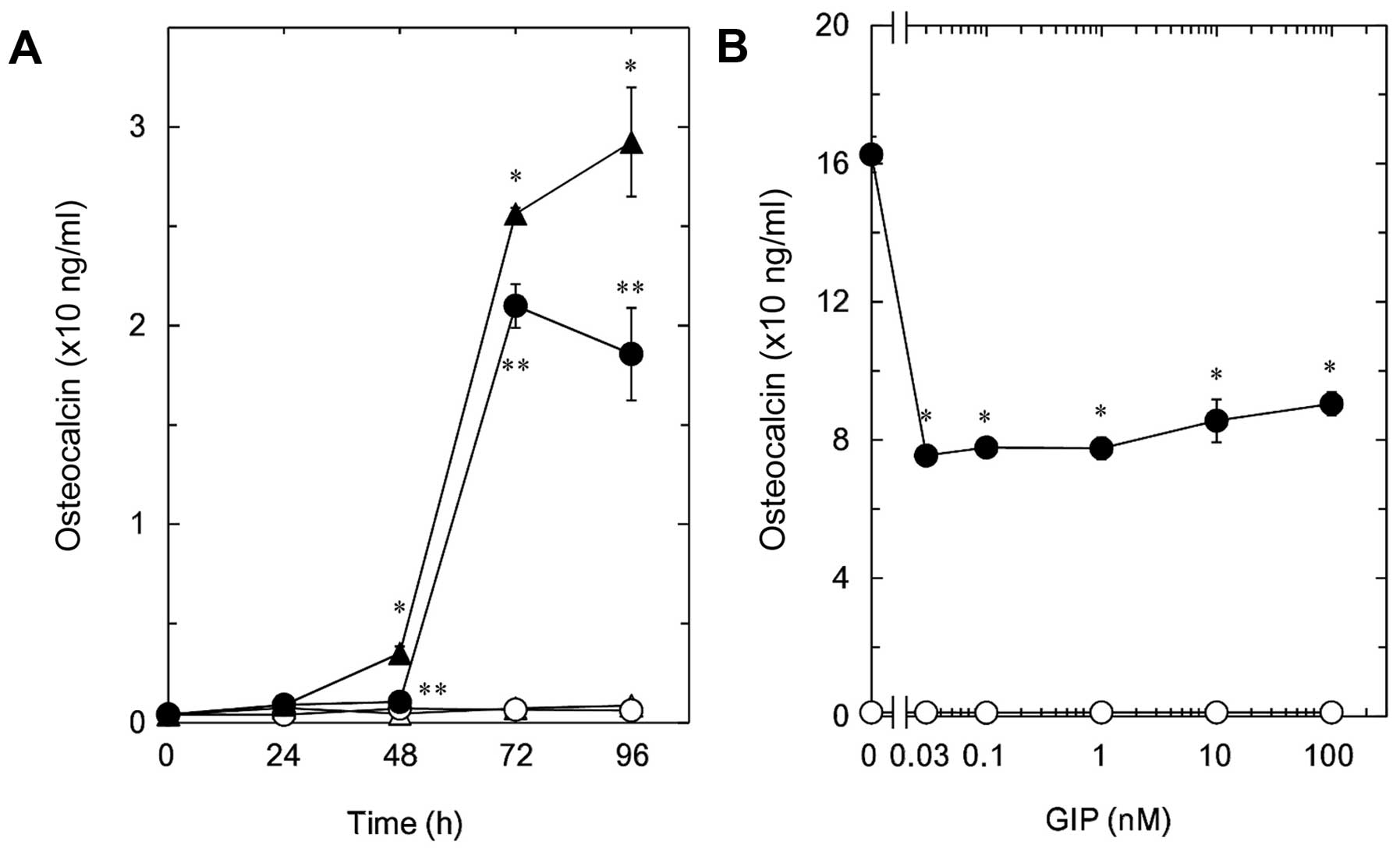
Effect of GIP on the
T3-stimulated osteocalcin release in MC3T3-E1 cells
We previously reported that T3 stimulates
the synthesis of osteocalcin from 48 to 96 h after stimulation in
osteoblast-like MC3T3-E1 cells (11).
In the present study, we first examined the effect of GIP, one of
the incretins, on the T3-stimulated osteocalcin release
in MC3T3-E1 cells. GIP, which alone did not affect the basal levels
of osteocalcin, significantly reduced the T3-stimulated
osteocalcin release (Fig. 1A). The
suppressive effect of GIP on the T3-stimulated
osteocalcin release was observed in the range between 0.03 and 100
nM (Fig. 1B). GIP at 0.03 nM caused an
~50% decrease in the T3-effect.
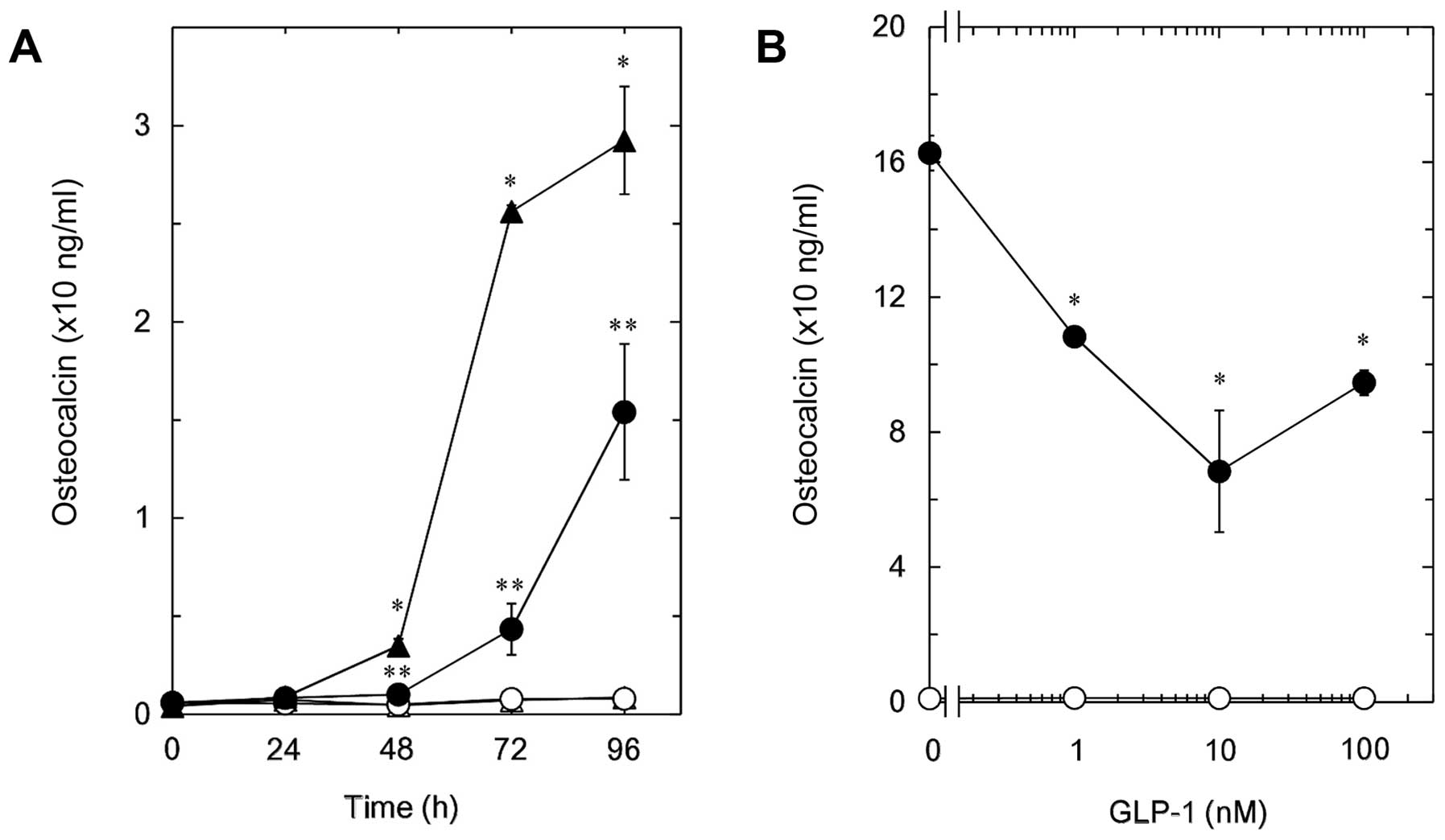
Effect of GLP-1 on the
T3-stimulated osteocalcin release in MC3T3-E1 cells
The effect of GLP-1, another incretin, was examined
on the T3-stimulated osteocalcin release in MC3T3-E1
cells. GLP-1, which by itself did not affect the osteocalcin
release, time-dependently reduced the release of osteocalcin from
48 h after the T3 stimulation ≤96 h (Fig. 2A). The inhibitory effect of GLP-1 on
the T3-induced osteocalcin release was observed in the
range between 1 and 100 nM (Fig. 2B).
A total of 10 nM of GLP-1 caused an ~60% suppression in the
T3-effect.
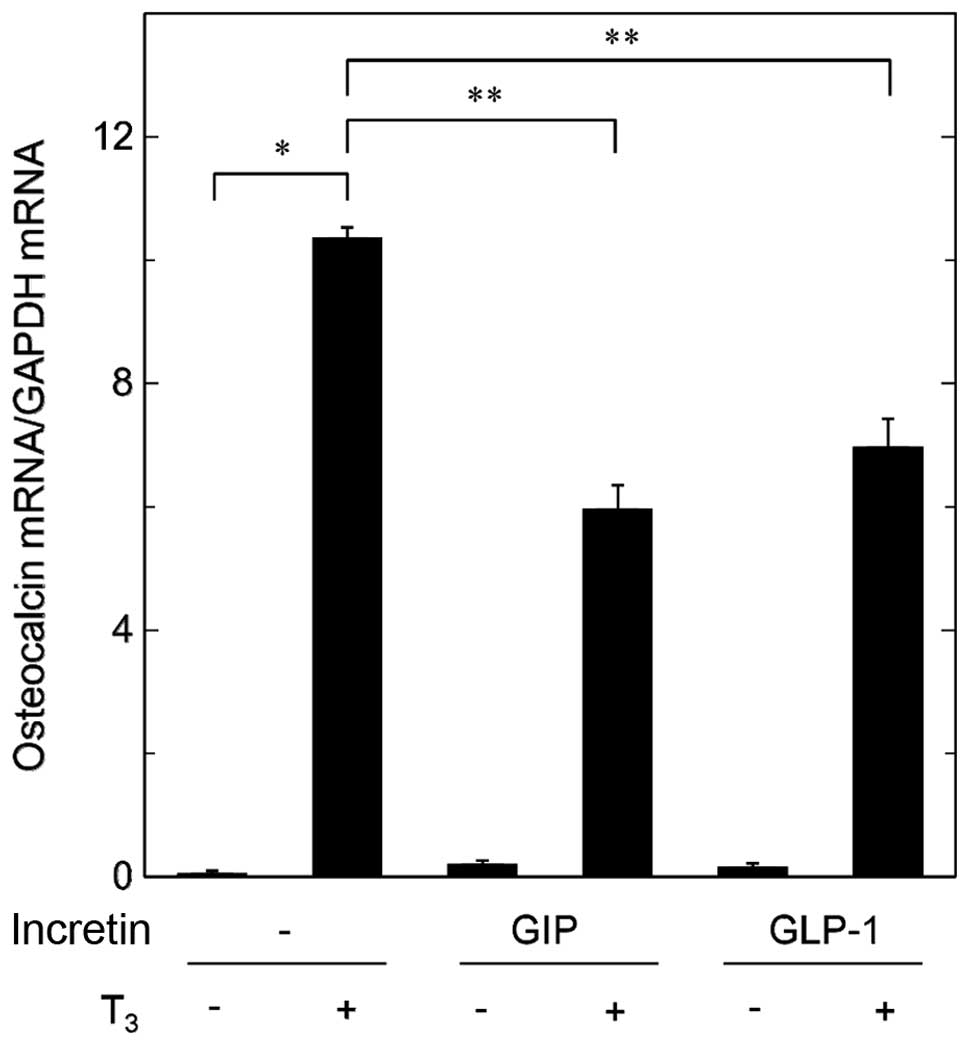
Effects of GIP or GLP-1 on the
T3-induced expression of osteocalcin mRNA in MC3T3-E1
cells
In order to clarify whether the suppressive effects
of incretins on the T3-stimulated osteocalcin release
are mediated through transcriptional events or not, we examined the
effects of GIP or GLP-1 on the T3-induced expression
levels of osteocalcin mRNA by RT-qPCR. Although GIP by itself had
little effect on the levels of osteocalcin mRNA, it significantly
attenuated the expression levels of osteocalcin mRNA induced by
T3 (Fig. 3). In addition,
GLP-1 reduced the T3-induced osteocalcin mRNA expression
(Fig. 3).
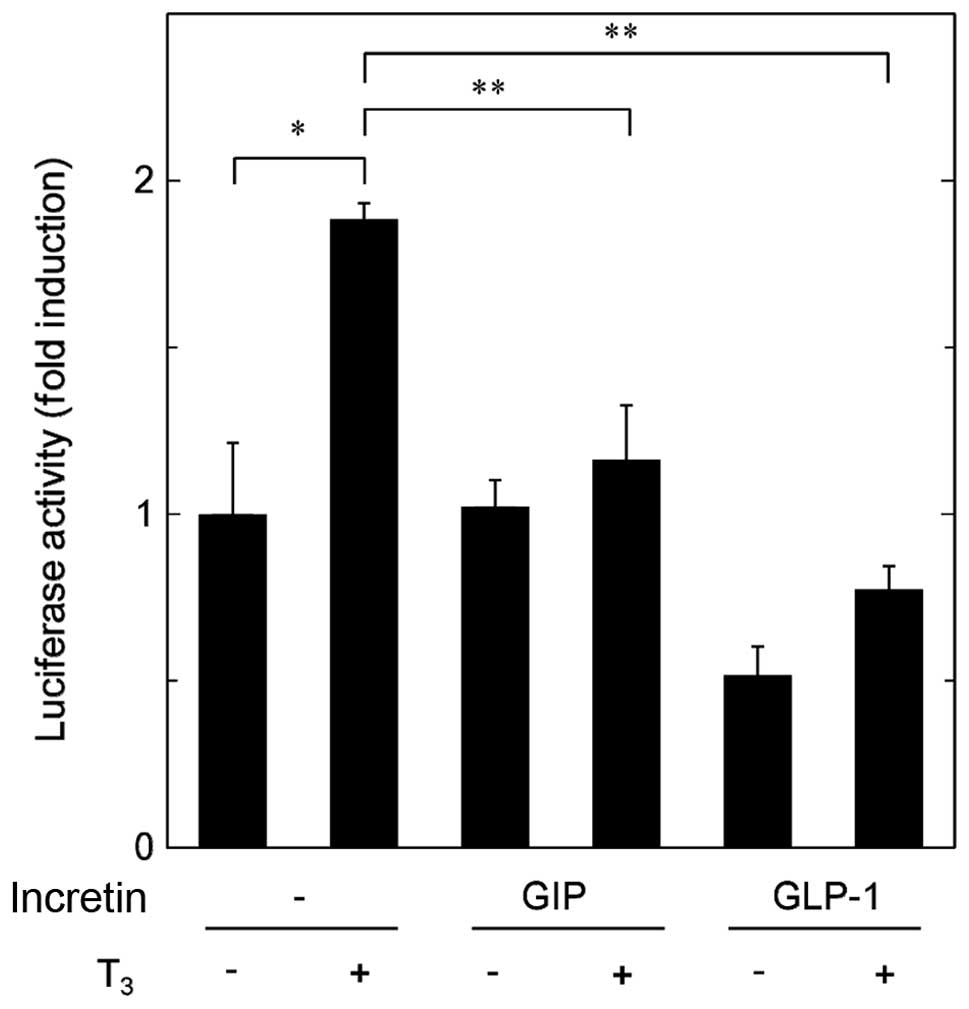
Effects of GIP or GLP-1 on the
T3-stimulated transactivation activity of thyroid
hormone responsive element in MC3T3-E1 cells
The thyroid hormone receptor belongs to the nuclear
receptor superfamily (8). Therefore,
we examined the effects of incretins on the
T3-stimulated transactivation of thyroid hormone
responsive element in osteoblast-like MC3T3-E1 cells using a
luciferase reporter assay. GIP markedly decreased the
T3-upregulated transactivation activity (Fig. 4). In addition, GLP-1 significantly
attenuated the transactivation activity stimulated by T3 (Fig. 4).
Discussion
GIP and GLP-1 are the two primary incretins secreted
from the small intestine in response to ingestion of glucose or
nutrients, resulting in the stimulation of insulin secretion from
pancreatic β-cells (13). Regarding
the effects of incretins on bone metabolism, it has been reported
that GIP stimulates osteoblast differentiation via its binding to
GIP-specific receptors expressed in osteoblasts (15). On the other hand, GLP-1 reportedly
affects receptors expressed in thyroid parafollicular cells,
resulting in upregulated synthesis of calcitonin and suppression of
osteoclastic bone resorption (22). In
the present study, we demonstrated that GIP and GLP-1 significantly
suppressed the T3-stimulated osteocalcin release in
osteoblast-like MC3T3-E1 cells. In addition, GIP and GLP-1 markedly
reduced the expression levels of osteocalcin mRNA upregulated by
T3. The biological functions of the thyroid hormone, one
of the nuclear receptor superfamily, are mediated by binding to
specific receptors in nucleus, and that the receptor-hormone
complex subsequently activates the transcription of target genes
(9). Thus, we examined the effect of
incretins on the T3-stimulated transactivation activity
of thyroid hormone responsive element assessed by a luciferase
reporter assay in osteoblast-like MC3T3-E1 cells. We showed that
GIP and GLP-1 significantly attenuated the T3-stimulated
transactivation activity. Taking our findings into account, it is
most likely that incretins reduce the T3-stimulated
synthesis of osteocalcin at a point upstream of the gene
transcription in osteoblast-like MC3T3-E1 cells.
Regarding the intracellular mechanism of incretins,
the specific receptors for GIP or GLP-1, which belong to
GTP-binding protein-coupled receptors, couple to adenylyl
cyclase-activating Gs, leading to the production of cAMP (23). With regard to osteoblasts, it has been
reported that both GIP and GLP-1 truly increase the intracellular
cAMP levels (15). We have previously
demonstrated that p38 MAP kinase is involved at least in part in
the T3-stimulated osteocalcin synthesis in
osteoblast-like MC3T3-E1 cells and that the adenylyl cyclase-cAMP
system regulates the osteocalcin synthesis via the suppression of
p38 MAP kinase activation (11,12). Based
on these findings, it is possible that incretins suppress the
T3-stimulated osteocalcin synthesis at least in part via
activation of the adenylyl cyclase-cAMP system in MC3T3-E1 cells.
Further investigation is necessary to elucidate the exact mechanism
of incretins in osteoblasts.
Osteocalcin is produced specifically in mature
osteoblasts, and embedded in bone matrix (3). The Gla residues contained in osteocalcin
are critical for the function of osteocalcin, and the osteocalcin
with their fully carboxylated state binds to hydroxyapatite with a
high affinity, resulting in the maintenance of calcification
(3). Since osteocalcin-deficient mice
reportedly present higher bone mass with strength, osteocalcin is
considered a determinant factor of bone formation (4). As for incretin-effects on bone
metabolism, it has been reported that incretins have inhibitory
effects on bone resorption (22,24). Based
on the present findings showing the inhibition by GIP or GLP-1 of
the T3-stimulated osteocalcin synthesis in osteoblasts,
it is probable that incretins upregulate bone formation by reducing
the osteocalcin levels. In addition, recent evidence suggests that
osteocalcin acts as a potent bone-derived hormone, resulting in
regulating glucose utilization and energy expenditure in myocytes
and adipocytes, and stimulating insulin secretion from pancreatic
β-cells (5,25). Taking these findings into account, it
is possible that incretins regulate whole energy metabolism by
modulating osteocalcin synthesis in osteoblasts. Additionally, GIP
and GLP-1 stimulate insulin secretion from pancreatic β-cells
(13,14). Therefore, incretins may play dual roles
in the regulation of whole body energy metabolism through
osteocalcin synthesis in osteoblasts and insulin secretion in
pancreatic β-cells. Further investigations are required to clarify
the detailed roles of incretins in bone metabolism.
In conclusion, our present findings strongly suggest
that incretins inhibit the T3-stimulated osteocalcin
synthesis in osteoblasts, and the suppressive effect of incretins
is mediated through transcriptional levels.
Acknowledgements
We are very grateful to Mrs. Yumiko Kurokawa for her
skillful technical assistance. This investigation was supported in
part by Grant-in-Aid for Scientific Research (grant nos. 19591042
and 26462289) from the Ministry of Education, Science, Sports and
Culture of Japan, the Research Funding for Longevity Sciences
(grant no. 26-12,25-4) from the National Center for Geriatrics and
Gerontology (Obu, Japan).
References
|
1
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hauschka PV, Lian JB, Cole DE and Gundberg
CM: Osteocalcin and matrix Gla protein: Vitamin K-dependent
proteins in bone. Physiol Rev. 69:990–1047. 1989.PubMed/NCBI
|
|
4
|
Ducy P, Desbois C, Boyce B, Pinero G,
Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, et
al: Increased bone formation in osteocalcin-deficient mice. Nature.
382:448–452. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karsenty G and Ferron M: The contribution
of bone to whole-organism physiology. Nature. 481:314–320. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gogakos AI, Bassett JH Duncan and Williams
GR: Thyroid and bone. Arch Biochem Biophys. 503:129–136. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vestergaard P and Mosekilde L:
Hyperthyroidism, bone mineral, and fracture risk - a meta-analysis.
Thyroid. 13:585–593. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng SY, Leonard JL and Davis PJ:
Molecular aspects of thyroid hormone actions. Endocr Rev.
31:139–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mullur R, Liu YY and Brent GA: Thyroid
hormone regulation of metabolism. Physiol Rev. 94:355–382. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasono K, Sato K, Han DC, Fujii Y,
Tsushima T and Shizume K: Stimulation of alkaline phosphatase
activity by thyroid hormone in mouse osteoblast-like cells
(MC3T3-E1): A possible mechanism of hyperalkaline phosphatasia in
hyperthyroidism. Bone Miner. 4:355–363. 1988.PubMed/NCBI
|
|
11
|
Ishisaki A, Tokuda H, Yoshida M, Hirade K,
Kunieda K, Hatakeyama D, Shibata T and Kozawa O: Activation of p38
mitogen-activated protein kinase mediates thyroid hormone-
stimulated osteocalcin synthesis in osteoblasts. Mol Cell
Endocrinol. 214:189–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanno Y, Ishisaki A, Yoshida M, Nakajima
K, Tokuda H, Numata O and Kozawa O: Adenylyl cyclase-cAMP system
inhibits thyroid hormone-stimulated osteocalcin synthesis in
osteoblasts. Mol Cell Endocrinol. 229:75–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baggio LL and Drucker DJ: Biology of
incretins: GLP-1 and GIP. Gastroenterology. 132:2131–2157. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holst JJ: The physiology of glucagon-like
peptide 1. Physiol Rev. 87:1409–1439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bollag RJ, Zhong Q, Phillips P, Min L,
Zhong L, Cameron R, Mulloy AL, Rasmussen H, Qin F, Ding KH, et al:
Osteoblast- derived cells express functional glucose-dependent
insulinotropic peptide receptors. Endocrinology. 141:1228–1235.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bollag RJ, Zhong Q, Ding KH, Phillips P,
Zhong L, Qin F, Cranford J, Mulloy AL, Cameron R and Isales CM:
Glucose-dependent insulinotropic peptide is an integrative hormone
with osteotropic effects. Mol Cell Endocrinol. 177:35–41. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanz C, Vázquez P, Blázquez C, Barrio PA,
Alvarez MM and Blázquez E: Signaling and biological effects of
glucagon-like peptide 1 on the differentiation of mesenchymal stem
cells from human bone marrow. Am J Physiol Endocrinol Metab.
298:E634–E643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2 in
osteoblast-like cells. Exp Cell Res. 198:130–134. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Yang N and Shi XM: Regulation of
mesenchymal stem cell osteogenic differentiation by
glucocorticoid-induced leucine zipper (GILZ). J Biol Chem.
283:4723–4729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simpson DA, Feeney S, Boyle C and Stitt
AW: Retinal VEGF mRNA measured by SYBR green I fluorescence: A
versatile approach to quantitative PCR. Mol Vis. 6:178–183.
2000.PubMed/NCBI
|
|
22
|
Seino Y and Yabe D: Glucose-dependent
insulinotropic polypeptide and glucagon-like peptide-1: Incretin
actions beyond the pancreas. J Diabetes Investig. 4:108–130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Godinho RO, Duarte T and Pacini ES: New
perspectives in signaling mediated by receptors coupled to
stimulatory G protein: The emerging significance of cAMP effux and
extracellular cAMP-adenosine pathway. Front Pharmacol. 6:582015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lecka-Czernik B: Safety of anti-diabetic
therapies on bone. Clin Rev Bone Miner Metab. 11:49–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee NK and Karsenty G: Reciprocal
regulation of bone and energy metabolism. Trends Endocrinol Metab.
19:161–166. 2008. View Article : Google Scholar : PubMed/NCBI
|