Introduction
Multicellular organisms are an aggregation of cells
that are responsible for particular tasks (1). The complexity of various different cell
types coming together in multicellular organisms requires
stringently regulated processes for cell division, proliferation
and differentiation, which are controlled by numerous signaling
pathways. The rate of cell division is controlled by apoptosis and
differentiation (2), with the
uncontrolled and unlimited growth of cells leading to cancer. Cell
proliferation results in tumor formation, which may spread to
distant sites by metastasis (3).
Canine mammary tumors (CMTs) are the most common
type of tumor, which may affect female dogs. The prevalence of this
tumor type in dogs is three times more than in humans. Today, CMT
comprises 50% of all tumors that affect female dogs, and 41–53% of
these appear to be malignant (4).
The initiation basis of all neoplasms is a lack of
response by natural growth inhibitors, as well as a lack of control
of the cell cycle. Cell death becomes a particularly vital
mechanism in preserving homeostasis and contributes to preventing
cancer pathogenesis. In addition to principle medical oncology
therapy, a number of substitute therapeutic strategies are being
applied to cancer cases. Factors generating apoptosis in cancer
cells or sensitizing the cells to routine cell-death mechanisms are
used as cancer therapeutics. Certain studies have reported adverse
effects of herbal supplements in different types of cancer
(5,6).
Herbal medicine has long been administered to treat malignancies in
Asian countries (7). The results of
studies using different cancer cell types propose that inhibition
of the cell cycle and growth are ordinary actions of phenolic
phytochemicals, determined by reduced cell viability and incidence
of cell cycle arrest (8). Various
studies have demonstrated that extracts from a number of herbal
plants exhibit anticancer activities in vitro and in
vivo (6–11).
Berberis vulgaris L. is a widely known plant,
with a history in traditional herbal medicine, which contains a
large quantity of berberine (BBR), an isoquinolone alkaloid. BBR is
a quaternary ammonium salt isolated from a variety of herbs,
including Coptidis rhizome (12). BBR
exerts various pharmacological and biochemical effects, such as
anti-diarrheal, anti-arrhythmic and anti-inflammatory activities
(13), and has become a point of
interest due to its particularly high antitumor activity in
vitro and in vivo.
As natural products of medicinal plants are
considered to be significant in controlling tumors and, as the role
of BBR in animal tumors is unclear, the current study examines the
effect of BBR (an isoquinolone alkaloid), as a phytochemical
anticancer agent, against proliferation of canine mammary tumor
cells (CF41.Mg) in vitro.
Materials and methods
Cell culture and chemicals
The canine mammary gland cancer cell line, CF41.Mg
(ATCC® CRL-6232™) was purchased from Pasteur Institute
of Iran (Tehran, Iran), and Sigma-Aldrich BBR chloride was obtained
from Merck Millipore (Darmstadt, Germany).
CF41.Mg cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat inactivated fetal bovine serum (FBS) and
100 mg/ml penicillin-streptomycin. The cells were maintained in an
incubator with a humidified atmosphere of 5% CO2 at
37°C.
Cell counting
The cells were counted using a hemocytometer. The
viability and growth of cells were estimated by direct counting of
0.4% trypan blue dye-excluding cells under a Nikon Eclipse Ti-S
inverted microscope (Nikon Corporation, Tokyo, Japan).
Cell harvesting and treatment
Subsequently, the cells at the third passage were
seeded into 96-well plates at a density of 12,000 cells/well. After
one day of incubation for attaching the cells, the phenol red-free
media with 10% FBS and different concentrations (10, 25, 50, 100
and 200 µM) of BBR chloride were added to the cells. In all
treatments, BBR was dissolved in 0.1% dimethyl sulfoxide (DMSO) and
made up to a maximum final concentration of 0.05% (v/v) in the
complete cell culture medium, so as not to affect cell growth.
Separate control (RPMI medium with 0.05% DMSO and cells), and blank
(only medium) wells were prepared. After BBR was added, the 96-well
plates were incubated for 24, 48 and 72 h at 37°C in an atmosphere
of 5% CO2.
Cell viability assay
Following treatment, the MTT assay was used to
detect cell viability. Briefly, 10 µM MTT (Thermo Fisher
Scientific, Inc.; at 5 mg/ml) was added to each well, at a final
concentration of 200 µg/ml and incubated for 4 h. The resulting
intracellular purple formazan was solubilized and quantified by
spectrophotometry. The absorbance of the sample was read at a
wavelength of 570 nm and cell viability was calculated according to
the following equation:
Cell viability (%) = mean absorbance of sample/mean
absorbance of control × 100 and activity (%) = 100 - cell viability
(%).
Statistical analysis
All of the experiments were repeated three times and
one-way analysis of variance followed by Dunnett's test were used
for data analysis. Data are presented as the mean ± standard
deviation of the mean and P<0.005 was considered to indicate a
statistically significant difference.
Results
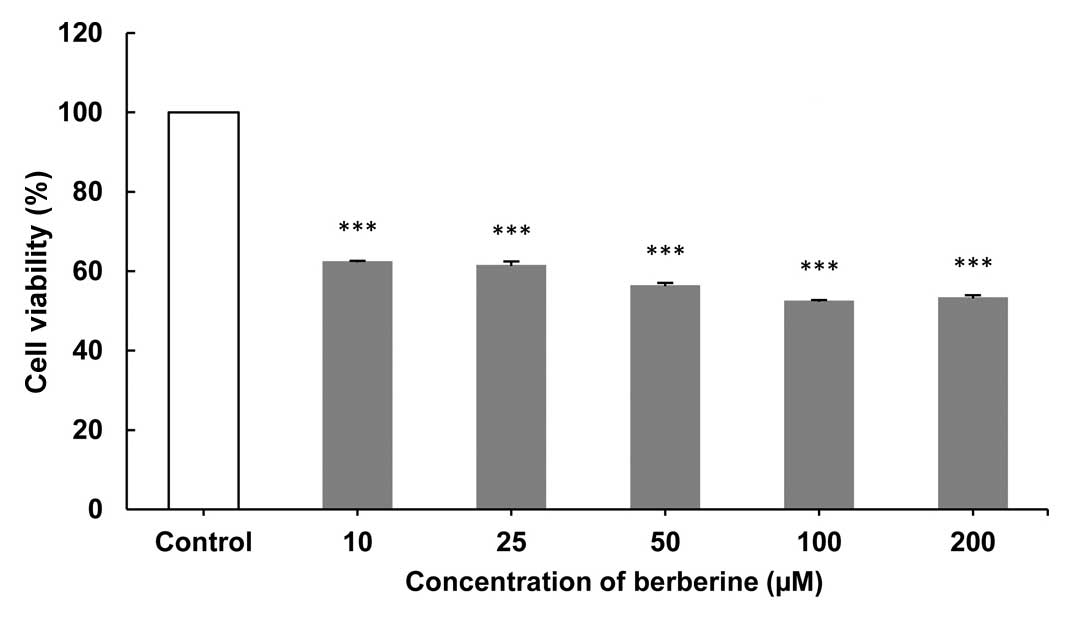
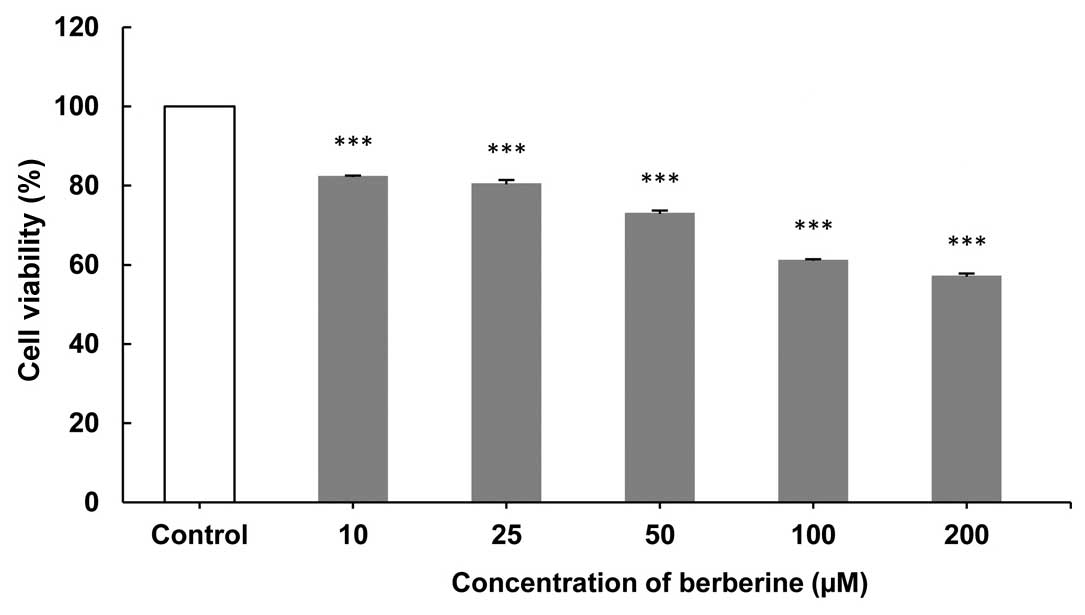
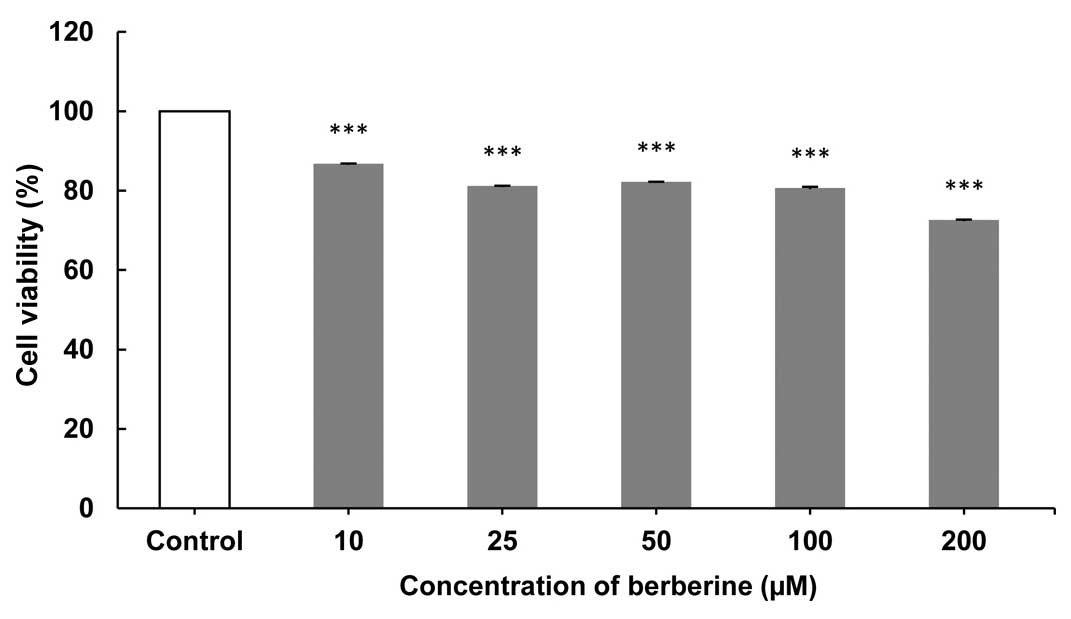
The growth of CF41.Mg cells treated with BBR at
concentrations ranging from 10 to 200 µM was monitored within 72 h
of culturing. To determine the cell viability following exposure to
different concentrations of BBR in the CF41.Mg cell line, the MTT
assay was performed. As shown in Figs.
1–3, the greatest inhibition of
cell proliferation was observed after 24- (Fig. 1), followed by 72- (Fig. 3) and 48-h (Fig. 2) exposures at concentrations of 200 and
100 µM, while no inhibition activity was observed in the control
group. The results indicate that BBR at concentrations of 100 and
200 µM had the highest antiproliferative effect after 24 h
(Fig. 1). Following a 48-h exposure to
different concentrations of BBR a delayed cytotoxic effect was
induced (Fig. 2). Data showed a
significant reduction of CMT cells that were treated with 100 and
200 µM BBR in comparison to the control group after 72 h of
exposure (P<0.005; Fig. 3).
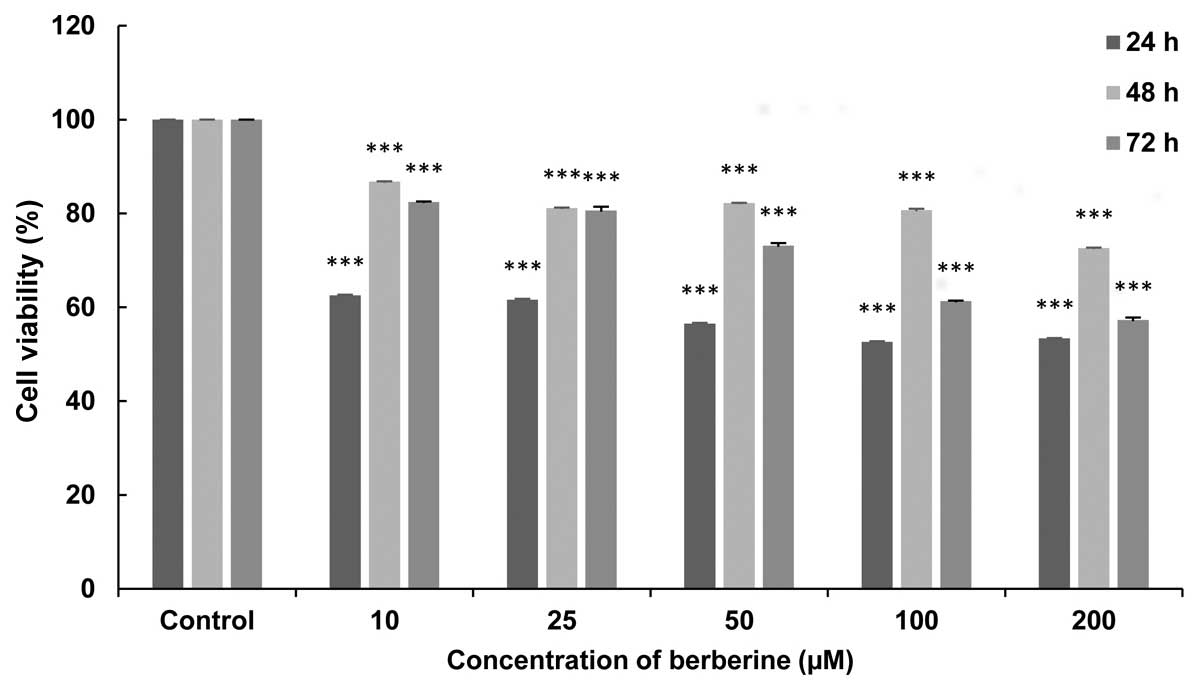
Briefly, the results indicated that BBR inhibited
the proliferation of canine mammary gland carcinoma cells, and that
treatment with 100 µM BBR for 24 h resulted in a significant
decrease in cell viability (P<0.005; Fig. 4).
Discussion
Cell homeostasis is regulated by various mechanisms,
including proliferation, growth arrest and apoptosis. Disturbance
of the equilibrium between cell growth and death often leads to
carcinogenesis (14). Cell
proliferation is regulated by the cell cycle, which is an involved
sequence of cell growth and replication (15).
Cancer is more exactly associated with being the
product of a breakdown in cell cycle regulation, such that injured
or mutated cells (that are usually destroyed) are permitted to
develop through the cell cycle accumulating mutations. Throughout
this process, the cells are tending towards genetic injuries.
Apoptosis is critical in the pathogenesis of human disease,
particularly in malignancies, while the factors controlling
apoptotic progression are suppressed, overexpressed or their
function (mutation, phosphorylation or acetylation) is modified
(16,17). Deficiency in its pathways promotes
cancer cell survival and confers resistance to antineoplastic
therapeutic agents. The number of studies regarding apoptosis is
rapidly increasing, which has led to the possibility of novel
therapeutic approaches for certain human diseases (18).
Many potential cancer-protective agents are broadly
categorized as blocking agents, which impede the initiation stage,
or suppressing agents, which arrest the promotion and progression
of tumors, presumably by affecting or disturbing crucial factors
that control cell proliferation (19).
Numerous studies in previous years have demonstrated the
anti-cancer activity of BBR against human lymphoma, leukaemia,
choriocarcinoma, melanoma, and breast, skin and prostatic cancers
(20).
In the present in vitro study, it was
indicated that BBR (10–200 µM) decreased the number of CF41.Mg
cells harvested, as evidenced by reductions in the cell viability.
These reductions, subsequent to 24-, 48- and 72-h exposure to BBR,
may be due to the inhibition of cell proliferation rather than to
the cytotoxicity of the compound used. After 24 h, the highest
tested concentrations (100 and 200 µM) demonstrated the greatest
antiproliferative effect, which was manifested by partial
degeneration of the CF41.Mg cell populations. BBR at different
concentrations, after 48 h, induced a delayed cytotoxic effect. The
inhibitory effect of BBR on the growth of CF41.Mg cells was
markedly greater after 72 h when compared with that at 48 h.
Briefly, in Fig. 4, BBR prevented the
proliferation of a markedly higher number of cells in a
concentration- (but not time-) dependent manner in the exposed
cells.
Previous studies indicated that BBR may lead to cell
death, and hence delay cell proliferation (19,21). In
addition, detailed investigations on molecular carcinogenesis
provided the potential for therapeutic intervention in cancer by
specifically targeting and sensitizing cancer cells to apoptosis
(22,23).
Herbal remedies, such as BBR act via interactions
with DNA and RNA, cell cycle arrest, and anti-inflammatory and
anti-angiogenesis activities (24).
The findings of the current study indicated that BBR may be more
active in the inhibition of tumor cell proliferation, although BBR
shows minor cytotoxicity against normal cells. For example, in the
study by Meeran et al (25) BBR
treatment enhanced reactive oxygen species (ROS) generation in
prostate cancer cells, but not in normal prostate epithelial cells,
which provides some explanation as to the antioxidative effect of
BBR in normal cells. Furthermore, the study by Sun et al
(20) indicated that various cell
lines display marked and varied reactivity to this particular
alkaloid. For example, Letasiová et al (26) demonstrated that the murine melanoma B16
cell line was more sensitive to BBR treatment than the human
promonocytic U937 cells (the values were 75–119 times lower). In
addition, different antiproliferative effects were apparent even in
the same category of tumor cells. A study by Jantova et al
(27) indicated that the cell
sensitivity to BBR, in increasing order, was as follows: B16, EAC,
V79, U937, L1210, NIH 3T3 and HeLa cells.
The results of the current study may be extrapolated
to human research, as CMTs are being considered as a spontaneous
animal model of human breast cancer. There are many similarities
between human and canine mammary cancers; the two species represent
a heterogeneous group, in terms of morphology and biological
behavior, and in human and canine mammary cancers similar
cancer-associated pathways are activated (in instances where the
two species live under similar environmental conditions) (28).
Since few therapeutic agents have been developed for
the treatment of different cancers, such as mammary neoplasms in
animals, and as BBR appears to be capable of treating tumor cells,
the current study indicates that BBR may potentially serve as a
naturally occurring compound for CMT therapy.
In conclusion, due to the antiproliferative effect
of BBR against CMT cells, the present in vitro study
proposes that BBR may be utilized as a candidate therapeutic agent
for the inhibition of CMT cell proliferation. However, further
investigation of the underlying biological mechanisms are required
to explicate the molecular interactions between BBR and cell
systems, and the potential of BBR administration in tumor cell
therapeutic strategies in vivo.
References
|
1
|
Croce CM: Oncogenes and cancer. N Engl J
Med. 358:502–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chandra D: Mitochondria as Targets for
Phytochemicals in Cancer Prevention and Therapy. Springer; New
York, USA: pp. 62–64. 2013
|
|
3
|
Cooper GM: The Cell: A Molecular Approach.
2nd. Sinauer Associates; Sunderland, MA: 2000
|
|
4
|
Andrade FH, Figueiroa FC, Bersano PR,
Bissacot DZ and Rocha NS: Malignant mammary tumor in female dogs:
Environmental contaminants. Diagn Pathol. 5:452010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Casey SC, Amedei A, Aquilano K, Azmi AS,
Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR,
et al: Cancer prevention and therapy through the modulation of the
tumor microenvironment. Semin Cancer Biol. 35:S199–S223. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seifu D, Assefa F and Abay SM: Medical
plants as antioxidant agents: Understanding their mechanism of
action and therapeutic efficacy. Research Signpost; Kerala, India:
pp. 97–145. 2012
|
|
7
|
Efferth T, Miyachi H and Bartsch H:
Pharmacogenomics of a traditional Japanese herbal medicine (Kampo)
for cancer therapy. Cancer Genomics Proteomics. 4:81–91.
2007.PubMed/NCBI
|
|
8
|
Hsu S, Yu FX, Huang Q, Lewis J, Singh B,
Dickinson D, Borke J, Sharawy M, Wataha J, Yamamoto T, et al: A
mechanism-based in vitro anticancer drug screening approach for
phenolic phytochemicals. Assay Drug Dev Technol. 1:611–618. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siriwatanametanon N, Fiebich BL, Efferth
T, Prieto JM and Heinrich M: Traditionally used Thai medicinal
plants: In vitro anti-inflammatory, anticancer and antioxidant
activities. J Ethnopharmacol. 130:196–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solowey E, Lichtenstein M, Sallon S,
Paavilainen H, Solowey E and Lorberboum-Galski H: Evaluating
medicinal plants for anticancer activity. ScientificWorldJournal.
2014:7214022014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenwell M and Rahman PK: Medicinal
Plants: Their Use in Anticancer Treatment. Int J Pharm Sci Res.
6:4103–4112. 2015.PubMed/NCBI
|
|
12
|
Gao S, Basu S, Yang G, Deb A and Hu M:
Oral bioavailability challenges of natural products used in cancer
chemoprevention. Pro Chem. 25:1553–1574. 2013.
|
|
13
|
Choi MS, Yuk DY, Oh JH, Jung HY, Han SB,
Moon DC and Hong JT: Berberine inhibits human neuroblastoma cell
growth through induction of p53-dependent apoptosis. Anticancer Res
28 (6A). 3777–3784. 2008.
|
|
14
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foster I: Cancer: A cell cycle defect.
Radiography. 14:144–149. 2008. View Article : Google Scholar
|
|
16
|
Thatte U and Dahanukar S: Apoptosis:
Clinical relevance and pharmacological manipulation. Drugs.
54:511–532. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pearson M, Carbone R, Sebastiani C, Cioce
M, Fagioli M, Saito S, Higashimoto Y, Appella E, Minucci S,
Pandolfi PP, et al: PML regulates p53 acetylation and premature
senescence induced by oncogenic Ras. Nature. 406:207–210. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vermeulen K, Berneman ZN and Van
Bockstaele DR: Cell cycle and apoptosis. Cell Prolif. 36:165–175.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang
HI, Wang CJ, Yang SF, Liou YS and Kuo WH: Berberine induces
apoptosis in SW620 human colonic carcinoma cells through generation
of reactive oxygen species and activation of JNK/p38 MAPK and FasL.
Arch Toxicol. 81:719–728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Y, Xun K, Wang Y and Chen X: A
systematic review of the anticancer properties of berberine, a
natural product from Chinese herbs. Anticancer Drugs. 20:757–769.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Serafim TL, Oliveira PJ, Sardao VA,
Perkins E, Parke D and Holy J: Different concentrations of
berberine result in distinct cellular localization patterns and
cell cycle effects in a melanoma cell line. Cancer Chemother
Pharmacol. 61:1007–1018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bremer E, van Dam G, Kroesen BJ, de Leij L
and Helfrich W: Targeted induction of apoptosis for cancer therapy:
Current progress and prospects. Trends Mol Med. 12:382–393. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin X, Zhou J, Jie C, Xing D and Zhang Y:
Anticancer activity and mechanism of Scutellaria barbata extract on
human lung cancer cell line A549. Life Sci. 75:2233–2244. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krey AK and Hahn FE: Berberine: Complex
with DNA. Science. 166:755–757. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meeran SM, Katiyar S and Katiyar SK:
Berberine-induced apoptosis in human prostate cancer cells is
initiated by reactive oxygen species generation. Toxicol Appl
Pharmacol. 229:33–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Letasiová S, Jantová S, Cipák L and
Múcková M: Berberine-antiproliferative activity in vitro and
induction of apoptosis/necrosis of the U937 and B16 cells. Cancer
Lett. 239:254–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jantova S, Cipak L and Letasiova S:
Berberine induces apoptosis through a mitochondrial/caspase pathway
in human promonocytic U937 cells. Toxicol In Vitro. 21:25–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Król M, Pawłowski KM, Szyszko K,
Maciejewski H, Dolka I, Manuali E, Jank M and Motyl T: The gene
expression profiles of canine mammary cancer cells grown with
carcinoma-associated fibroblasts (CAFs) as a co-culture in vitro.
BMC Vet Res. 8:352012. View Article : Google Scholar : PubMed/NCBI
|