Introduction
Against the background of the westernization of
eating habits and the decrease in the rate of Helicobacter
pylori (H. pylori) infection in Japan, gastroesophageal
reflux disease (GERD) is showing a tendency to increase in
incidence (1). GERD symptoms,
including heartburn and regurgitation, are known to cause a
decrease in the quality of life (QOL) of patients with GERD
(2). Functional dyspepsia (FD) is a
commonly encountered disease in clinical practice, and the symptoms
of FD are also known to reduce QOL (3). Among the therapeutic drugs available for
GERD and FD, proton pump inhibitors (PPIs) are predominantly used
as a first-line drug (4,5); however, a substantial proportion of
patients are resistant to treatment using PPIs (6). Recently, a new potassium-competitive acid
blocker (PCAB) drug, termed vonoprazan (VPZ), was developed in
Japan (7). A report has been published
describing the effects of PCAB administration on promoting the
healing of esophageal mucosal injury (8); however, there have been few reports about
the efficacy of PCAB for improving the symptoms of GERD and FD. The
aim of the present study was to investigate the efficacy of PCAB
for producing symptomatic improvements in patients with GERD and
FD.
Materials and methods
Study design
A hospital-based, retrospective study of outpatients
was performed in our department (Department of Gastroenterology,
University of Juntendo, Tokyo, Japan) between March 2015 and August
2016. The patients were asked to complete the global overall
symptom (GOS) scale to determine symptom severity at baseline, and
after 4 weeks of VPZ therapy by one specialist (D.A.), who was a
member of the Japan Society of Gastroenterology. Patients also had
to have heartburn, acid regurgitation, gastric pain, and/or a heavy
feeling in the stomach of at least moderate severity (GOS scale
score of 4) at baseline. The patients were administered with 20 mg
VPZ once daily for 4 weeks. The efficacy of the improvement in the
symptoms attributable to VPZ was assessed by evaluating the GOS
scale scores for heartburn, acid regurgitation, gastric pain, and a
heavy feeling in the stomach recorded by patients at baseline, and
subsequently after the administration of VPZ for 4 weeks. The GOS
scale has been validated for the assessment of upper
gastrointestinal symptoms in a clinical trial setting (9), and has been used in clinical studies to
assess the symptoms of GERD and FD (10,11). The GOS
scale measures the severity of eight symptoms (heartburn, acid
regurgitation, gastric pain, stomach feeling heavy, early satiety,
feeling queasy, burping, and feeling of fullness) on a 7-point
scale, from 1 [‘no problem’ (no symptoms)] to 7 [‘very severe
problem’ (cannot be ignored, markedly limits my daily activities
and often requires rest)] (9). The
four symptoms (heartburn, acid regurgitation, gastric pain, and
stomach feeling heavy) measured by the GOS scale were investigated
in order to perform symptom-based evaluations in the present
study.
Patients of either gender who were aged ≥20 years
were eligible for inclusion if information on all of the following
aspects were provided from their medical records: Patient profile
[age, gender, body mass index (BMI), alcohol intake, and smoking];
H. pylori infection status (negative, positive, or negative
after eradication therapy); and findings of upper gastrointestinal
endoscopy [hiatal hernia and endoscopic gastric mucosal atrophy
(EGA)]. BMI was calculated as body weight divided by the square of
body height in meters (kg/m2). H. pylori
infection status was assessed by the 13C-urea breath
test (12) and/or the presence of
serum antibodies against H. pylori. A positive result for
any of these tests was defined as being positive for H.
pylori infection. A result of ‘negative after eradication’ was
also defined as the 13C-urea breath test being negative
for H. pylori infection at 4–8 weeks following eradication
therapy. In upper gastrointestinal endoscopy, a hiatal hernia was
defined as an apparent separation of the esophagogastric junction
and diaphragm impression by >2 cm. EGA was classified as C-0
(normal), C-1, C-2, C-3, O-1, O-2 or O-3 using the Kimura-Takemoto
classification system (13), which
identifies the location of the endoscopic atrophic border. Overall,
EGA was scored as 0 for C-0 type, 1 for C-1 type, 2 for C-2 type, 3
for C-3 type, 4 for O-1 type, 5 for O-2 type, and 6 for O-3 type.
Patients with the following were excluded: Those who had
gastrectomy, peptic ulcer, gastric or esophageal malignant disease,
or successful eradication of H. pylori within the previous 6
months. Additionally, patients who were currently or previously
treated with non-steroidal anti-inflammatory drugs and low-dose
aspirin were excluded.
In the present study, the study patients were
divided into 3 groups (RE, NERD, and FD). ‘RE’ patients were
defined as those who had findings of RE of grades A, B, C, and D
according to the Los Angeles Classification system (14), and also had heartburn and/or acid
regurgitation of at least moderate severity (GOS scale score of 4)
at baseline. ‘NERD’ patients were defined as those who had
exhibited findings without RE and also had heartburn and/or acid
regurgitation of at least moderate severity (GOS scale score of 4)
at baseline. ‘FD’ patients were defined as those who had findings
without RE, and also had gastric pain and/or a heavy feeling in the
stomach of at least moderate severity (GOS scale score of 4) at
baseline. However, the patients for whom the scores for heartburn
and/or acid regurgitation were higher than the scores for gastric
pain and/or a heavy feeling in the stomach were excluded from the
FD group. As part of a subgroup analysis, in each group (RE, NERD,
and FD), the patients who were administered with VPZ as an initial
therapy were defined as ‘f-RE’, ‘f-NERD’, and ‘f-FD’ patients,
respectively. Among the RE and NERD patients, those who had
heartburn and/or acid regurgitation of at least moderate severity
(GOS scale score of 4) after having received PPI therapy for >8
weeks were defined as ‘r-RE’ and ‘r-NERD’ patients, respectively.
For the FD patients, the patients who had gastric pain and/or a
heavy feeling in the stomach of at least moderate severity (GOS
scale score of 4) after receiving PPI therapy for >8 weeks were
defined as ‘r-FD’ patients. Furthermore, the total points of the
GOS scale scores for both heartburn and acid regurgitation were
defined as a ‘GERD score’, and the total points of the GOS scale
scores of both gastric pain and a heavy feeling in the stomach were
also defined as an ‘FD score’.
First, the proportions of patients with RE, NERD,
and FD who achieved an improvement in their symptoms, and who were
assigned a GOS scale score of 1 [‘no problem’ (no symptoms)] or 2
[‘minimal problem’ (can be easily ignored without effort)] after
the administration of VPZ for 4 weeks, were evaluated. As a
subgroup analysis, the proportions of f-RE, f-NERD, f-FD, r-RE,
r-NERD, and r-FD patients achieving an improvement in their
symptoms after the administration of VPZ were evaluated.
Secondly, changes in the GERD score in patients with
RE and NERD, and in the FD score in FD patients, during 4 weeks of
VPZ therapy were evaluated. The presence of adverse events was
investigated throughout the administration of VPZ, and these were
assessed according to whether or not they were serious.
The present study was performed in accordance with
the principles of the Declaration of Helsinki. The Juntendo
University Ethics Committee approved the study, and the study
protocol (reference no. 16-098). With regard to the informed
consent of participants, the Juntendo University Ethics Committee
made a decision based on the Ethical Guidelines for Medical and
Health Research Involving Human Subjects, which states that
non-intervention studies are deemed exempt from patients' consent
and, instead, researchers must notify the study subjects about the
information regarding study contents on a home page, and guarantee
an opportunity when the study subjects could refuse it.
Consequently, the decision of the Juntendo University Ethics
Committee was put into practice in the present study.
Statistical analysis
The clinical characteristics were evaluated using
the χ2 test and Fisher's exact test. Changes in the GERD
and FD scores in before and after 4-weeks of VPZ therapy were
evaluated using Student's t-test. All statistical analyses were
performed using SPSS version 19 software (IBM SPSS, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics
The clinical characteristics of the 88 eligible
cases, in total [30 men (34.1%) and 58 women (65.9%); mean age,
60.2±14.7; and mean BMI, 21.9±3.3] are summarized in Table I. The rates of alcohol intake and
smoking were 25.0 and 10.2%, respectively. Cases who were H.
pylori negative, H. pylori positive, and H.
pylori negative following eradication therapy numbered 52
(59.1%), 8 (9.1%), and 28 (31.8%), respectively. There were 20
(22.7%) cases of RE, and 9, 7, 3, and 1 of these cases had Los
Angeles grades A, B, C, and D, respectively. Hiatal hernia was
observed in 51 (58.0%) cases. The mean EGA was 1.7±1.8. The numbers
of cases of EGA classified as C-0, C-1, C-2, C-3, O-1, O-2, and O-3
were 35, 16, 9, 6, 16, 3, and 3, respectively. In the present
study, RE, NERD, and FD patients numbered 20, 25, and 43,
respectively. During the 4-week administration of VPZ, four
patients had mild constipation and one patient had mild diarrhea;
however, none of these patients discontinued the treatment since
their symptoms of constipation and diarrhea were only mild.
 | Table I.Clinical characteristics of study
subjects (n=88). |
Table I.
Clinical characteristics of study
subjects (n=88).
| Characteristic | Total (n=88) | RE (n=20) | NERD (n=25) | FD (n=43) |
|---|
| Patient profile |
|
|
|
|
| Age
(years) | 60.2
(±14.7)b | 59.1
(±15.1)b | 61.7
(±15.2)b | 59.8
(±14.4)b |
|
Gender |
|
|
|
|
|
Male | 30
(34.1)a | 13
(65.0)a | 7 (28.0)a | 10
(23.3)a |
|
Female | 58
(65.9)a | 7 (35.0)a | 18
(72.0)a | 33
(76.7)a |
| BMI
(kg/m2) | 21.9
(±3.3)b | 24.0
(±2.6)b | 22.3
(±2.9)b | 20.8
(±3.4)b |
|
Smoking |
|
|
|
|
|
Smoker | 9
(10.2)a | 4
(20.0)a | 2
(8.0)a | 3
(7.0)a |
|
Non-smoker | 79
(89.8)a | 16
(80.0)a | 23
(92.0)a | 40
(93.0)a |
| Alcohol
consumption |
|
|
|
|
|
Drinker | 22
(25.0)a | 9
(45.0)a | 6
(24.0)a | 7
(16.3)a |
|
Non-drinker | 66
(75.0)a | 11
(55.0)a | 19
(76.0)a | 36
(83.7)a |
| H.
pylori |
|
|
|
|
| H.
pylori infection |
|
|
|
|
|
Positive | 8
(9.1)a | 1
(5.0)a | 4
(16.0)a | 3
(7.0)a |
|
Negative | 52
(59.1)a | 15
(75.0)a | 11
(44.0)a | 26
(60.5)a |
|
Negative after
eradication | 28
(31.8)a | 4
(20.0)a | 10
(40.0)a | 14
(32.5)a |
| Upper GI
findings |
|
|
|
|
| Reflux
esophagitis |
|
Yes | 20
(22.7)a |
|
|
|
|
No | 68
(77.3)a |
|
|
|
|
LA-grade |
|
|
|
|
|
A | 9 |
|
|
|
|
B | 7 |
|
|
|
|
C | 3 |
|
|
|
|
D | 1 |
|
|
|
| Hiatal
hernia |
|
|
|
|
|
Yes | 51
(58.0)a | 18
(90.0)a | 7
(28.0)a | 26
(60.5)a |
|
No | 37
(42.0)a | 2
(10.0)a | 18
(72.0)a | 17
(39.5)a |
|
Endoscopic gastric mucosal
atrophy | 1.7
(±1.8)b | 1.3
(±1.6)b | 1.8
(±1.8)b | 1.8
(±1.9)b |
RE patients
The clinical characteristics of the 20 eligible
patients with RE [13 men (65.0%) and 7 women (35.0%); mean age,
59.1±15.1 years; and mean BMI, 24.0±2.6) are shown in Table I. The rates of alcohol intake and
smoking were 45.0 and 20.0%, respectively. Cases who were H.
pylori negative, H. pylori positive, and H.
pylori negative following eradication therapy numbered 15
(75.0%), 1 (5.0%), and 4 (20.0%), respectively. Among the cases of
RE, 9, 7, 3, and 1 cases had Los Angeles grades A, B, C, and D,
respectively. Hiatal hernia was observed in 18 (90.0%) cases. The
mean EGA was 1.3±1.6. The numbers of cases of EGA classified as
C-0, C-1, C-2, C-3, O-1, O-2, and O-3 were 9, 5, 1, 2, 2, 1, and 0,
respectively. Of the 20 RE patients, 11 cases had f-RE and 9 cases
had r-RE. The proportion of RE patients who achieved an improvement
in their reflux symptoms after 4 weeks of VPZ therapy was 75.0%
(15/20). The proportions of patients with f-RE and r-RE who
achieved an improvement in their reflux symptoms after 4 weeks of
VPZ therapy were 90.9% (10/11) and 55.6% (5/9), respectively. The
GERD scores at baseline and after 4 weeks of VPZ therapy in
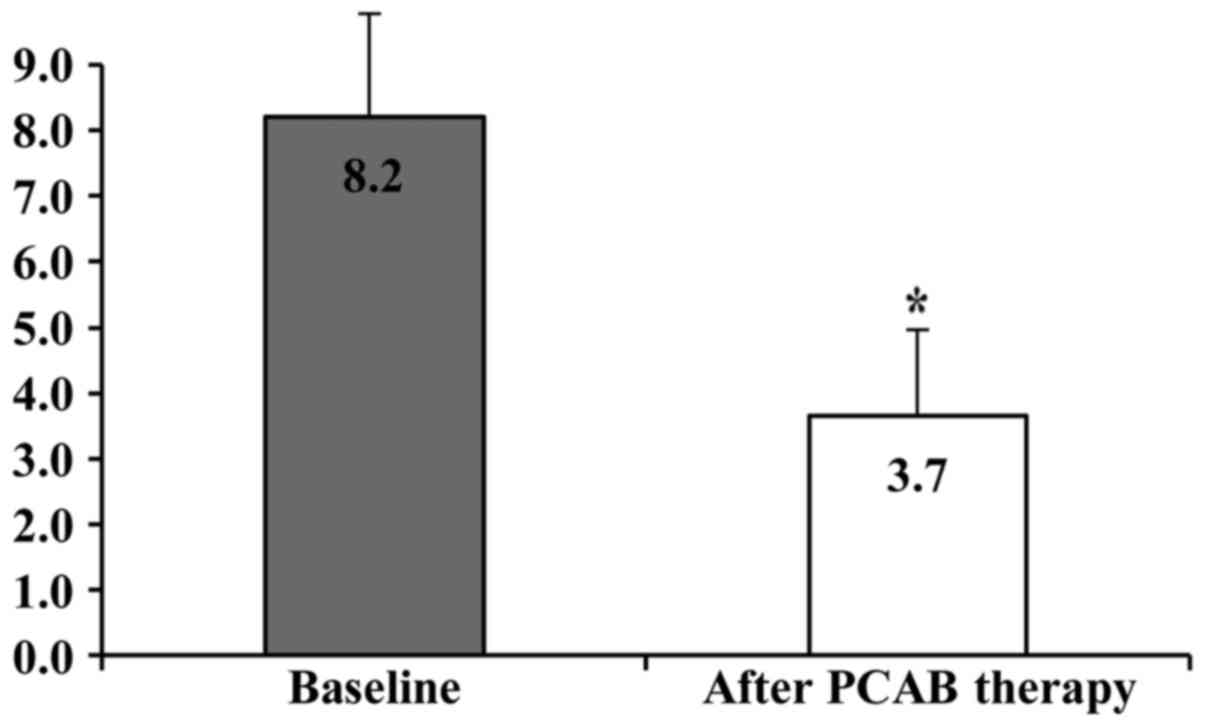
patients with RE were 8.2±1.7 and 3.7±1.4, respectively, and this
difference was statistically significant (P<0.01; Fig. 1).
NERD patients
The clinical characteristics of the 25 eligible
patients with NERD [7 men (28.0%) and 18 women (72.0%); mean age
61.7±15.2, and mean BMI, 22.3±2.9] are shown in Table I. The rates of alcohol intake and
smoking were 24.0 and 8.0%, respectively. Cases who were H.
pylori negative, H. pylori positive, and H.
pylori negative following eradication therapy numbered 11
(44.0%), 4 (16.0%), and 10 (40.0%), respectively. Hiatal hernia was
observed in 7 (28.0%) cases. The mean EGA was 1.8±1.8. The numbers
of cases of EGA classified as C-0, C-1, C-2, C-3, O-1, O-2, and O-3
were 9, 4, 3, 2, 6, 0, and 1, respectively. Of the 25 patients with
NERD, 12 cases had f-NERD and 13 cases had r-NERD. The proportion
of the total NERD patients who achieved an improvement in their
reflux symptoms after 4 weeks of VPZ therapy was 60.0% (15/25). The
proportions of patients with f-NERD and r-NERD who achieved an
improvement in their reflux symptoms after 4 weeks of VPZ therapy
were 66.7% (8/12) and 53.8% (7/13), respectively. The GERD scores
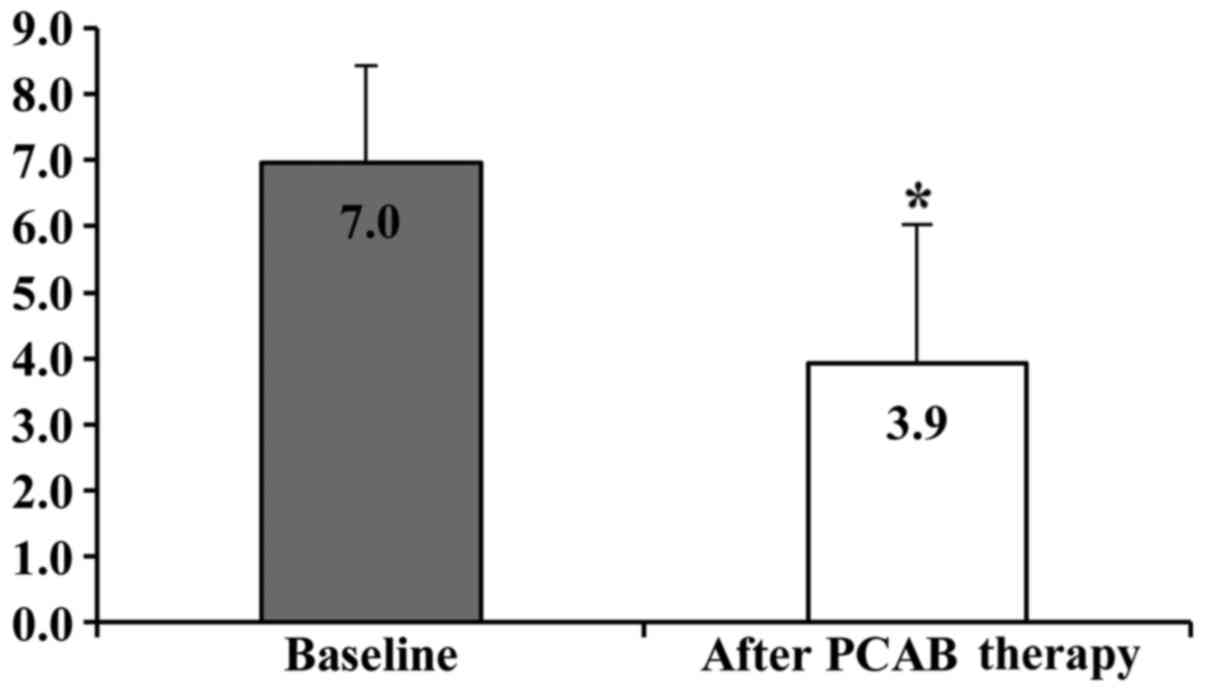
at baseline and after 4 weeks of VPZ therapy in patients with NERD
were 7.0±1.6 and 3.9±2.0, and this difference was statistically
significant (P<0.01; Fig. 2).
FD patients
The clinical characteristics of the 43 eligible
patients with FD [10 men (23.3%) and 33 women (76.7%); mean age,
59.8±14.4; and mean BMI, 20.8±3.4] are shown in Table I. Alcohol intake and smoking were 16.3
and 7.0%, respectively. Cases who were H. pylori negative,
H. pylori positive, and H. pylori negative after
eradication therapy numbered 26 (60.5%), 3 (7.0%), and 14 (32.6%),
respectively. Hiatal hernia was observed in 26 (60.5%) cases. The
mean EGA was 1.8±1.9. The numbers of cases of EGA classified as
C-0, C-1, C-2, C-3, O-1, O-2, and O-3 were 17, 7, 5, 2, 8, 2, and
2, respectively. Of the 43 patients with FD, 17 cases had f-FD and
26 cases had r-FD. The proportion of FD patients who achieved an
improvement in their dyspepsia symptoms after 4 weeks of VPZ
therapy was 48.8% (21/43). The proportions of f-FD and r-FD
patients who achieved an improvement in their dyspepsia symptoms
after 4 weeks of VPZ therapy were 58.8% (10/17) and 42.3% (11/26),
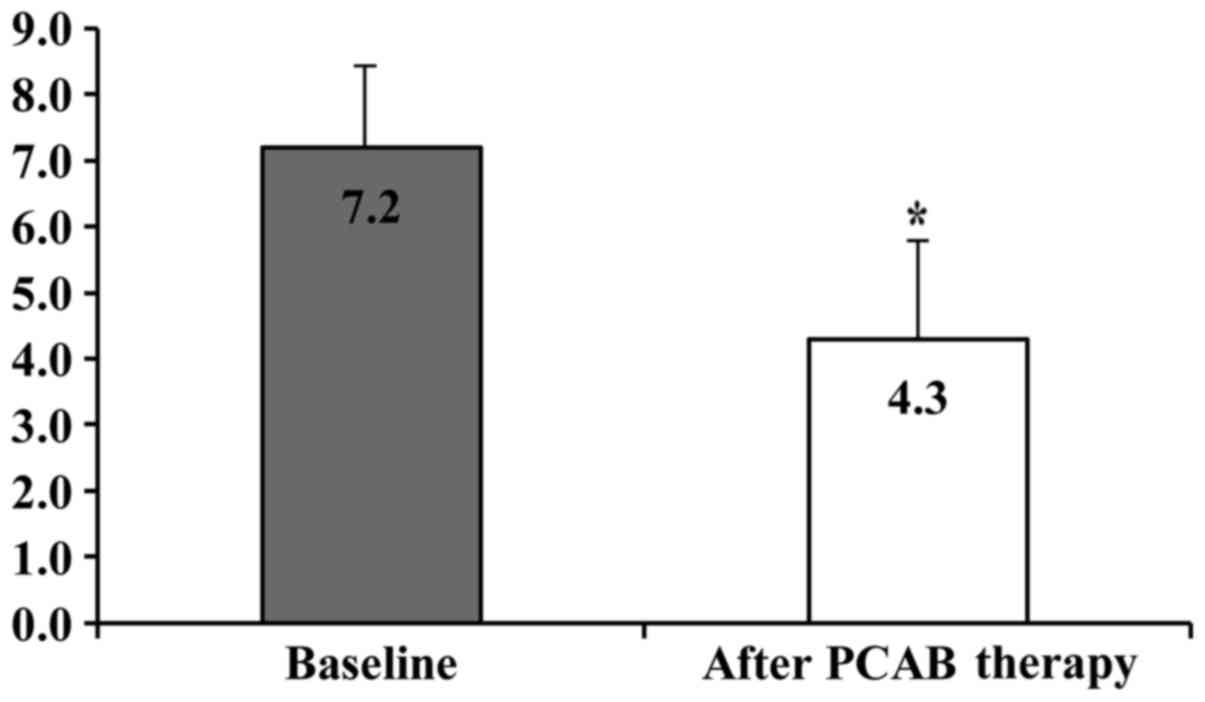
respectively. The FD scores at baseline and after 4 weeks of VPZ
therapy in patients with FD were 7.2±1.6 and 4.3±1.8, respectively,
and this difference was statistically significant (P<0.01;
Fig. 3).
Discussion
The present retrospective study is, to the best of
our knowledge, the first study on efficacy of a PCAB for improving
the symptoms of patients with RE, NERD, and FD. In the present
study, patients were administered with a new PCAB, termed VPZ, for
4 weeks. Following this treatment, compared with baseline, the
rates of symptomatic improvement in patients with RE, NERD, and FD
were 75.0, 60.0, and 48.8%, respectively, and the GERD score in
patients with RE and NERD, and the FD score in patients with FD,
were both decreased. In cases that were administered VPZ as an
initial therapy, the rates of symptomatic improvement were 90.9,
66.7, and 58.8% in patients with RE, NERD, and FD, respectively. In
cases that were resistant to 8 weeks of PPI therapy, the rates of
symptomatic improvement were 55.6, 53.8, and 42.3% in patients with
RE, NERD, and FD, respectively. The GERD score in patients with RE
and NERD, and the FD score in patients with FD, were both decreased
after 4 weeks of VPZ therapy.
PPIs have been used as the first-line drugs for the
treatment of GERD in Japan since it became evident from the results
of multiple Japanese studies, and those from outside Japan, that
PPIs exhibit superior protective effects against the esophageal
mucosal injury associated with RE compared with those of
histamine-2 receptor antagonists (4,15). However,
it has become clear during the use of PPIs in patients with GERD
that there are certain cases in which GERD symptoms remain
following treatment with PPIs (16).
It was reported that the symptoms of GERD are not proportional to
the degree of the esophageal mucosal injury (17), and these symptoms reduce the QOL of
GERD patients (18). Therefore, in
addition to healing the esophageal mucosal injury, it is also
important to achieve symptomatic relief for GERD. In a multicenter,
randomized trial in Japanese patients with RE, the proportions of
patients achieving sufficient relief of their reflux symptoms
(heartburn and regurgitation) were similar (~80%) in 2 groups who
were treated with 20 mg omeprazole and 10 mg rabeprazole,
respectively, for 4 weeks (19). In
the present study, patients who were administered VPZ as an initial
therapy had a relatively high rate of symptomatic improvement
(90.9%) in cases of RE, while the rate of improvement in patients
with NERD tended to be lower (66.7%). Previous reports have
revealed that not only the regurgitation of gastric acid, but also
hyperesthesia and psychological factors participate in generating
the subjective symptoms of NERD (20,21).
Therefore, in patients with NERD, a strong suppression of gastric
acid secretion might be less likely to succeed compared with that
in RE patients. On the other hand, in patients with RE and NERD who
were resistant to 8 weeks of PPI therapy, the rates of improvement
for their GERD symptoms were 55.6 and 53.8%, respectively. PPIs are
known to have certain disadvantages, including being affected by
genetic polymorphisms of CYP2C19, and being inactivated easily
under acidic conditions (22). In
comparison with PPIs, PCABs bind to the proton pump continuously in
gastric parietal cells to reduce gastric acid secretion without
requiring activation or being deactivated by the gastric acid.
Furthermore, it was reported that PCABs are not affected by genetic
polymorphisms of CYP2C19 (23,24). In patients with GERD whose gastric acid
secretion was not suppressed sufficiently well by the
administration of a PPI, it may be possible to control the
secretion further by administering a PCAB. PCABs may become a novel
choice of therapy for patients with GERD who are resistant to PPI
therapy.
The etiology of FD is complicated, and FD may be
caused by various factors (25).
According to the Japanese guidelines for the treatment of FD, the
major therapeutic drugs for FD are gastric acid secretion
inhibitors and gastroprokinetic drugs (5,26). As for
gastric acid secretion inhibitors, in a multicenter,
double-blinded, randomized, placebo-controlled trial in Japanese
patients with FD, it was reported that treatment with 20 mg
rabeprazole once daily for 8 weeks achieved a higher rate of
satisfactory symptom relief compared with a placebo treatment (45.3
vs. 28.2%) (27). As for
gastroprokinetic drugs, a multicenter, randomized, 4-week
placebo-controlled trial in Japanese patients with FD reported that
52.2% of those receiving acotiamide, and 34.8% of those in the
placebo group, were classified as responders (28). In the present study, patients with FD
who were administered with VPZ for 4 weeks as an initial treatment
had a relatively high response rate (58.8%), although the present
study is a retrospective study, rather than a placebo-controlled
study. In addition, the subgroup analysis revealed that, even for
patients with FD who were resistant to 8 weeks of PPI therapy, the
rate of symptomatic improvement was 42.3%. Therefore, PCABs may
become novel therapeutic drugs for patients with FD who are
PPI-therapy-resistant. However, for ~60% of the
PPI-therapy-resistant FD patients in the present study, VPZ
treatment did not succeed, and the influence of a mechanism other
than gastric acid reflux was considered to be an etiological factor
in these patients. In addition, the present study did not consider
the disease duration of the patients with FD. The rate of
symptomatic improvement elicited by the administration of PCAB to
patients with FD according to the Rome III criteria (29), which categorize the disease duration of
FD, has yet to be elucidated.
The present study had several limitations. First, it
was a hospital-based, single-center, retrospective study of
outpatients who were asked to complete a questionnaire to determine
the severity of their symptoms. Secondly, the study procedures were
conducted by one specialist (D.A.) who was a member of the Japan
Society of Gastroenterology; therefore, the data might not
represent the general population. Furthermore, the sample size of
the present study was relatively small; therefore, further larger,
randomized multicenter prospective studies will be required to
clarify the efficacy of PCAB for improving the symptoms of patients
with RE, NERD, and FD. Finally, it was not possible to investigate
dietary intake, beverages, waist circumference, visceral fat area,
exercise, eating habits, or sleep, which are all able to affect the
pathophysiology of RE, NERD, and FD.
This retrospective study is the first report, to the
best of our knowledge, that has examined the efficacy of PCAB for
improving the symptoms of patients with GERD and FD. In GERD and FD
patients, the possibility has materialized that PCAB might be
useful as a novel therapeutic drug, not only as an initial therapy,
but also for patients who are not satisfied with their treatment
after 8 weeks of therapy with conventional PPIs. Symptomatic relief
by PCABs might also increase the QOL of patients with GERD and FD.
However, additional, large and prospective multicenter trials are
required to further clarify the efficacy of PCABs for improving the
symptoms of patients with GERD and FD.
References
|
1
|
Fujiwara Y and Arakawa T: Epidemiology and
clinical characteristics of GERD in the Japanese population. J
Gastroenterol. 44:518–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dimenas E: Methodological aspects of
evaluation of quality of life in upper gastrointestinal diseases.
Scand J Gastroenterol Suppl. 199:181993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meineche-Schmidt V, Talley NJ, Pap A,
Kordecki H, Schmid V, Ohlsson L, Wahlqvist P, Wiklund I and
Bolling-Sternevald E: Impact of functional dyspepsia on quality of
life and health care consumption after cessation of antisecretory
treatment. A multicentre 3-month follow-up study. Scand J
Gastroenterol. 34:566–574. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiba N, De Gara CJ, Wilkinson JM and Hunt
RH: Speed of healing and symptom relief in grade II to IV
gastroesophageal reflux disease: A meta-analysis. Gastroenterology.
112:1798–1810. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Talley NJ, Meineche-Schmidt V, Paré P,
Duckworth M, Räisänen P, Pap A, Kordecki H and Schmid V: Efficacy
of omeprazole in functional dyspepsia: Double-blind, randomized,
placebo-controlled trials (the Bond and Opera studies). Aliment
Pharmacol Ther. 12:1055–1065. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chey WD, Mody RR and Izat E: Patient and
physician satisfaction with proton pump inhibitors (PPIs): Are
there opportunities for improvement? Dig Dis Sci. 55:3415–3422.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hori Y, Imanishi A, Matsukawa J, et al: 1-
[5- (2- Fluorophenyl)- 1- (pyridin- 3- ylsulfonyl)- 1H- pyrrol- 3-
yl]- N- methylmethanamine monofumarate (TAK- 438), a novel and
potent potassium- competitive acid blocker for the treatment of
acid- related diseases. J Pharmacol Exp Ther. 335:231–238. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashida K, Sakurai Y, Nishimura A, Kudou K,
Hiramatsu N, Umegaki E, Iwakiri K and Chiba T: Randomised clinical
trial: A dose-ranging study of vonoprazan, a novel
potassium-competitive acid blocker, vs. lansoprazole for the
treatment of erosive oesophagitis. Aliment Pharmacol Ther.
42:685–695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Zanten SJ Veldhuyzen, Chiba N,
Armstrong D, Barkun AN, Thomson AB, Mann V, Escobedo S, Chakraborty
B and Nevin K: Validation of a 7-point global overall symptom scale
to measure the severity of dyspepsia symptoms in clinical trials.
Aliment Pharmacol Ther. 23:521–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakurai K, Nagahara A, Inoue K, Akiyama J,
Mabe K, Suzuki J, Habu Y, Araki A, Suzuki T, Satoh K, et al:
Efficacy of omeprazole, famotidine, mosapride and teprenone in
patients with upper gastrointestinal symptoms: an
omeprazole-controlled randomized study (J-FOCUS). BMC
Gastroenterol. 12:422012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Zanten SV, Armstrong D, Chiba N, Flook
N, White RJ, Chakraborty B and Gasco A: Esomeprazole 40 mg once a
day in patients with functional dyspepsia: The randomized,
placebo-controlled ‘ENTER’ trial. Am J Gastroenterol.
101:2096–2106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Savarino V, Vigneri S and Celle G: The 13C
urea breath test in the diagnosis of Helicobacter pylori infection.
Gut. 45:(Suppl 1). I18–I22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 3:87–97. 1969. View Article : Google Scholar
|
|
14
|
Armstrong D, Bennett JR, Blum AL, Dent J,
De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE,
Spechler SJ, et al: The endoscopic assessment of esophagitis: A
progress report on observer agreement. Gastroenterology. 111:85–92.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soga T, Matsuura M, Kodama Y, Fujita T,
Sekimoto I, Nishimura K, Yoshida S, Kutsumi H and Fujimoto S: Is a
proton pump inhibitor necessary for the treatment of lower-grade
reflux esophagitis? J Gastroenterol. 34:435–440. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Serag H, Becher A and Jones R:
Systematic review: Persistent reflux symptoms on proton pump
inhibitor therapy in primary care and community studies. Aliment
Pharmacol Ther. 32:720–737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fennerty MB and Johnson DA: Heartburn
severity does not predict disease severity in patients with erosive
esophagitis. MedGenMed. 8:62006.PubMed/NCBI
|
|
18
|
Pace F, Negrini C, Wiklund I, Rossi C and
Savarino V: ITALIAN ONE INVESTIGATORS STUDY GROUP: Quality of life
in acute and maintenance treatment of non-erosive and mild erosive
gastro-oesophageal reflux disease. Aliment Pharmacol Ther.
22:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagahara A, Suzuki T, Nagata N, Sugai N,
Takeuchi Y, Sakurai K, Miyamoto M, Inoue K, Akiyama J, Mabe K, et
al: A multicentre randomised trial to compare the efficacy of
omeprazole versus rabeprazole in early symptom relief in patients
with reflux esophagitis. J Gastroenterol. 49:1536–1547. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fass R: Erosive esophagitis and nonerosive
reflux disease (NERD): Comparison of epidemiologic, physiologic,
and therapeutic characteristics. J Clin Gastroenterol. 41:131–137.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagahara A, Miwa H, Minoo T, Hojo M,
Kawabe M, Osada T, Kurosawa A, Asaoka D, Terai T, Ohkusa T, et al:
Increased esophageal sensitivity to acid and saline in patients
with nonerosive gastro-esophageal reflux disease. J Clin
Gastroenterol. 40:891–895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adachi K, Katsube T, Kawamura A, Takashima
T, Yuki M, Amano K, Ishihara S, Fukuda R, Watanabe M and Kinoshita
Y: CYP2C19 genotype status and intragastric pH during dosing with
lansoprazole or rabeprazole. Aliment Pharmacol Ther. 14:1259–1266.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin JM, Inatomi N, Munson K, Strugatsky
D, Tokhtaeva E, Vagin O and Sachs G: Characterization of a novel
potassium-competitive acid blocker of the gastric H, K-ATPase,
1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine
monofumarate (TAK-438). J Pharmacol Exp Ther. 339:412–420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hori Y, Matsukawa J, Takeuchi T, Nishida
H, Kajino M and Inatomi N: A study comparing the antisecretory
effect of TAK-438, a novel potassium-competitive acid blocker, with
lansoprazole in animals. J Pharmacol Exp Ther. 337:797–804. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tack J, Piessevaux H, Coulie B, Caenepeel
P and Janssens J: Role of impaired gastric accommodation to a meal
in functional dyspepsia. Gastroenterology. 115:1346–1352. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Talley NJ, Tack J, Ptak T, Gupta R and
Giguère M: Itopride in functional dyspepsia: Results of two phase
III multicentre, randomised, double-blind, placebo-controlled
trials. Gut. 57:740–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwakiri R, Tominaga K, Furuta K, Inamori
M, Furuta T, Masuyama H, Kanke K, Nagahara A, Haruma K, Kinoshita
Y, et al: Randomised clinical trial: Rabeprazole improves symptoms
in patients with functional dyspepsia in Japan. Aliment Pharmacol
Ther. 38:729–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsueda K, Hongo M, Tack J, Saito Y and
Kato H: A placebo-controlled trial of acotiamide for meal-related
symptoms of functional dyspepsia. Gut. 61:821–828. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drossman DA: The functional
gastrointestinal disorders and the Rome III process.
Gastroenterology. 130:1377–1390. 2006. View Article : Google Scholar : PubMed/NCBI
|