Introduction
Approximately one-third of breast cancer patients
develop recurrent tumors and subsequently succumb to the disease.
The treatment strategy for recurrent breast cancer is generally
determined based on information from the pathological diagnosis of
the primary lesion. However, tumor phenotype, as represented by
estrogen receptor (ER), progesterone receptor (PR) and human
epidermal growth factor 2 (HER2) status occasionally changes at
recurrence (1–4). Therefore, pathological assessment of
recurrent tumors may provide important information to guide the
therapeutic strategy.
In the present study, the status of hormonal
receptors, ER, PR and HER2, and the Ki67 index were compared in
primary and recurrent tumors, as well as those of metastatic lymph
nodes that were dissected at the time of primary tumor evaluation.
In addition, correlations between alterations in tumor status and
patient survival were examined. To the best of our knowledge, this
is the first study to report survival risk upon recognition of
alterations in hormonal receptors.
Patients and methods
Patients
A total of 70 breast cancer patients with a date of
original diagnosis between August 1990 and January 2012 were
enrolled in the present study. The initial surgical procedures for
the primary lesions were performed at Aichi Medical University
Hospital (Nagakute, Japan) for 54 patients, at Marumo Hospital
(Nagoya, Japan) for 13, at Kato Clinic (Nagoya, Japan) for 2, and
at Nagoya City University Hospital (Nagoya, Japan) for 1 patient.
All the patients were subsequently continuously followed-up at
Aichi Medical University Hospital. During the follow-up period, the
attending physician of the outpatient clinic planned for annual
mammography. Computed tomography of the total body and/or blood
tests were performed if indicated by the patient's clinical
condition or at the discretion of the attending physician. When
recurrence was diagnosed, the lesion was biopsied or excised for
pathological confirmation. For 15 patients, fine needle aspiration
cytology was used to diagnose metastatic lesions. For
immunohistochemical examination of cytology specimens, cell blocks
were prepared using cyto-puncture materials as described by Kumar
et al (5). Clinical data of
the study patients were obtained from their medical records.
Tumor pathology
Pathological assessment of recurrent lesions in all
patients was performed by the Department of Pathology at the Aichi
Medical University Hospital. Diagnosis of the primary lesion was
made at Aichi Medical University Hospital for 59 patients. For the
remaining 11 patients, pathological examination of the primary
lesion was performed at the institution where surgery was
performed. The histologic type was determined according to the
World Health Organization criteria (6). Histopathological grading was as
described by TNM classification of the 6th edition (7). Primary and metastatic lesions were
compared based on the ER, PR and HER2 status, as well as the Ki67
index, a marker of proliferation. ER or PR positivity was defined
as an Allred score of ≥3 and moderate-to-intense nuclear staining
of ≥10%, respectively, while for cell block specimens, moderate to
intense staining of ≥10% of all countable tumor cells was
considered to indicate ER or PR positivity (8,9).
Immunohistochemical assessment of HER2 expression levels was
conducted using an anti-HER2 monoclonal antibody (A0485; Agilent
Technologies, Inc., Santa Clara, CA, USA). HER2 overexpression was
scored as 0 (negative), 1+ (incomplete membrane staining in any
proportion of tumor cells), 2+ (complete membrane staining that was
either nonuniform or weak in intensity but with obvious
circumferential distribution in ≥10% tumor cells, or invasive
tumors with intense, complete membrane staining of ≤30% tumor
cells) and 3+ (uniform, intense membrane staining of >30%
invasive tumor cells), in accordance with the guidelines of the
American Society of Clinical Oncology (ASCO)/College of American
Pathologists (CAP) (10). Scores of 0
and 1+ were considered negative while 3+ was considered positive
(10). When the immunohistochemical
score was 2+, fluorescence in situ hybridization (FISH) was
performed using the PathVysion HER-2 DNA Probe kit (Abbott
Pharmaceutical Co. Ltd., Lake Bluff, IL, USA) and the results were
assessed according to the manufacturer's instructions. A FISH score
>2 was defined as positive. The same procedure was used for the
evaluation of HER2 in cell block specimens. The Ki67 index was
expressed as the proportion of positive cells within at least 500
tumor cells. Interpretation of Ki67 staining and scoring was based
upon the recommendations described by Dowsett et al
(11). Similarly, the proportion of
cells with moderate to intense staining, using the anti-Ki67
antibody (M7240; Agilent Technologies, Inc.), within all countable
tumor cells in the cell block specimen was interpreted as the Ki67
index. Lymph node metastases were observed in 39 patients at the
time of initial surgery. The same pathological assessments were
performed for metastatic lymph nodes for 30 patients for whom
paraffin blocks could be made.
Immunohistochemistry
Primary tumors, metastatic lymph nodes and
histologically proven metastases were subjected to
immunohistochemical analysis with anti-ER (1:1; 107925) and anti-PR
(1:1; 102333) (both from Roche Diagnostics, Basel, Switzerland),
anti-HER2 (1:100; A0485) and anti-Ki67 (1:100; M7240) (both from
Agilent Technologies, Inc.) antibodies according to routine
protocols. Briefly, 4-µm sections of paraffin-embedded tissue and
cell block specimens were deparaffinized 3 times in xylene,
rehydrated in graded alcohol, and rinsed in Tris-buffered saline
(TBS). To improve antigen retrieval, dewaxed sections were immersed
in 0.1 M Tris-buffer, pH 9.5 (for ER and PR) or citrate buffer, pH
6.0 (for HER2 and Ki67), heated for 7 min in a pressure cooker,
cooled at room temperature for 5 min, and washed 3 times in TBS.
All subsequent steps were performed at room temperature. Endogenous
peroxidase activity was blocked by incubation for 15 min in
methanol containing 0.3% hydrogen peroxidase, followed by 3 washes
in TBS. Non-specific binding was blocked by incubating the sections
for 10 min in phosphate-buffered saline containing 5% skim milk.
After removing excess blocking agents, the primary antibodies were
applied and the sections were incubated in a moist chamber (20CG;
Cosmo Bio Co., Ltd., Tokyo, Japan) for 1 h at room temperature to
enable primary antibody binding. Following several rinses, labeling
was detected by administering dextran coupled with peroxidase
molecules and goat secondary antibody molecules against rabbit and
mouse immunoglobulin (1:1) of a Dako EnVision peroxidase kit
(K1491; Agilent Technologies, Inc.) for 1 h. Subsequently, the
sections were rinsed and a chromogenic substrate, prepared by
mixing 20 µl 3,3′-diaminobenzidine tetrahydrochloride and 1 ml
imidazole-buffered solution containing hydrogen peroxide (Dako
Liquid DAB Substrate Chromogen System; K3465; Agilent Technologies,
Inc.), was applied for 1.5 min for ER, 2 min for PR, 3 min for HER2
and 2 min for Ki67 staining. The slides were lightly counterstained
with hematoxylin to provide cellular details. Positive expression
of the hormonal receptors (ER and PR) was defined as nuclear
staining of cancer cells at any intensity. Ki67 staining was
recognized in nucleus of the cancer cell at all cell cycle phases
other than G0 phase according to a previous method described by
Gerdes et al (12). For HER2,
membrane staining was evaluated in accordance with the guidelines
of ASCO/CAP. The evaluation of immunostained slides was performed
in random order by a single pathologist blinded to the other data
of the paired samples. The assessment of slides was performed by
optical microscopy.
Statistical analysis
The association between changes in hormone receptor
status and other characteristics was evaluated using the c2 test.
Student's t-test and the Mann-Whitney U test were performed to
assess the Ki67 index. Student's t-test was applied when the Ki67
index exhibited a normal distribution and the Mann-Whitney U test
was used to evaluate the remaining data. Disease-free survival was
defined as the time from initial surgery to pathological
confirmation of recurrence. Overall survival was defined as the
time from initial surgery to mortality. Overall survival was
analyzed using the Kaplan-Meier function, the log-rank test and Cox
regression. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using IBM SPSS Statistics, version 20 (IBM Corporation, Armonk, NY,
USA).
Results
ER and PR status
The clinicopathological characteristics of the
patients are presented in Table I.
The data in Table I are divided into
four groups according to primary tumor hormonal receptor status. No
significant difference was present in the distribution among the
four groups (c2 test). Of the 70 patients, 48 (68.5%) were positive
for ER and 38 (54.2%) were positive for PR at the primary site. The
conversion rate for ER, from positive in the primary lesion to
negative in the metastatic lesion (denoted as ER+/−), was 19.8%.
The PR+/− conversion rate was 39.5%. Positive conversions, i.e.,
ER−/+ and PR−/+, occurred in 27.2 and 31.2% of all patients
enrolled, respectively. One of the 32 patients, who was negative
for PR at the primary site, was excluded from the assessment due to
paraffin block deterioration. Among the 30 patients with lymph node
metastasis, for whom pathological re-examination could be
performed, hormone receptor status conversion in a metastatic lymph
node occurred in only one patient with an ER-positive primary
lesion (4.0%), in 18.8% of 16 patients with a PR-positive primary
lesion and in 35.7% of 14 patients with a PR-negative primary
lesion (Table II). ER status
conversion in metastatic lymph nodes was rarely observed, whereas
the PR status frequently differed at a metastatic site. For PR,
conversion occurs relatively easily at recurrent sites, as well as
in metastatic lymph nodes. No statistically significant
associations were observed between changes in hormone receptor
status and adjuvant treatment (Table
II). In addition, metastatic locations were divided into two
groups (lymph nodes, local metastases and bone metastases versus
other sites, such as the lung, liver, brain and gastrointestinal
tract, indicating visceral crisis). Patients exhibiting positive
receptor status at metastatic sites were predominantly in the
former group, with 61.8% for ER and 69.2% for PR. By contrast,
approximately half of patients with negative receptor status at
metastatic sites had visceral crisis (48.0% for ER and 44.3% for
PR). These findings imply that tumors with negative hormone
receptor status at metastatic sites demonstrate aggressive
progression.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
|
| Primary tumor
status |
|---|
|
|
|
|
|---|
|
|
| Estrogen
receptor |
| Progesterone
receptor |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | All patients | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| Patients (n) | 70 | 48 | 22 |
| 38 | 32 |
|
| Age (mean ± SD) | 54.5±15.5 | 55.8±15.5 | 51.8±15.6 | 0.320 |
53.6±17.3 | 55.6±13.3 | 0.603 |
| Menopausal status (at
original diagnosis) |
|
|
|
|
|
|
|
|
Premenopausal | 32 | 23 | 9 | 0.585 | 21 | 11 | 0.081 |
|
Postmenopausal | 38 | 25 | 13 |
| 17 | 21 |
|
| Pathological
type |
|
|
|
|
|
|
|
| Invasive
ductal carcinoma | 61 | 41 | 20 | 0.213 | 32 | 29 | 0.398 |
| Invasive
lobular carcinoma | 2 | 2 | 0 |
| 2 | 0 |
|
| Invasive
micropapillary carcinoma | 3 | 3 | 0 |
| 2 | 1 |
|
| Medullary
carcinoma | 2 | 0 | 1 |
| 0 | 1 |
|
| Spindle
cell carcinoma | 1 | 0 | 1 |
| 0 | 1 |
|
| Ductal
carcinoma in situ | 1 | 1 | 0 |
| 1 | 0 |
|
| Surgical
procedure |
|
|
|
|
|
|
|
|
Mastectomy | 54 | 39 | 15 | 0.227 | 30 | 24 | 0.695 |
| Partial
resection | 16 | 9 | 7 |
| 8 | 8 |
|
| Histological
grade |
|
|
|
|
|
|
|
| 1 | 8 | 6 | 2 | 0.001 | 5 | 3 | 0.314 |
| 2 | 35 | 30 | 5 |
| 22 | 13 |
|
| 3 | 18 | 10 | 8 |
| 8 | 10 |
|
|
Unknown | 9 | 2 | 7 |
| 3 | 6 |
|
| TNM stage |
|
|
|
|
|
|
|
|
Tis | 1 | 1 | 0 | 0.801 | 1 | 0 | 0.568 |
| T1 | 21 | 12 | 9 |
| 10 | 11 |
|
| T2 | 33 | 24 | 9 |
| 20 | 13 |
|
| T3 | 7 | 5 | 2 |
| 2 | 5 |
|
| T4 | 5 | 4 | 1 |
| 3 | 2 |
|
|
Unknown | 3 | 2 | 1 |
| 2 | 1 |
|
| N0 | 29 | 16 | 13 | 0.198 | 15 | 14 | 0.673 |
| N1 | 34 | 25 | 9 |
| 19 | 15 |
|
| N2 | 3 | 3 | 0 |
| 1 | 2 |
|
| N3 | 2 | 2 | 0 |
| 1 | 1 |
|
|
Unknown | 2 | 2 | 0 |
| 2 | 0 |
|
| Stage |
|
|
|
|
|
|
|
| 0 | 1 | 1 | 0 | 0.803 | 1 | 0 | 0.818 |
| I | 14 | 17 | 7 |
| 7 | 7 |
|
| II | 39 | 27 | 12 |
| 22 | 17 |
|
|
III | 13 | 11 | 2 |
| 6 | 7 |
|
|
Unknown | 3 | 2 | 1 |
| 2 | 1 |
|
 | Table II.Distribution of changes in hormone
receptor status at metastatic sites. |
Table II.
Distribution of changes in hormone
receptor status at metastatic sites.
|
| Primary tumor
receptor status |
|---|
|
|
|
|---|
|
| Estrogen
receptor | Progesterone
receptor |
|---|
|
|
|
|
|---|
| Variable | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative |
|---|
| Patients (n) | 48 | – | – | 22 | 38 | – | – | 32 |
| Receptor
status |
|
|
|
|
|
|
|
|
|
Metastatic lymph nodes from
the primaries |
|
|
|
|
|
|
|
|
| Number
of patients (%) | 24 (96.0) | 1 (4.0) | 0 | 5 (100) | 13 (81.2) | 3 (18.8) | 5 (35.7) | 9 (64.3) |
|
Metastatic site |
|
|
|
|
|
|
|
|
| Number
of patients (%) | 39 (81.2) | 9 (19.8) | 6 (27.2) | 16 (82.8) | 23 (60.5) | 15 (39.5) | 10 (31.2) | 21 (65.6) |
| Adjuvant treatment,
n (%) |
|
|
|
|
|
|
|
|
|
Hormonal treatment only | 17 (43.6) | 4 (44.4) | 0 | 1 (6.2) | 10 (43.5) | 6 (40.0) | 2 (20.0) | 1 (4.8) |
|
Hormonal treatment and/or
chemotherapy | 19 (48.7) | 5 (55.6) | 6 (100) | 11 (68.8) | 12 (52.2) | 8 (53.3) | 7 (70.0) | 15 (71.4) |
|
None | 1 (2.6) | 0 | 0 | 4 (25.0) | 1 (4.3) | 0 | 0 | 5 (23.8) |
|
Unknown | 2 (5.1) | 0 | 0 | 0 | 0 | 1 (6.7) | 1 (10.0) | 0 |
|
P-value | 0.858 |
| 0.297 |
| 0.529 |
| 0.106 |
|
| Metastatic site, n
(%) |
|
|
|
|
|
|
|
|
| Lymph
nodes | 19 (30.1) | 3 (15.8) | 1 (6.3) | 8 (24.3) | 10 (27.0) | 7 (38.9) | 4 (25.0) | 8 (17.4) |
| Local
site | 15 (23.8) | 2 (10.5) | 1 (6.3) | 3 (9.1 | 10 (27.0) | 2 (11.1) | 3 (18.5) | 7 (15.2) |
|
Bone | 8 (12.7) | 4 (21.0) | 3 (18.7) | 6 (18.2) | 7 (18.9) | 2 (11.1) | 2 (12.5) | 8 (17.4) |
| Lung
and pleura | 7 (11.1) | 3 (15.8) | 4 (25.0) | 3 (9.1) | 3 (8.1) | 2 (11.1) | 3 (18.7) | 6 (13.0) |
|
Liver | 7 (11.1) | 4 (21.0) | 3 (18.7) | 7 (21.2) | 3 (8.1) | 3 (16.7) | 4 (25.0) | 8 (17.4) |
|
Brain | 3 (4.8) | 1 (5.3) | 2 (12.5) | 4 (12.1) | 2 (5.4) | 0 | 0 | 5 (10.9) |
|
Gastrointestinal tract | 1 (1.6) | 1 (5.3) | 2 (12.5) | 1 (3.0) | 1 (2.7) | 1 (5.5) | 0 | 3 (6.5) |
|
Other | 3 (4.8) | 1 (5.3) | 0 | 1 (3.0) | 1 (2.7) | 1 (5.5) | 0 | 1 (2.2) |
Ki67 index
Overall, the mean Ki67 index of recurrent lesions
was significantly greater than that of primary lesions (P=0.019).
However, Ki67 index of synchronous metastatic lymph nodes exhibited
almost the same value with that of primary lesions (P=0.927). This
trend was only observed for patients with positive hormone receptor
status in their primary lesion. By contrast, the Ki67 index of
patients who had primary lesions that were hormone receptor
negative did not change significantly with metastasis, which may
indicate that hormone-negative tumors maintain high proliferative
activity. Regarding changes in the Ki67 index with respect to
changes in hormone receptor status, the Ki67 index of patients with
primary lesions that were hormone receptor-positive exhibited
increased proliferative activity with metastasis, but not in
patients with primary lesions that were hormone receptor-negative
(Fig. 1).
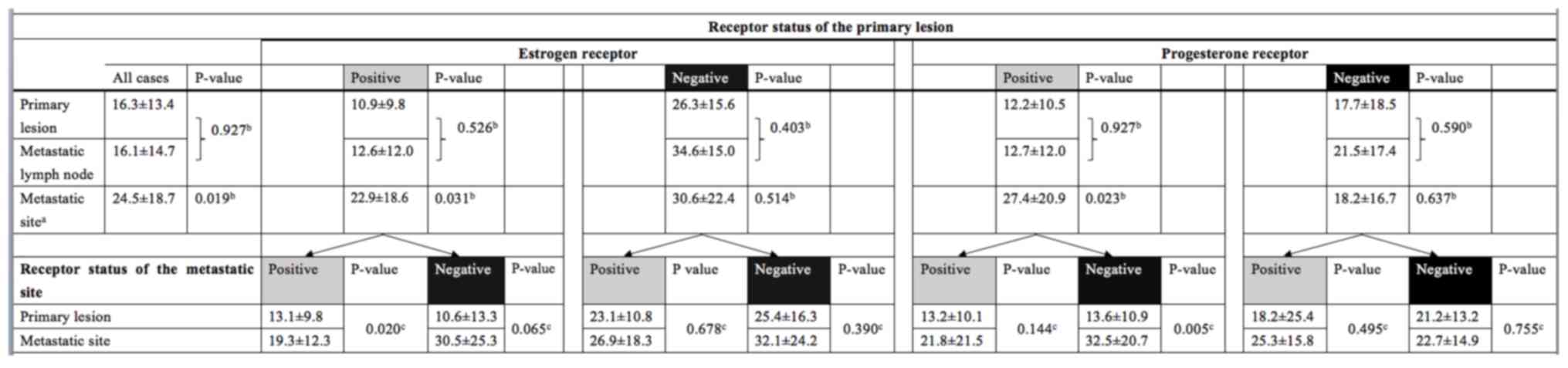
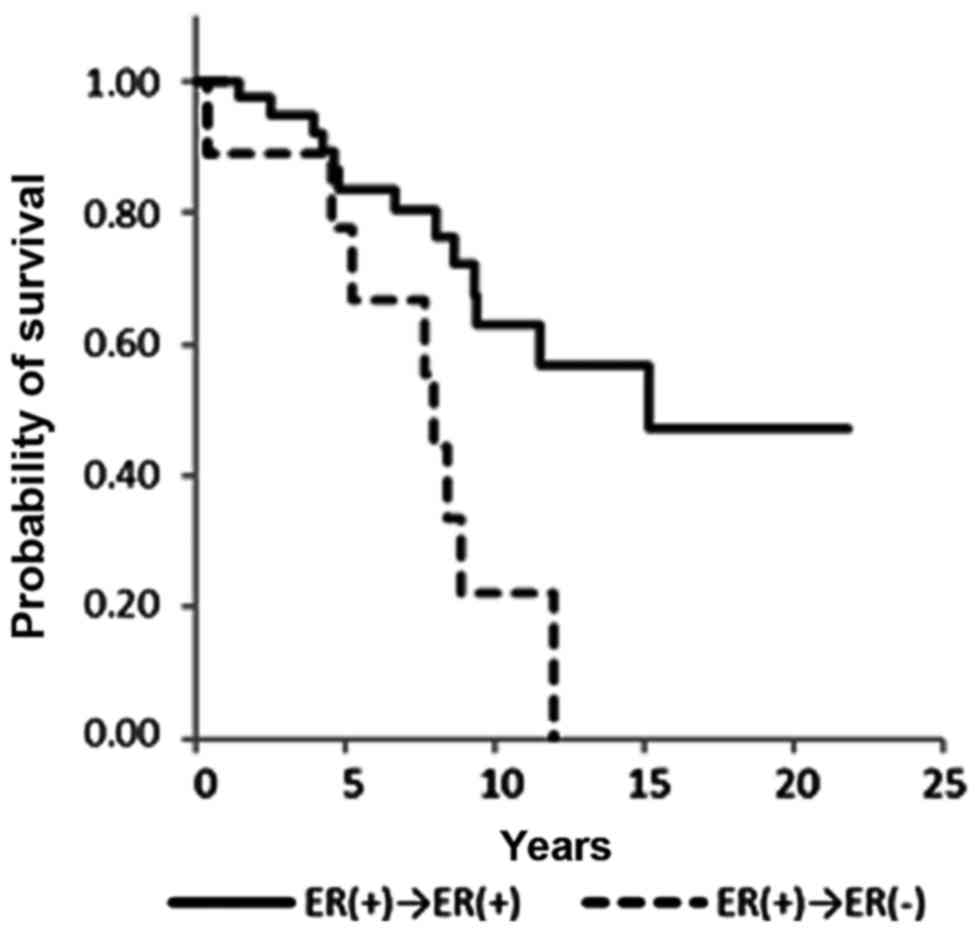
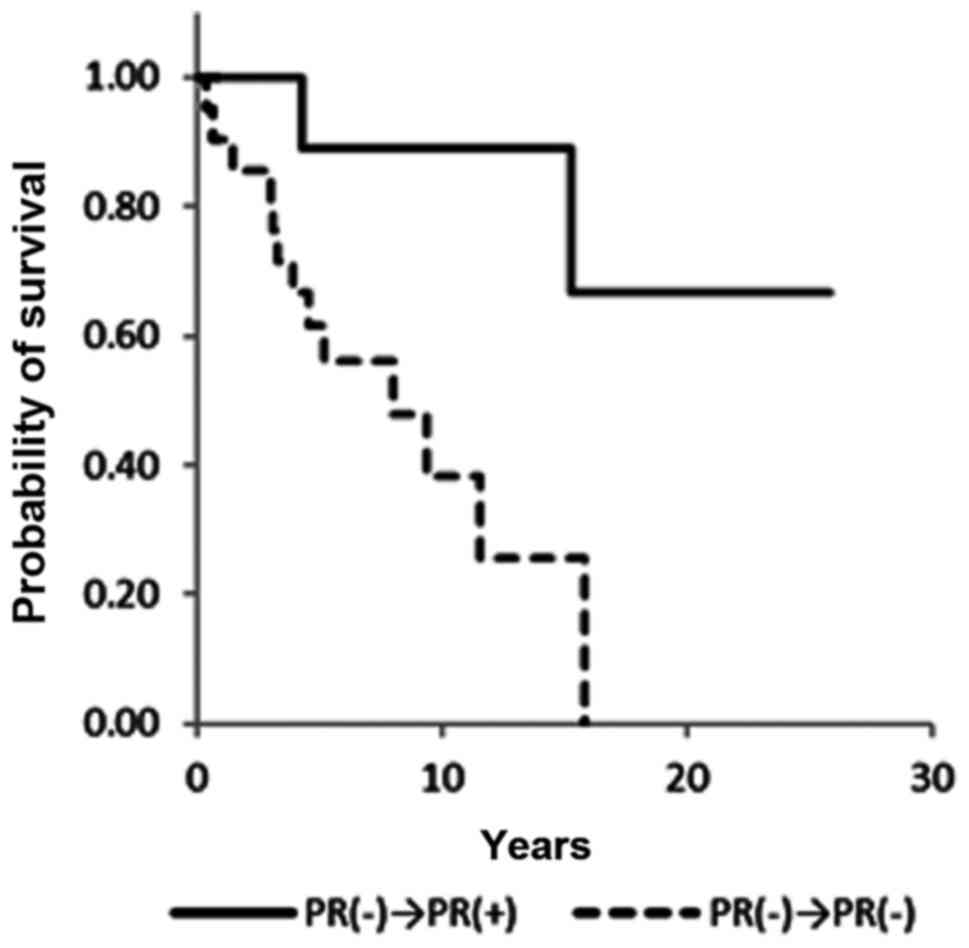
Overall survival
A significant difference in overall survival was
observed between ER+/+ and ER+/− patients [hazard ratio (HR), 0.29;
95% confidence interval (CI), 0.12–0.73; P=0.008] and PR+/+ and
PR+/− patients (HR, 0.12; 95% CI 0.03–0.43; P=0.001) (Figs. 2 and 3;
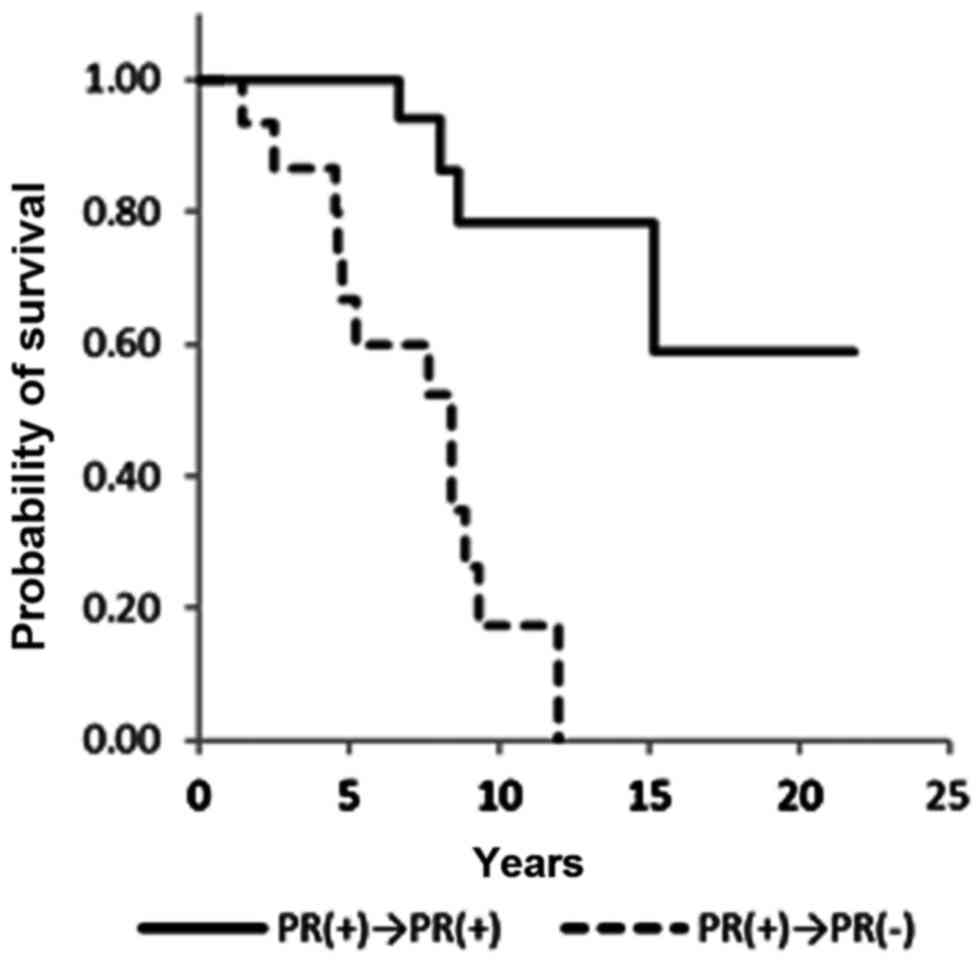
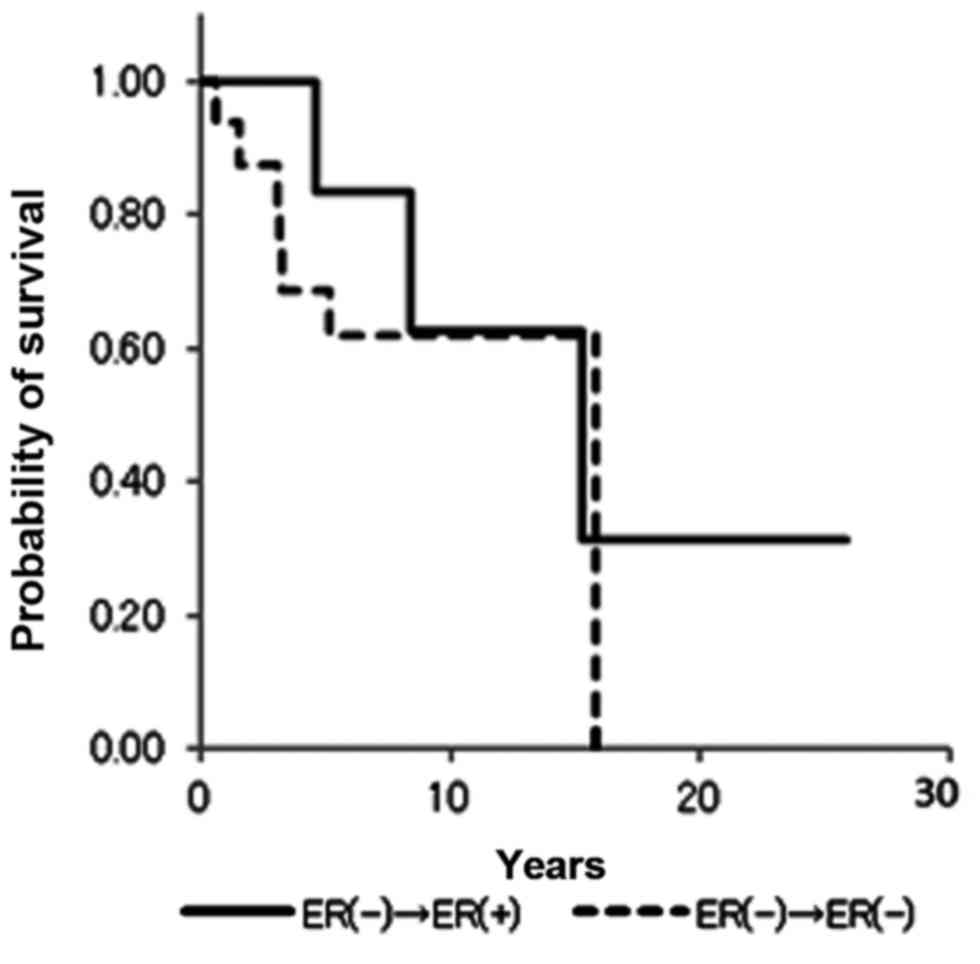
Table III). ER−/+ and PR−/+
conversion was not associated with a significant difference in
overall survival (Figs. 4 and
5). Notably, no statistical
differences in overall survival were identified between the ER+/+
and ER−/+ groups (data not shown). Positive conversion from
negative hormone receptor status in the primary lesion to positive
status in the metastatic lesion improved overall survival (Table III).
 | Table III.Log-rank test and Cox proportional
HRs for overall survival. |
Table III.
Log-rank test and Cox proportional
HRs for overall survival.
| Receptor
status |
|
|
|
|
|
|---|
|
|
|
|
|
|
|---|
| Primary tumor | Metastatic
tumor | Disease-free
interval Means ± SE (years) | Overall survival
Means ± SE (years) | P-value | HR (95% CI) | P-value |
|---|
|
Estrogen receptor |
|
|
|
|
|
|
|
|
|
|
|
|
Positive | Positive | 5.4±0.8 | 14.8±1.4 | 0.005 | 1 | 0.008 |
|
| Negative | 4.1±1.2 | 7.4±1.2 |
| 3.44
(1.36–8.33) |
|
|
Negative | Positive | 6.5±2.6 | 15.3±3.7 | 0.542 | 1 | 0.544 |
|
| Negative | 2.7±0.6 | 10.8±1.7 |
| 1.56
(0.38–6.66) |
|
|
Progesterone receptor |
|
|
|
|
|
|
|
|
|
|
|
|
Positive | Positive | 6.0±1.0 | 17.5±1.7 | 0.000 | 1 | 0.001 |
|
| Negative | 3.7±0.0 | 7.3±0.8 |
|
| 8.33
(2.32–33.3) |
|
Negative | Positive | 7.7±2.1 | 19.3±3.6 | 0.116 | 1 | 0.018 |
|
| Negative | 2.7±0.0 | 9.6±1.4 |
|
| 6.66
(1.36–33.3) |
HER2 status
A total of 12 patients (17.1%) exhibited positivity
for HER2 in the primary lesion. For the metastatic lesion, 19
patients (27.1%), including 7 patients whose HER2 expressions were
negative in the primary lesions, exhibited overexpression of HER2
(data not shown). Therefore, a total of 7 patients (10.0%)
exhibited increases in HER2 expression levels from 0, 1+ or 2+ in
the primary lesion to 3+ or FISH score >2 in the metastatic
lesion (Table IV). No downregulation
of HER2 expression was observed in the present study, whereas
Niikura et al (13) previously
observed loss of HER2 in 24% of 182 metastatic breast cancer cases.
The location of recurrence in these seven patients included
loco-regional sites, bone, lung and liver. The mean Ki67 index of
the primary lesion was 13.6±10.8 and that of the metastatic site
was 15.1±15.9 (P=0.406, Mann-Whitney U test). The mean overall
survival of these patients was 8.4±2.6 years, which is similar to
the survival of patients with negative conversion, particularly
ER+/− and PR+/−.
 | Table IV.Changes in HER2 status. |
Table IV.
Changes in HER2 status.
|
| Primary tumor | Metastatic
tumor |
|---|
|
|
|
|
|---|
| Age at initial
surgery (years) | Pathology | Hormone receptor
status (ER, PR) | Hormonal status
HER2 status | (ER, PR) | HER2 status |
|---|
| 77 | Invasive ductal
carcinoma | ER(+)/PR(+) | 1+ | ER(+)/PR(+) | 3+ |
| 34 | Invasive ductal
carcinoma | ER(+)/PR(+) | 1+ | ER(+)/PR(+) | 3+ |
| 66 | Invasive ductal
carcinoma | ER(+)/PR(+) | 0 | ER(+)/PR(−) | 3+ |
| 52 | Invasive
micropapillary carcinoma | ER(+)/PR(−) | 1+ | ER(−)/PR(−) | 3+ |
| 31 | Invasive ductal
carcinoma | ER(−)/PR(+) | 1+ | ER(+)/PR(−) | 3+ |
| 40 | Invasive ductal
carcinoma | ER(+)/PR(+) | 2+
(1.03)a | ER(+)/PR(+) | 3+ |
| 50 | Invasive ductal
carcinoma | ER(+)/PR(+) | 1+ | ER(−)/PR(−) | 2+
(2.23)a |
Discussion
When considering the treatment strategy for patients
with metastatic breast cancer, oncologists are eager to achieve an
adequate antitumor effect while maintaining a high quality of life
for the patient. Chemotherapy, hormonal therapy or radiotherapy may
be the treatment of choice. Personalized treatment strategies are
presently required, with clinicians making meticulous treatment
plans that take into account the tumor phenotype. Various studies
have reported that the receptor status of metastatic breast cancer
may differ from that of the primary tumor (1–4). Aitken
et al (14) reported that
metastatic lymph node receptor status may be a more accurate
parameter for guiding adjuvant therapy. Thus, it is necessary to
understand the alterations in tumor phenotype for further
therapeutic decision making.
In the present study, hormone receptor and HER2
status conversion were assessed at recurrence. In approximately
20–40% of cases, hormone receptor status conversion was observed.
Conversion from positive to negative status was associated with a
significantly worse overall survival (HR, 3.44; 95%CI, 1.36–8.33;
P=0.008 for ER and HR, 8.33; 95% CI, 2.32–33.3; P=0.001 for PR).
The difference in HR may be due to biological differences between
ER and PR. Strong ER expression is associated with good response to
hormonal treatment and strong PR expression favors survival,
particularly in patients who are also ER positive (15,16). The
Kaplan-Meier curves in the present study demonstrate that PR status
is closely correlated with overall survival (Figs. 2 and 4).
PR−/+ conversion appears to improve overall survival. Observing
larger cohorts of metastatic or recurrent breast cancer patients
with longer follow-up may demonstrate that the PR-/+ group
significantly differs in survival when compared with the PR-/-
group. Discordance of HER2 status was observed only in seven
patients (10%) in the present study. The discordance rate ranges
from 14 to 17% across studies (17,18). Six
patients in the present study received intensive chemotherapy,
including taxane and anthracycline, before changes in HER2 status
were recognized. The etiology of hormone receptor and HER2 status
discordance remains unknown. A variety of adjuvant treatments that
were administered following initial surgery may have influenced the
hormone receptor and HER2 status. The most plausible hypothesis is
that the distribution of cancer cell types was altered, as chemo-
and/or hormone-sensitive tumor cells were eliminated by certain
types of treatment. Yang et al (19) demonstrated that chemo-sensitive tumor
cells were targeted and killed in the setting of neoadjuvant
chemotherapy; consequently, insensitive tumor cells with different
biological properties survived in the residual lesion prior to
surgery. Furthermore, Keen et al (20) revealed that tumor progression may
induce genetic drift and also treatment-associated clone selection.
For example, ER-negative cancer cells generally respond to
chemotherapy better than ER-positive cancer cells. The
chemotherapeutic agents preferentially eliminate chemo-sensitive
cancer cells, such as ER-negative tumor cells and consequently the
nest of breast cancer cells is rearranged into the new nest
consisting of ER-positive dominant cancer cells (21). Niikura et al (13) reported that the discordance of HER2
status between primary and metastatic tumor sites increased
following chemotherapy. However, hormonal treatment may induce
receptor discordance at the metastatic sites. It remains
controversial as to whether endocrine therapy with tamoxifen may
influence the receptor discordance of the metastatic tumors.
Johnston et al (22)
demonstrated the ER expression levels in metastatic tumors were
significantly reduced in the patients that had undergone tamoxifen
treatment prior to cancer recurrence, whereas other studies
identified that no significant correlation was observed between
endocrine therapy and ER discordance rate (23,24). In
the present study, only two cases received tamoxifen treatment
alone as an adjuvant setting prior to recurrence. Almost all the
patients experienced adjuvant chemotherapy postoperatively.
Unfortunately, the effect of tamoxifen on the receptor discordance
cannot be discussed using the present data due to the lack of
applicable patients. In the present study, PR expression levels are
reduced with the metastasis in 55% of ER+/− conversion and 5.1% of
ER+/+. It is comprehensible that the downregulation of PR occurs
concomitantly with the reduction of ER expression levels in the
metastatic sites, as ER regulates PR expression levels. For the two
cases (5.1%) that demonstrated reduced PR expression with ER+/+
conversion, the influence of growth factors may be considered. It
has been reported that the phosphatidylinositol-3 kinase/Akt
signaling pathway, which is occasionally activated in metastatic
breast cancer cells, may deactivate PR expression, as the
application of inhibitors for this signaling pathway may reverse PR
downregulation (25).
The Ki67 index generally increases with metastasis
or recurrence. In the present study, the Ki67 index of metastatic
lesions was significantly upregulated when compared with that of
the primary lesions. Notably, this trend was observed in patients
with primary lesions that were hormone receptor-positive. The Ki67
index of patients who were hormone receptor-negative remained high
during the life of the tumor, indicating high levels of
proliferative activity.
There were certain limitations of the present study.
A biopsy of recurrent tumors was performed at the discretion of the
treating physicians. However, to assess recurrent tumors, a biopsy
of the first recurrent site must be performed. Furthermore, the
biopsy specimen of a recurrent tumor may not fully represent all of
its biological features. As metastatic spread of breast cancer
typically occurs systemically, a biopsy of all recurrent tumors is
difficult due to limited accessibility.
In conclusion, the biological features of metastatic
breast cancer may differ from those of primary breast cancer. When
a recurrent tumor loses hormone receptor positivity, survival may
be substantially worsened. To establish an appropriate treatment
plan for a patient with recurrent disease, a biopsy of the
recurrent tumor should be mandatory if access is feasible.
References
|
1
|
Holdaway IM and Bowditch JV: Variation in
receptor status between primary and metastatic breast cancer.
Cancer. 52:479–485. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hull DF III, Clark GM, Osborne CK,
Chamness GC, Knight WA III and McGuire WL: Multiple estrogen
receptor assays in human breast cancer. Cancer Res. 43:413–416.
1983.PubMed/NCBI
|
|
3
|
Li BD, Byskosh A, Molteni A and Duda RB:
Estrogen and progesterone receptor concordance between primary and
recurrent breast cancer. J Surg Oncol. 57:71–77. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimizu C, Fukutomi T, Tsuda H,
Akashi-Tanaka S, Watanabe T, Nanasawa T and Sugihara K: c-erbB-2
protein overexpression and p53 immunoreaction in primary and
recurrent breast cancer tissues. J Surg Oncol. 73:17–20. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar SK, Gupta N, Rajwanshi A, Joshi K
and Singh G: Immunochemistry for oestrogen receptor, progesterone
receptor and HER2 on cell blocks in primary breast carcinoma.
Cytopathology. 23:181–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
The World Health Organization:
Histological typing of breasr tumors. Neoplasma. 30:113–123.
1983.PubMed/NCBI
|
|
7
|
Sobin LH and Witterkind C: TNM
Classification of Malignant Tumours. 6th edition. Wiley; New York:
pp. 131–141. 2002
|
|
8
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
9
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College Of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American Society of Clinical Oncology; College of
American Pathologists: American Society of Clinical
Oncology/College of American Pathologists guideline recommendations
for human epidermal growth factor receptor 2 testing in breast
cancer. J Clin Oncol. 25:118–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dowsett M, Nielsen TO, A'Hern R, Bartlett
J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et
al: International Ki-67 in breast cancer working group: Assessment
of Ki67 in breast cancer: Recommendations from the International
Ki67 in breast cancer working group. J Natl Cancer Inst.
103:1656–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|
|
13
|
Niikura N, Liu J, Hayashi N, Mittendorf
EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN
and Ueno NT: Loss of human epidermal growth factor receptor 2
(HER2) expression in metastatic sites of HER2-overexpressing
primary breast tumors. J Clin Oncol. 30:593–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aitken SJ, Thomas JS, Langdon SP, Harrison
DJ and Faratian D: Quantitative analysis of changes in ER, PR and
HER2 expression in primary breast cancer and paired nodal
metastases. Ann Oncol. 21:1254–1261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu S, Chia SK, Mehl E, Leung S, Rajput A,
Cheang MCU and Nielsen TO: Progesterone receptor is a significant
factor associated with clinical outcomes and effect of adjuvant
tamoxifen therapy in breast cancer patients. Breast Cancer Res
Treat. 119:53–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Purdie CA, Quinlan P, Jordan LB, Ashfield
A, Ogston S, Dewar JA and Thompson AM: Progesterone receptor
expression is an independent prognostic variable in early breast
cancer: A population-based study. Br J Cancer. 110:565–572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishimura R, Osako T, Okumura Y, Tashima
R, Toyozumi Y and Arima N: Changes in the ER, PgR, HER2, p53 and
Ki-67 biological markers between primary and recurrent breast
cancer: Discordance rates and prognosis. World J Surg Oncol.
9:1312011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ibrahim T, Farolfi A, Scarpi E, Mercatali
L, Medri L, Ricci M, Nanni O, Serra L and Amadori D: Hormonal
receptor, human epidermal growth factor receptor-2, and Ki67
discordance between primary breast cancer and paired metastases:
Clinical impact. Oncology. 84:150–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang YF, Liao YY, Li LQ, Xie SR, Xie YF
and Peng NF: Changes in ER, PR and HER2 receptors status after
neoadjuvant chemotherapy in breast cancer. Pathol Res Pract.
209:797–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keen JC and Davidson NE: The biology of
breast carcinoma. Cancer. 97 Suppl:825–833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong Y, Han EY, Guo M, Pusztai L and
Sneige N: Stability of estrogen receptor status in breast
carcinoma: A comparison between primary and metastatic tumors with
regard to disease course and intervening systemic therapy. Cancer.
117:705–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnston SRD, Saccani-Jotti G, Smith IE,
Salter J, Newby J, Coppen M, Ebbs SR and Dowsett M: Changes in
estrogen receptor, progesterone receptor, and pS2 expression in
tamoxifen-resistant human breast cancer. Cancer Res. 55:3331–3338.
1995.PubMed/NCBI
|
|
23
|
Broom RJ, Tang PA, Simmons C, Bordeleau L,
Mulligan AM, O'Malley FP, Miller N, Andrulis IL, Brenner DM and
Clemons MJ: Changes in estrogen receptor, progesterone receptor and
Her-2/neu status with time: Discordance rates between primary and
metastatic breast cancer. Anticancer Res. 29:1557–1562.
2009.PubMed/NCBI
|
|
24
|
Lower EE, Glass EL, Bradley DA, Blau R and
Heffelfinger S: Impact of metastatic estrogen receptor and
progesterone receptor status on survival. Breast Cancer Res Treat.
90:65–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui X, Schiff R, Arpino G, Osborne CK and
Lee AV: Biology of progesterone receptor loss in breast cancer and
its implications for endocrine therapy. J Clin Oncol. 23:7721–7735.
2005. View Article : Google Scholar : PubMed/NCBI
|