Introduction
1α,25-dihydroxyvitamin D3
[1,25(OH)2D3] has been originally known for
its action on calcium and phosphorus homeostasis as well as bone
mineralization. As a pleiotropic hormone with numerous regulatory
effects, 1,25(OH)2D3 has multiple
physiological and pathological roles beyond the regulation of
mineral metabolism, including the regulation of the immune system
(1,2) and
cardiovascular functions (3). The
levels of vitamin D and 25-hydroxyvitamin D are significantly and
inversely associated with blood pressure (4–6).
Furthermore, emerging evidence for the important roles of vitamin D
in the pathogenesis of metabolic disorders, such as obesity and
diabetes, is accumulating (7). An
increasing number of studies have shown that low plasma vitamin
D3 levels are an independent risk factor for a variety
of cancer types, including colorectal (8,9), breast
(10) and prostate cancer (11). Vitamin D3 inhibits the
proliferation and migration of a variety of cancer cell types,
induce their differentiation and apoptosis, and has a potential
anticancer effect (12,13).
Epithelial-mesenchymal transition (EMT) is a process
by which epithelial cells are converted into mesenchymal cells. It
occurs as part of several pathological processes, including tumor
invasion and organ fibrosis, including renal fibrosis (7). Several studies have demonstrated the
regulatory role of 1,25(OH)2D3 in renal EMT
(14–18). 1,25(OH)2D3 was
found to inhibit EMT via inducing a variety of target genes that
encode cell adhesion and polarity proteins responsible for the
epithelial phenotype, as well as through the repression of key EMT
inducers (14). In renal
tubulointerstitial fibrosis (RTF), EMT is known to represent the
pathological basis of renal interstitial fibrosis characterized by
downregulation of E-cadherin and upregulation of α-smooth muscle
actin (α-SMA). In a mouse model of chronic kidney disease (CKD),
unilateral ureteral obstruction (UUO)-induced RTF was ameliorated
by administration of 1,25(OH)2D3 (15). Other studies have found that
1,25(OH)2D3 can attenuate the progression of
renal interstitial fibrosis via inhibiting transforming growth
factor (TGF)-β and high glucose-induced EMT (16–18). In the
present study, the effects of 1,25(OH)2D3 on
the EMT of NRK-52E rat proximal tubular epithelial cell line
induced by high glucose were investigated. Furthermore, the effects
on the expression of vitamin D receptor (VDR) and angiotensin (Ang)
II were assessed with the aim to explore the possible underlying
mechanisms and to provide novel targets and strategies to delay the
progress of renal interstitial fibrosis.
Materials and methods
Cell line and culture
The NRK-52E normal rat kidney epithelial cell line
was purchased from the American Type Culture Collection (CRL-1571;
Manassas, VA, USA). The NRK-52E cells were cultured in Dulbecco's
modified Eagle's medium (low glucose; Gibco, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 5% fetal bovine
serum (FBS; Gibco), 1% streptomycin (100 µg/ml) and penicillin (100
U/ml), and incubated in a humidified atmosphere containing 5%
CO2 at 37°C. Upon reaching 90% confluency, the cells
were passaged following detachment with 0.05%
trypsin-ethylenediaminetetraacetate (Gibco), which was performed
every 2 days.
Groups and treatments
One day prior to treatment, the NRK-52E cells were
incubated with serum-free media for 24 h to synchronize the cell
growth. The cells were randomly divided into the following five
groups: control group (5.5 mmol/l glucose), high-glucose group (25
mmol/l glucose), high glucose with low dose of
1,25(OH)2D3 group [25 mmol/l glucose with
10−9 mol/l 1,25(OH)2D3], high
glucose with a medium dose of 1,25(OH)2D3
group [25 mmol/l glucose with 10−8 mol/l
1,25(OH)2D3] and high glucose with a high
dose of 1,25(OH)2D3 group [25 mmol/l glucose
with 10−7 mol/l 1,25(OH)2D3].
Subsequent analyses were performed following for 24 h.
Morphological observation was performed under a microscope (JS-500
binocular biological microscope; LIOO, Beijing, China) at ×40
magnification before the cells were lysed.
Western blot analysis
Following washing in cold phosphate-buffered saline
(PBS), the cells were lysed with radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
containing protease and phosphatase inhibitor (both from Roche,
Mannheim, Germany). A Bradford Protein Assay (Beyotime Institute of
Biotechnology) was performed to determine the total protein
content. Twenty milligrams of total protein from each sample were
separated by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto a polyvinylidene difluoride
membrane (Wuhan Boster Biological Technology, Ltd., Wuhan, China).
Following incubation in 5% bovine serum albumin for 2 h at room
temperature to block non-specific binding, the membranes were
incubated overnight at 4°C with primary antibodies against α-SMA
(cat. no. ab32575; 1:2,000 dilution), E-cadherin (cat. no. ab76055;
1:1,000 dilution) or VDR (cat. no. ab109234; 1:1,000 dilution) (all
from Abcam, Cambridge, MA, USA). Following three washes in
Tris-buffered saline containing 0.1% Tween-20 for 10 min each,
membranes were incubated with peroxidase-conjugated AffiniPure goat
anti-rabbit immunoglobulin G (cat. no. 111035003; 1:10,000
dilution; Jackson ImmunoResearch Laboratories, Inc., West Grove,
PA, USA) secondary antibody for at least 1 h at room temperature.
Blots were visualized using an Enhanced Chemiluminescence Western
Blotting Detection System (GE Healthcare Life Sciences, Chalfont,
UK). The membrane was re-blotted with a rabbit anti-rat β-actin
monoclonal antibody (cat. no. 4970; 1:1,000 dilution; Cell
Signaling Technology, Inc.) to verify equal protein loading in each
lane.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from NRK-52E cells was extracted using
TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. After determining the
purity and quality of the total RNA, E-cadherin, α-SMA and VDR mRNA
were quantified by RT-qPCR using the QuantiTect Reverse
Transcription kit and the RNA SYBR-Green kit (both from Qiagen,
Inc., Valencia, CA, USA) according to the manufacturer's
instructions. PCR amplification was performed using the ABI 7500
Fast Real-Time PCR System (Applied Biosystems, Thermo Fisher
Scientific, Inc.). The amplification program was 95°C for 15 min
followed by 40 cycles consisting of 95°C for 10 sec and 60°C for 35
sec. The ABI PRISM 7900HT Sequence Detection System (Applied
Biosystems) was used to analyze the data, and the ΔΔCq method was
used to calculate the relative expression of the respective sample
gene (19). The sequences of the
primers, which were designed using Primer Premier (v5.0; Premier
Biosoft International, Palo Alto, CA, USA) based on the relevant
sequences deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/), are listed in
Table I.
 | Table I.Primer sequences for polymerase chain
reaction. |
Table I.
Primer sequences for polymerase chain
reaction.
| Gene | Sequence | Length (bp) |
|---|
| α-SMA | F
5′-CTGTTCCAGCCATCCTTCAT-3′ | 70 |
|
| R
5′-TCATGATGCTGTTGTAGGTGGT-3′ |
|
| E-cadherin | F
5′-CCAAAGCCTCAGGTCATTAAACA-3′ | 126 |
|
| R
5′-TTCTTGGGTTGGGTCGTTGTAC-3′ |
|
| VDR | F
5′-TGCTATGACCTGTGAAGGCT-3′ | 159 |
|
| R
5′-ATCATGCCGATGTCCACACA-3′ |
|
| GAPDH | F
5′-TGACATCAAGAAGGTGGTGA-3′ | 177 |
|
| R
5′-TCATACCAGGAAATGAGCTT-3′ |
|
Enzyme-linked immunosorbent assay
(ELISA)
The Ang II secreted into the culture supernatant by
NRK-52E cells was detected by ELISA. The culture supernatant was
collected and centrifuged at 8,000–12,000 × g for 20 min. ELISA
plates (Nalge Nunc International, Thermo Fisher Scientific, Inc.)
were coated with anti-Ang II monoclonal antibody (MoAb; Ziker
Biological Technology, Ltd., Shenzhen, China) according to the
instructions of the Rat Ang II ELISA kit (100 µl of a 10 µg/ml
solution in 0.07 M NaCl buffered with 0.1 M borate, pH 8.2) and
left overnight at 4°C, followed by four washes with 200 µl of wash
solution per well (0.9% w/v NaCl containing 0.05% v/v Tween-20).
Subsequent to blocking with 200 µl PBS containing bovine serum
albumin (1.0% w/v) and 0.05% v/v Tween-20 for 60 min at room
temperature, wells were washed as described above. Centrifuged
culture supernatant or serum samples (100 µl/well) were added,
followed by incubation for 30 min at 37°C. Subsequently, wells were
incubated with horseradish peroxidase-conjugated streptavidin
(1:4,000 dilution) for 30 min at 37°C and tetramethylbenzidine
(both from Zymed Laboratories, Inc., South San Francisco, CA, USA)
as a substrate. The color reaction was allowed to develop for 30
min at 4°C in the dark and was stopped by addition of 100 µl 0.2 M
H2SO4 per well (Baker, Mexico City, Mexico).
The optical density of each well at a wavelength of 450 nm (OD 450)
was determined using an ELISA processor (Benchmark Microplate
Reader; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
concentration of Ang II in the culture supernatant was determined
from the OD 450 using a standard curve and the experiments were
performed in triplicate.
Statistical analysis
Values are expressed as the mean ± standard
deviation of results of experiments performed in triplicate.
Statistical analysis was performed using SPSS 20.0 software
(International Business Machines Corp., Armonk, NY, USA). The mean
values were analyzed for their normality by using the Shapiro-Wilk
normality test. All data passed the normality test and were tested
for significant differences by one-way analysis of variance or
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference between values. Each
experiment was repeated three times.
Results
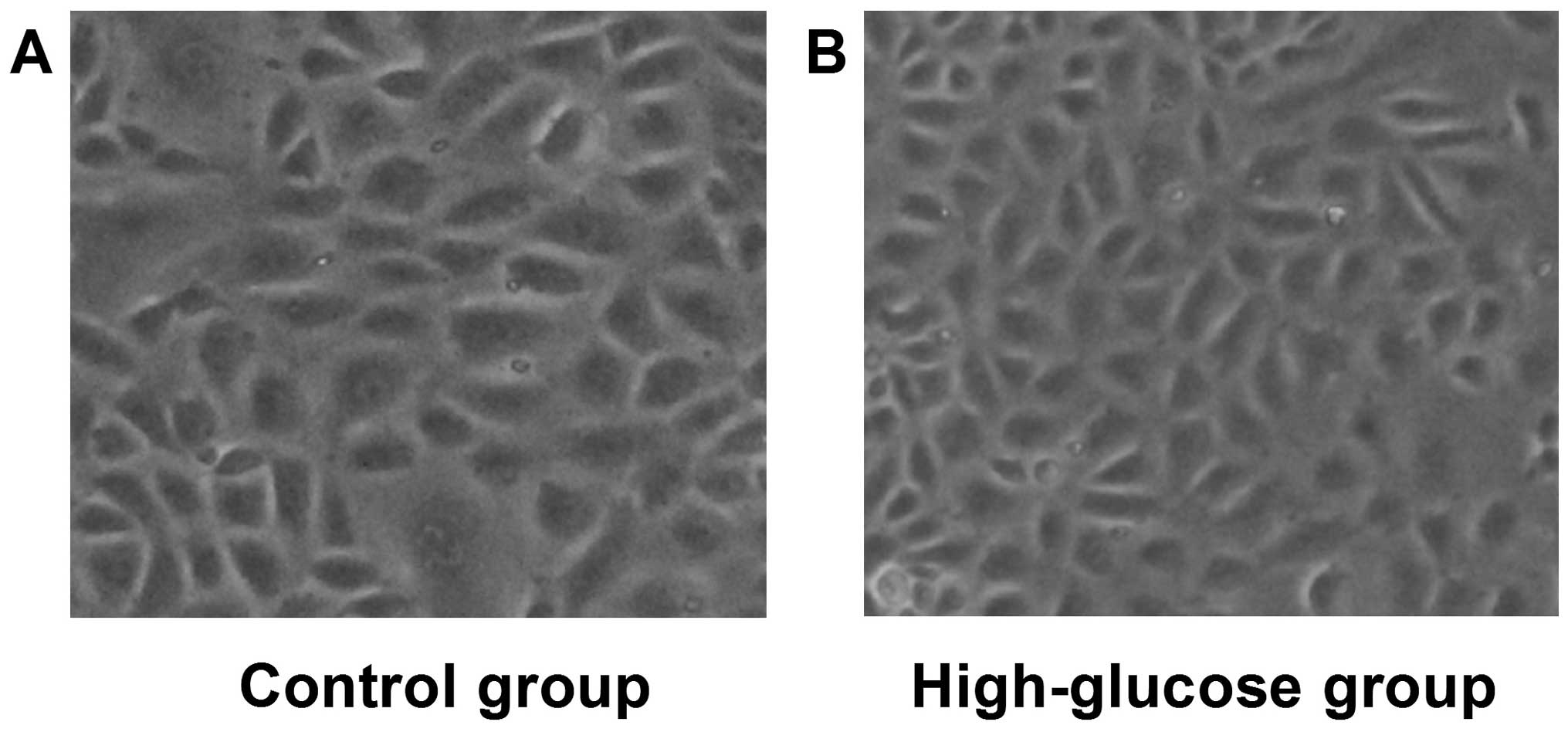
High glucose induces morphological
changes of kidney cells characteristic of EMT
Initially, the effects of high glucose on the
morphology of NRK-52E cells were assessed, which were observed by
light microscopy following incubation with high glucose (25 mmol/l)
for 24 h (Fig. 1). Compared to the
control cells, those in the high-glucose group showed obvious
transdifferentiation with cell polarity disappearing and the shape
changing from circular to elliptic, suggesting that high glucose
can induce cell morphological changes associated with EMT.
High glucose induces expression of
EMT-associated molecular markers and the secretion of Ang II by
kidney cells
As high-glucose treatment resulted in cell
morphological changes indicative of EMT, changes in the expression
of molecular markers associated with epithelial and mesenchymal
phenotypes by NRK-52E cells following high-glucose treatment were
assessed. As determined by RT-qPCR and western blot analyses,
respectively, the mRNA and protein levels of E-cadherin were
significantly increased, while those of α-SMA were significantly
reduced following high-glucose treatment (P<0.01) (Fig. 2A-C). Furthermore, ELISA revealed that
the Ang II content in the culture supernatant of the high-glucose
group (105.9±6.6 pg/l) was significantly higher than that in the
control group (4.3±1.8 pg/l; P<0.001) (Fig. 2D).
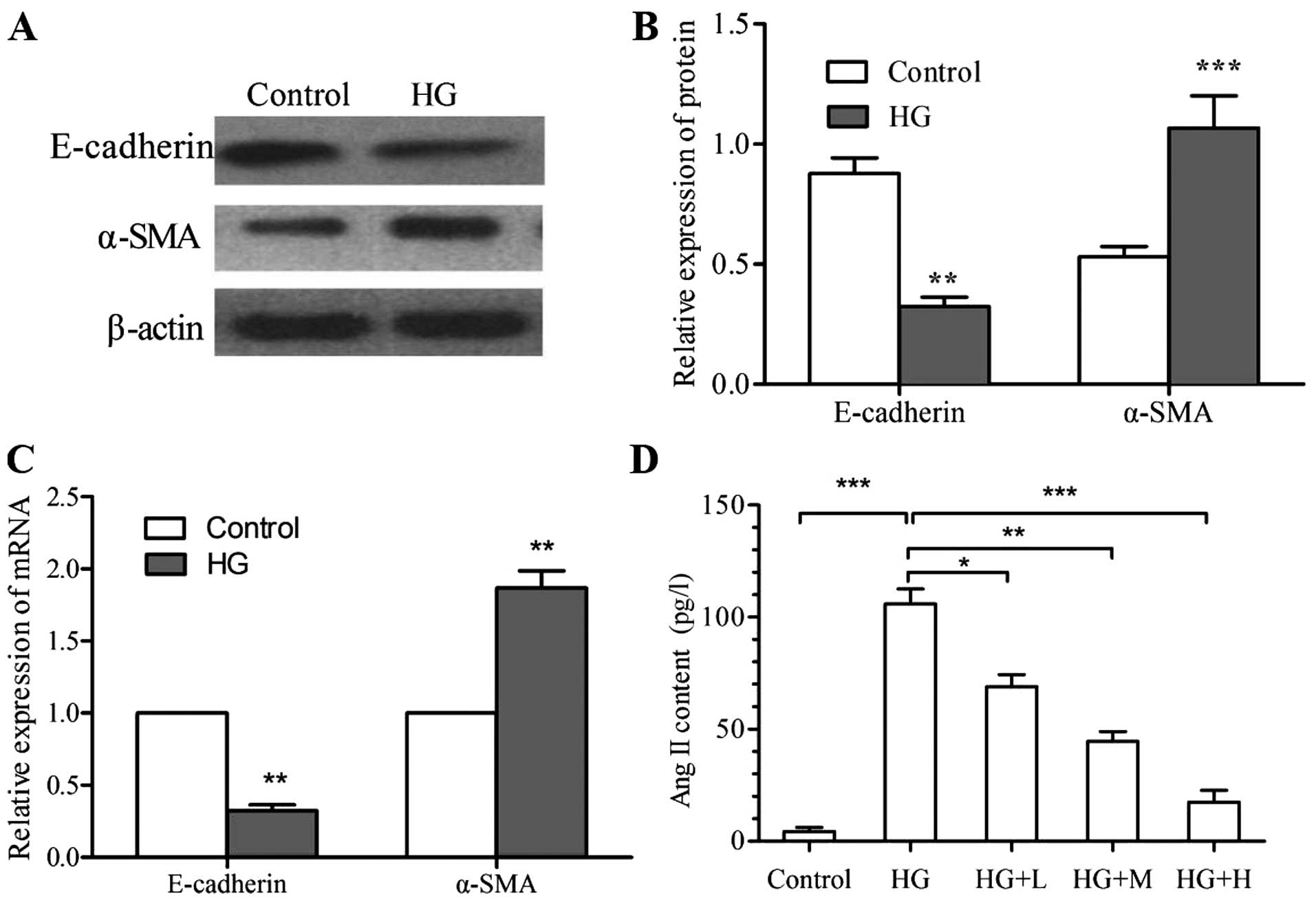 | Figure 2.Effects of HG on the expression of
molecular markers of the EMT transition and the secretion of Ang
II. The expression of E-cadherin and α-SMA was assessed by (A and
B) western blot analysis and (C) RT-qPCR analysis. (D) Ang II was
detected in the culture supernatant by ELISA. Values are expressed
as the mean ± standard deviation of results of experiments
performed in triplicate. *P<0.05; **P<0.01; ***P<0.001 vs.
control or as indicated. Groups: HG, high glucose (25 mmol/l);
HG+L, high glucose with a low dose of
1,25(OH)2D3 (10−9 mol/l); HG+M,
high glucose with a medium dose of
1,25(OH)2D3 (10−8 mol/l); HG+H,
high glucose with a high dose of 1,25(OH)2D3
(10−7 mol/l). HG, high glucose; EMT,
epithelial-mesenchymal transition; Ang, angiotensin; α-SMA,
α-smooth muscle actin; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; ELISA, enzyme-linked immunosorbent
assay; 1,25(OH)2D3, 1α,25-dihydroxyvitamin
D3. |
1,25(OH)2D3
abrogates high glucose-induced EMT of kidney cells
In order to assess the inhibitory effects of
1,25(OH)2D3 on the EMT of kidney cells, the
mRNA and protein expression of α-SMA and E-cadherin by NRK-52E
cells was detected after incubation with various concentrations of
1,25(OH)2D3 and high glucose. As shown in
Fig. 3, the high glucose-mediated
reduction of E-cadherin in NRK-52E cells was significantly and
dose-dependently attenuated by treatment with
1,25(OH)2D3. Furthermore,
1,25(OH)2D3 suppressed the high
glucose-induced expression of α-SMA in a dose-dependent manner.
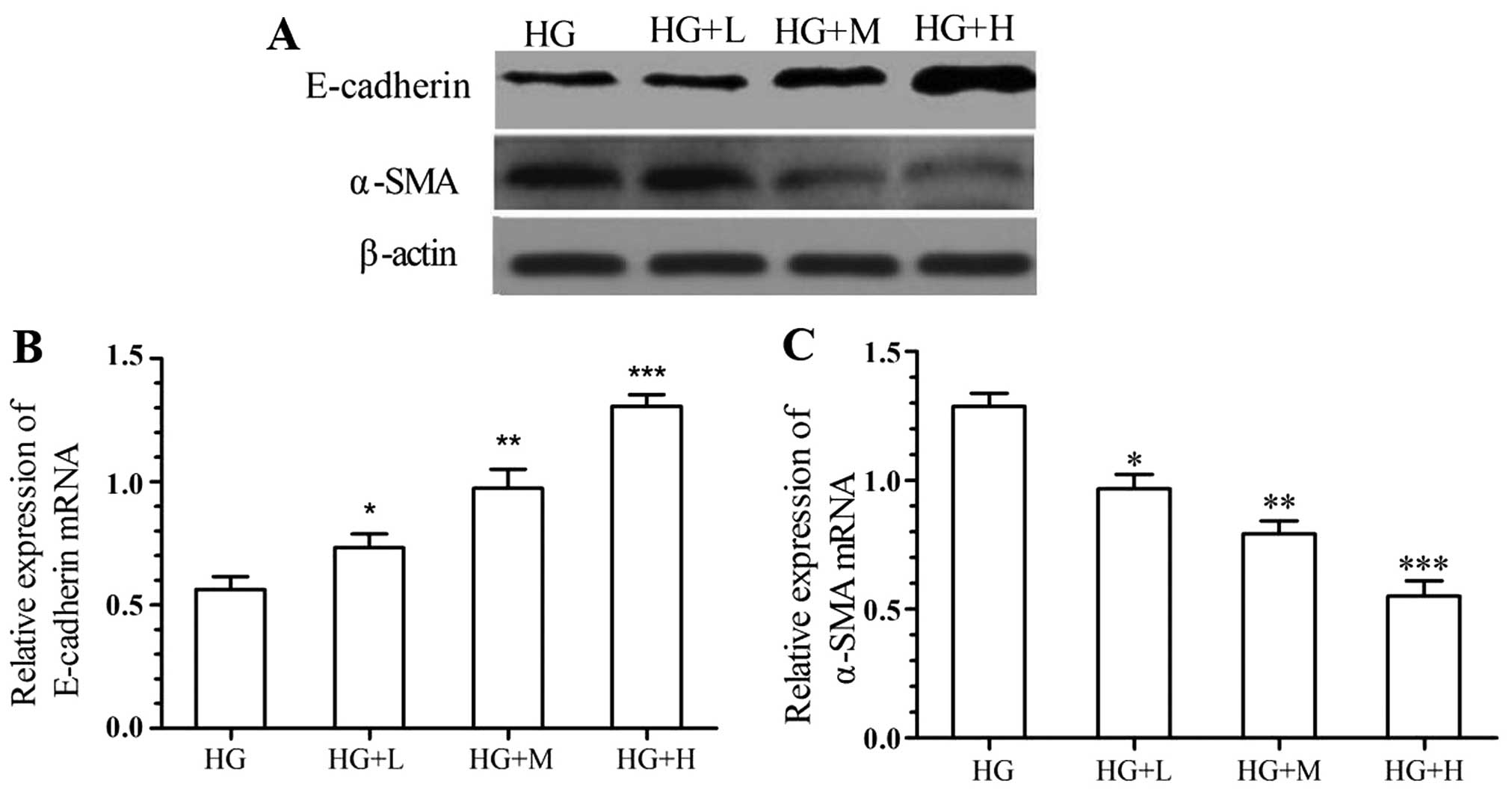 | Figure 3.Inhibitory effects of
1,25(OH)2D3 on molecular markers of the EMT.
The expression of E-cadherin and α-SMA was assessed by (A) western
blot analysis and (B and C) RT-qPCR analysis. Values are expressed
as the mean ± standard deviation of results of experiments
performed in triplicate. *P<0.05; **P<0.01; ***P<0.001 vs.
HG group. Groups: HG, high glucose (25 mmol/l); HG+L, high glucose
with a low dose of 1,25(OH)2D3
(10−9 mol/l); HG+M, high glucose with a medium dose of
1,25(OH)2D3 (10−8 mol/l); HG+H,
high glucose with a high dose of 1,25(OH)2D3
(10−7 mol/l). 1,25(OH)2D3,
1α,25-dihydroxyvitamin D3; EMT, epithelial-mesenchymal
transition; α-SMA, α-smooth muscle actin; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; HG, high
glucose. |
Effects of
1,25(OH)2D3 on the expression of VDR and Ang
II
To further investigate the mechanisms by which
1,25(OH)2D3 inhibits high glucose-induced
EMT, the mRNA and protein expression of VDR was analyzed using
RT-qPCR and western blot analysis, while Ang II was detected by
ELISA. The results showed that VDR expression was significantly
downregulated by high-glucose stimulation, while
1,25(OH)2D3 significantly inhibited this
effect in a dose-dependent manner (Fig.
4). Furthermore, the Ang II content in the high-glucose group
(105.9±6.6 pg/l) was significantly reduced by low, medium and high
dose of 1,25(OH)2D3 in a dose-dependent
manner (68.9±5.5, 44.6±4.3 and 17.4±5.23 pg/l, respectively)
P<0.001 (Fig. 2D).
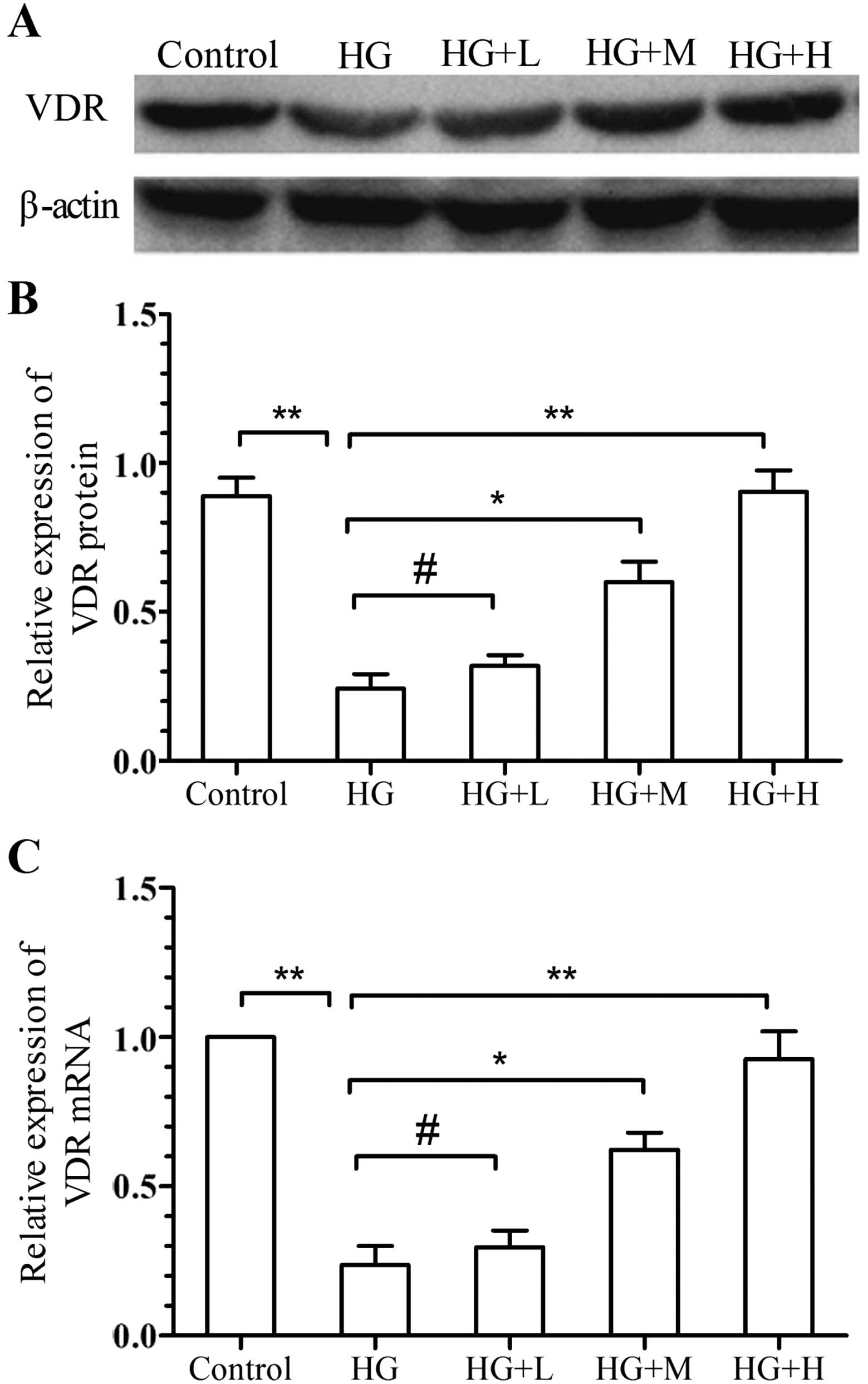 | Figure 4.Effects of
1,25(OH)2D3 on the expression of VDR. The
expression of VDR was assessed by (A and B) western blot analysis
and (C) RT-qPCR analysis. Values are expressed as the mean ±
standard deviation of results of experiments performed in
triplicate. #P>0.05; *P<0.05, **P<0.01 vs. HG
group. Groups: HG, high glucose (25 mmol/l); HG+L, high glucose
with a low dose of 1,25(OH)2D3
(10−9 mol/l); HG+M, high glucose with a medium dose of
1,25(OH)2D3 (10−8 mol/l); HG+H,
high glucose with a high dose of 1,25(OH)2D3
(10−7 mol/l). 1,25(OH)2D3,
1α,25-dihydroxyvitamin D3; VDR, vitamin D receptor;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; HG, high glucose. |
Discussion
1,25(OH)2D3, the most active
vitamin D3 metabolite, is generated by its initial
hydroxylation in the liver and subsequently in the kidney and other
tissues. Within the last two decades, the endocrine and paracrine
effects of vitamin D have been widely recognized.
1,25(OH)2D3 has also gained attention for its
cytoprotective effects in the kidney, and has been indicated to be
important for maintaining podocyte health, preventing EMT
transformation and suppressing the expression of renin as well as
inflammation. Tian et al (20)
revealed that vitamin D3 exerts protective effects on
the kidney in a rat model of diabetic nephropathy, probably by
downregulating the expression of inflammatory factors, including
TGF-β1, connective tissue growth factor and monocyte
chemoattractant protein-1. Maquigussa et al (21) indicated that calcitriol supplementation
may be a strategy to reduce renal damage induced by proteinuria in
kidney disease. A number of studies have demonstrated the
protective effects of 1,25(OH)2D3 on the
kidneys (18,20–22). In the
present study, treatment of NRK-52E cells with high glucose for 24
h led to cell-morphological changes resulting in elliptic shapes,
decreases of expression E-cadherin and increases of a-SMA
expression, suggesting that the rat renal tubular cells underwent
EMT. Simultaneously, addition of 1,25(OH)2D3
inhibited high glucose-induced downregulation of E-cadherin and
upregulation of α-SMA in a dose-dependent manner. These results
provided evidence at the molecular level that
1,25(OH)2D3 inhibits high glucose-induced
EMT.
VDR, a member of the superfamily of nuclear
receptors, is expressed in various kidney cell types, including
proximal and distal tubular epithelial cells, glomerular parietal
epithelial cells and collecting duct cells (23). VDR exerts multiple physiological and
pathological functions by modulating the transcription of vitamin
D-regulated genes via binding to vitamin D-responsive element. In a
mouse model of UUO to simulate early CKD, the expression of VDR was
significantly decreased, which was likely to be mediated by
pro-inflammatory TNF-α, and late administration of active vitamin D
was effective in restoring VDR expression (24). Wang et al (18) found that VDR(−/−) mice developed severe
renal fibrosis following UUO, while
1,25(OH)2D3 suppressed high glucose-induced
apoptosis of podocytes. Ito et al (16) provided strong evidence that VDR
suppresses renal fibrosis by inhibiting TGF-β-SMAD signal
transduction. All of these studies, confirmed the critical role of
1,25(OH)2D3-VDR signaling in renal
protection. In the present study, high-glucose stimulation reduced
the mRNA and protein expression of VDR in NRK-52E cells, which was
dose-dependently inhibited by
1,25(OH)2D3.
The renin-angiotensin-aldosterone system (RAAS) is a
regulatory cascade with Ang II as the central effector.
Dysregulation of the RAAS has a critical role in the pathogenesis
of CKD. Diabetic kidney disease is the leading cause of CKD and
blockade of the RAAS slows their progression to end-stage renal
disease (25). Zhang et al
(26) demonstrated that inhibition of
Ang II activity with losartan reduced the development of renal
fibrosis in mice subjected to UUO. In the present study,
overproduction of Ang II was found in NRK-52E cells which underwent
high glucose-induced EMT, which was inhibited by
1,25(OH)2D3 in a dose-dependent manner. Of
note, a previous study demonstrated that the inhibitory function of
1,25(OH)2D3 on Ang II production was almost
completely abrogated after silencing of the VDR gene (17). It is speculated that VDR inactivation
leads to activation of the RAAS and that overproduction of Ang II
and RAAS can be blocked by vitamin D/VDR signaling to attenuate
renal fibrosis. The major mechanisms underlying the negative
modulation of the RAAS by 1,25(OH)2D3 appears
to be: i) Suppression of renin gene transcription by blocking the
activity of the cyclic adenosine monophosphate response element in
the renin gene promoter (27); and ⅱ)
suppression of high glucose-induced angiotensinogen expression in
kidney cells by blocking the nuclear factor-κB pathway (28).
In conclusion, the present study showed that
1,25(OH)2D3 inhibits the transdifferentiation
of rat renal tubular epithelial cells (RTECs) induced by high
glucose in a dose-dependent manner, which may be associated with
the inhibition of high glucose-induced upregulation of Ang II and
downregulation of VDR. These results provided an experimental basis
for further exploring the mechanisms of the renoprotective effects
of 1,25(OH)2D3 as well as a novel approach
for the clinical treatment of renal fibrosis.
References
|
1
|
Baeke F, van Etten E, Gysemans C,
Overbergh L and Mathieu C: Vitamin D signaling in immune-mediated
disorders: Evolving insights and therapeutic opportunities. Mol
Aspects Med. 29:376–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kikuta J and Ishii M: Current topics on
Vitamin D. The effects of vitamin D on the immune system. Clin
Calcium. 25:359–365. 2015.(In Japanese). PubMed/NCBI
|
|
3
|
Verstuyf A, Carmeliet G, Bouillon R and
Mathieu C: Vitamin D: A pleiotropic hormone. Kidney Int.
78:140–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Afzal S and Nordestgaard BG: Low vitamin D
and hypertension: A causal association? Lancet Diabetes Endocrinol.
2:682–684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmitz KJ, Skinner HG, Bautista LE,
Fingerlin TE, Langefeld CD, Hicks PJ, Haffner SM, Bryer-Ash M,
Wagenknecht LE, Bowden DW, et al: Association of 25-hydroxyvitamin
D with blood pressure in predominantly 25-hydroxyvitamin D
deficient Hispanic and African Americans. Am J Hypertens.
22:867–870. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Margolis KL, Ray RM, Van Horn L, Manson
JE, Allison MA, Black HR, Beresford SA, Connelly SA, Curb JD, Grimm
RH Jr, et al: Women's Health Initiative Investigators: Effect of
calcium and vitamin D supplementation on blood pressure: The
Women's Health Initiative Randomized Trial. Hypertension.
52:847–855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakashima A, Yokoyama K, Yokoo T and
Urashima M: Role of vitamin D in diabetes mellitus and chronic
kidney disease. World J Diabetes. 7:89–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin L, Grandi N, Raum E, Haug U, Arndt V
and Brenner H: Meta-analysis: Longitudinal studies of serum vitamin
D and colorectal cancer risk. Aliment Pharmacol Ther. 30:113–125.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baron JA, Barry EL, Mott LA, Rees JR,
Sandler RS, Snover DC, Bostick RM, Ivanova A, Cole BF, Ahnen DJ, et
al: A trial of calcium and vitamin D for the prevention of
colorectal adenomas. N Engl J Med. 373:1519–1530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reimers LL, Crew KD, Bradshaw PT, Santella
RM, Steck SE, Sirosh I, Terry MB, Hershman DL, Shane E, Cremers S,
et al: Vitamin D-related gene polymorphisms, plasma
25-hydroxyvitamin D, and breast cancer risk. Cancer Causes Control.
26:187–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shui IM, Mondul AM, Lindström S, Tsilidis
KK, Travis RC, Gerke T, Albanes D, Mucci LA, Giovannucci E and
Kraft P: Breast and Prostate Cancer Cohort Consortium Group:
Circulating vitamin D, vitamin D-related genetic variation, and
risk of fatal prostate cancer in the National Cancer Institute
Breast and Prostate Cancer Cohort Consortium. Cancer.
121:1949–1956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deeb KK, Trump DL and Johnson CS: Vitamin
D signalling pathways in cancer: Potential for anticancer
therapeutics. Nat Rev Cancer. 7:684–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leyssens C, Verlinden L and Verstuyf A:
Antineoplastic effects of 1,25(OH)2D3 and its
analogs in breast, prostate and colorectal cancer. Endocr Relat
Cancer. 20:R31–R47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Larriba MJ, García de Herreros A and Muñoz
A: Vitamin D and the epithelial to mesenchymal transition. Stem
Cells Int. 2016:62138722016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Zhou G, Gui T, Shimokado A,
Nakanishi M, Oikawa K, Sato F and Muragaki Y: Elevated serum
1,25(OH)2-vitamin D3 level attenuates renal
tubulointerstitial fibrosis induced by unilateral ureteral
obstruction in kl/kl mice. Sci Rep. 4:65632014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito I, Waku T, Aoki M, Abe R, Nagai Y,
Watanabe T, Nakajima Y, Ohkido I, Yokoyama K, Miyachi H, et al: A
nonclassical vitamin D receptor pathway suppresses renal fibrosis.
J Clin Invest. 123:4579–4594. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao P, Liu Y, Lin M, Chen YM, Shui H, Yao
T and Wu XY: Effects of 1,25-(OH)2D3 on the
expression of vitamin D receptor and angiotensin IIII in human
proximal tubular epithelial cells induced by high glucose. Chin J
Diab. 5:495–499. 2013.
|
|
18
|
Wang Y, Deb DK, Zhang Z, Sun T, Liu W,
Yoon D, Kong J, Chen Y, Chang A and Li YC: Vitamin D receptor
signaling in podocytes protects against diabetic nephropathy. J Am
Soc Nephrol. 23:1977–1986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian Y, Lv G, Yang Y, Zhang Y, Yu R and
Zhu J, Xiao L and Zhu J: Effects of vitamin D on renal fibrosis in
diabetic nephropathy model rats. Int J Clin Exp Pathol.
7:3028–3037. 2014.PubMed/NCBI
|
|
21
|
Maquigussa E, Arnoni CP, Pereira LG and
Boim MA: Calcitriol ameliorates renal damage in a pre-established
proteinuria model. Mol Med Rep. 12:1009–1015. 2015.PubMed/NCBI
|
|
22
|
Cozzolino M, Brunini F, Capone V, Ricca F,
Kwaidri Y, Montanari E and Cusi D: Role of vitamin D in the
pathogenesis of chronic kidney disease. Recenti Prog Med.
104:33–40. 2013.(In Italian). PubMed/NCBI
|
|
23
|
Wang Y, Borchert ML and Deluca HF:
Identification of the vitamin D receptor in various cells of the
mouse kidney. Kidney Int. 81:993–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong M, Gong J, Liu Y, Xiang R and Tan X:
Loss of vitamin D receptor in chronic kidney disease: A potential
mechanism linking inflammation to epithelial-to-mesenchymal
transition. Am J Physiol Renal Physiol. 303:F1107–F1115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gurley SB and Coffman TM: The
renin-angiotensin system and diabetic nephropathy. Semin Nephrol.
27:144–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Kong J, Deb DK, Chang A and Li
YC: Vitamin D receptor attenuates renal fibrosis by suppressing the
renin-angiotensin system. J Am Soc Nephrol. 21:966–973. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan W, Pan W, Kong J, Zheng W, Szeto FL,
Wong KE, Cohen R, Klopot A, Zhang Z and Li YC:
1,25-dihydroxyvitamin D3 suppresses renin gene
transcription by blocking the activity of the cyclic AMP response
element in the renin gene promoter. J Biol Chem. 282:29821–29830.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto
FL, Wong KE, Kong J and Li YC: 1,25-Dihydroxyvitamin D3
suppresses high glucose-induced angiotensinogen expression in
kidney cells by blocking the NF-κB pathway. Am J Physiol Renal
Physiol. 296:F1212–F1218. 2009. View Article : Google Scholar : PubMed/NCBI
|