Introduction
Acute lymphoblastic leukemia (ALL) is the most
prevalent malignancy in children and constitutes approximately 75%
of pediatric acute leukemias (1).
While the etiology of ALL is not fully understood, previous reports
have indicated that genetic factors serve a role in the development
of childhood ALL (2–5).
Non-coding RNAs, comprising microRNAs and long
non-coding RNAs (lncRNAs), do not encode protein sequences, yet are
involved in various biological processes (6–8). In
particular, lncRNAs, as transcripts of >200 nucleotides in
length that lack protein-coding potential, regulate gene expression
at various levels, including at the chromatin remodeling (9), transcription and post-transcriptional
processing stages (10,11).
Paired-box gene 8 (PAX8) encodes a transcription
factor required for cell growth and differentiation during
embryonic development (12).
Overexpression of PAX8 has been identified in various cancers
(13–17). Though the precise role of PAX8 in
cancer remains uncertain, it has been proposed that PAX8
contributes to the development and progression of specific cancers
by maintaining tissue specific stem cells, by inhibiting terminal
differentiation and apoptosis (18).
LncRNA PAX8 antisense RNA 1 (PAX8-AS1) is mapped to
chromosome 2q13 in the upstream region of PAX8 (19). An expression quantitative trait loci
(eQTL) is a locus containing a genetic variant that influences the
expression level of a gene (20).
PAX8-AS1, a potential regulator of PAX8, may contain polymorphisms
that represent eQTLs for PAX8 (21).
In particular, previous bioinformatics analyses have revealed that
the polymorphisms rs4848320 C>T and rs1110839 G>T in PAX8-AS1
may be eQTLs for PAX8 (21).
Furthermore, it has been suggested that rs4848320 and rs1110839 may
affect the function or expression of PAX8-AS1, thereby influencing
PAX8 expression (22,23). Few previous studies have evaluated the
impact of PAX8-AS1 variants on cancer risk. Han et al
(19) reported that rs4848320 and
rs1110839 variants of PAX8-AS1 significantly decreased the risk of
cervical cancer (19). Ma et
al (24) identified that the two
variants of PAX8-AS1 were significantly associated with the
prognosis of hepatocellular carcinoma (HCC). However, to the best
of our knowledge, no previous study has investigated the impact of
PAX8-AS1 polymorphisms on childhood ALL. Based on the previous
findings on PAX8-AS1 and cancer risk, it was hypothesized that
polymorphisms of PAX8-AS1 may affect the risk of childhood ALL by
disturbing the interaction between PAX8-AS1 and PAX8, to in turn
influence PAX8 expression. PAX8 has been demonstrated to serve an
important role in the pathogenesis of cancer by inhibiting cell
differentiation and apoptosis (18).
Therefore, the present study aimed to assess the possible
association between the PAX8-AS1 polymorphisms rs4848320 C>T,
rs1110839 G>T and rs6726151 T>G and the risk of childhood ALL
in a Southeast Iranian population sample. In the analysis, the
polymorphisms which have been implicated as potential risk factors
for cancer were selected (19,24), while
the rs6726151 T>G variant, with a minor allele frequency of
0.486 (25), was examined for the
first time. The findings of the present study highlight the
potential role of PAX8-AS1 variants in the pathogenesis of
childhood ALL.
Materials and methods
Patients
A total of 230 subjects including 110 children
diagnosed with ALL and 120 age- and sex-matched healthy children
were enrolled in the present case-control study. The study design
including the enrolled patients has been reported previously by our
group (2,8). The local Ethics Committee of Zahedan
University of Medical Sciences (Zahedan, Iran) approved the project
(approval no. IR.ZAUMS.REC.1395.270) and informed consent was
obtained from the parents of all participants. Extraction of
genomic DNA from whole blood was performed using the salting out
method as described previously (26).
Genotyping
Polymorphism genotyping was performed through the
polymerase chain reaction-restriction fragment length polymorphism
(PCR-RFLP) method. The primer sequences and restriction enzymes are
summarized in Table I. The primers
were produced by Bioneer Corp., (Daejeon, Korea). Into a 0.20 ml
PCR reaction tube, 1 µl genomic DNA (~100 ng/ml), 1 µl (10 µM each)
forward and reverse primers, 10 µl 2X Prime Taq Premix, all from
Genet Bio, Inc., (Daejeon, Korea), and 7 µl ddH2O were
added. The PCR conditions were as follows: Preheating for 6 min at
95°C; 30 cycles of 95°C for 30 sec, 64°C for rs1110839 and
rs4848320 for 30 sec or 62°C for rs6726151 for 30 sec, and 72°C for
30 sec; followed by a final extension step for 5 min at 72°C.
Subsequently, 10 µl of amplified product was digested with the
appropriate restriction enzyme (New England BioLabs, Inc., Ipswich,
MA, USA), resolved on 2.5% agarose gel containing 0.5 µg/ml
ethidium bromide, observed under a UV transilluminator (DigiDoc
H101; UVP, LLC, Upland, CA, USA) and photographed. For quality
control, 15% randomly selected samples were regenotyped and the
outcome revealed 100% concordance.
 | Table I.Primers and restriction enzymes used
in the detection of paired-box gene 8 antisense RNA 1
polymorphisms. |
Table I.
Primers and restriction enzymes used
in the detection of paired-box gene 8 antisense RNA 1
polymorphisms.
| Polymorphism | Sequence, 5′-3′ | Restriction
enzyme | Product size, bp |
|---|
| rs4848320 C>T | F:
CTGCTTAGCATGTGCTTGGTGATG | PstI | T allele: 222; |
|
| R:
GAAACACTGAGAACTAAGAGAAGCCTGCA |
| C allele: 195+27 |
| rs1110839
G>T | F:
TCATCTCCCCAGGAGAGGTCCTCAGC | HhaI | T allele: 270; |
|
| R:
ACAGTCCGGTTGGAGACTG C |
| G allele:
244+26 |
| rs6726151
T>G | F:
CCCAAAGACCAGCACACA | MboI | G allele: 371; |
|
| R:
AGACCCACCATTTCCATAACA |
| T allele:
211+160 |
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM, Corp., Armonk, NY, USA). The categorical and
continuous data were analyzed using χ2 and t-test,
respectively. Individual single nucleotide polymorphism (SNP)
associations with childhood ALL risk were assessed using
unconditional logistic regression analyses, in which odds ratios
(ORs) and 95% confidence intervals (CIs) were determined for
codominant, dominant, recessive, overdominant and allele
inheritance models. P<0.05 was considered to indicate a
statistically significant difference. Haplotype and linkage
disequilibrium analyses were conducted using SNPStats (https://www.snpstats.net/snpstats) (27) and Haploview 4.2 software both from
Broad Institute (Cambridge, MA, USA) (28), respectively. Linkage disequilibrium
between the PAX8-AS1 polymorphisms was estimated through
calculation of D' (correlation coefficient between pairs of loci)
and r2 (square of the correlation coefficient between
two indicator variables) with Haploview 4.2.
Results
Patient characteristics
The demographic characteristics of the patients
considered in the present study are reported previously (2,8).
Genotyping of the variants
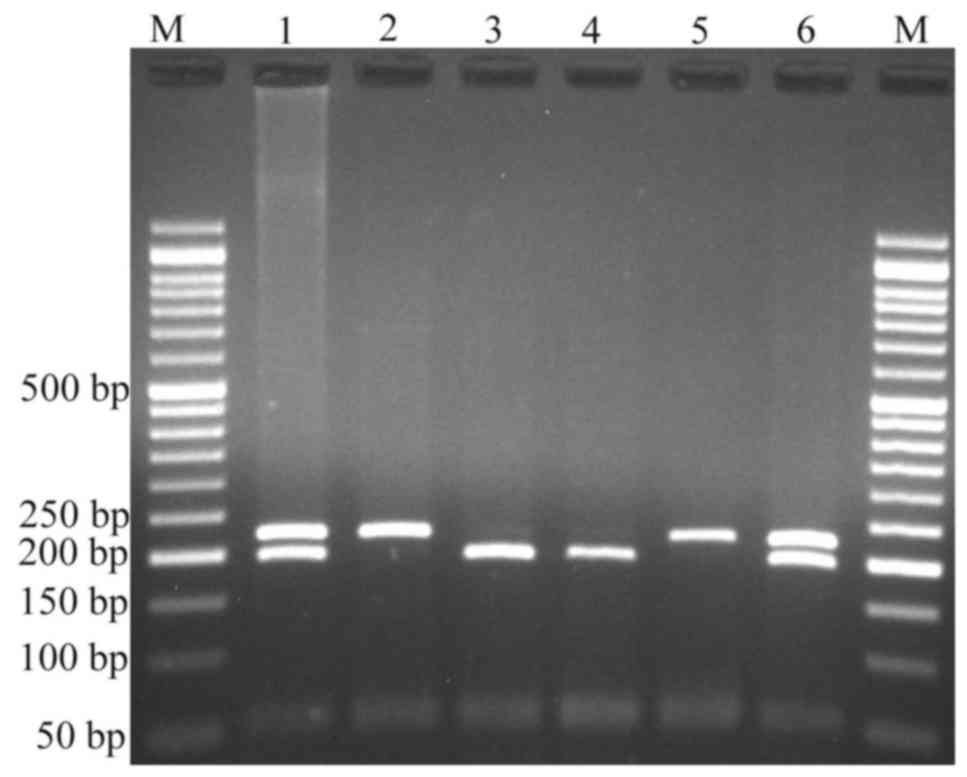
Variants were genotyped by PCR-RFLP. When genotyping
the rs4848320 variant, digestion of the PCR product (222 bp)
yielded a fragment of 195 bp (and presumed 27 bp fragment not
visible on agarose gel) for the C allele, and remained undigested
for the T allele (Fig. 1). Regarding
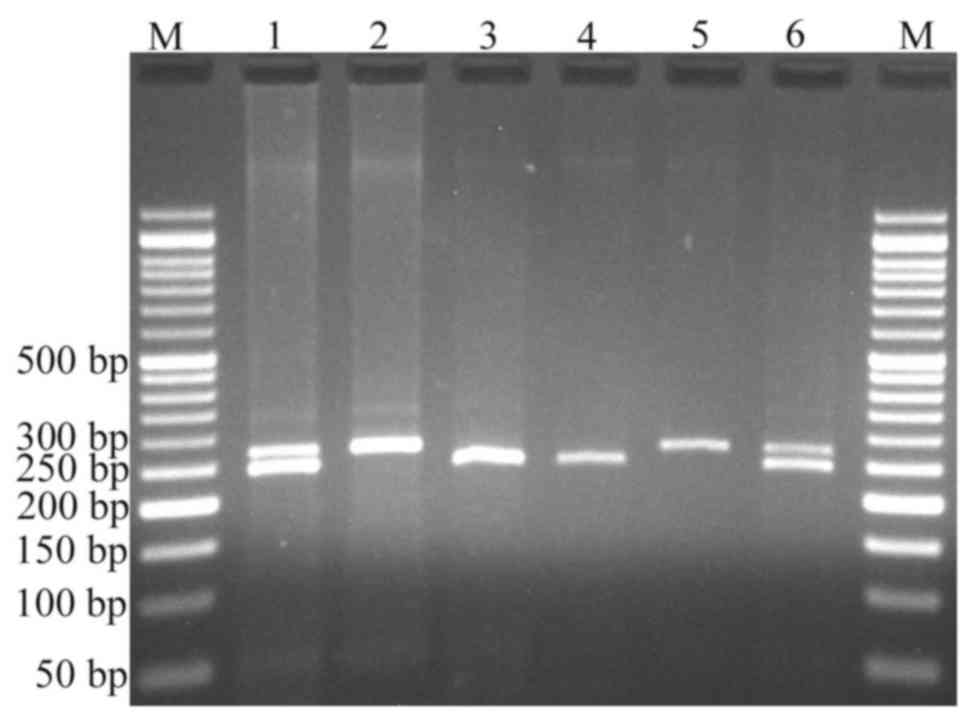
the rs1110839 variant, the T allele remained undigested (270 bp),
while the G allele was digested and produced a fragment of 244 bp
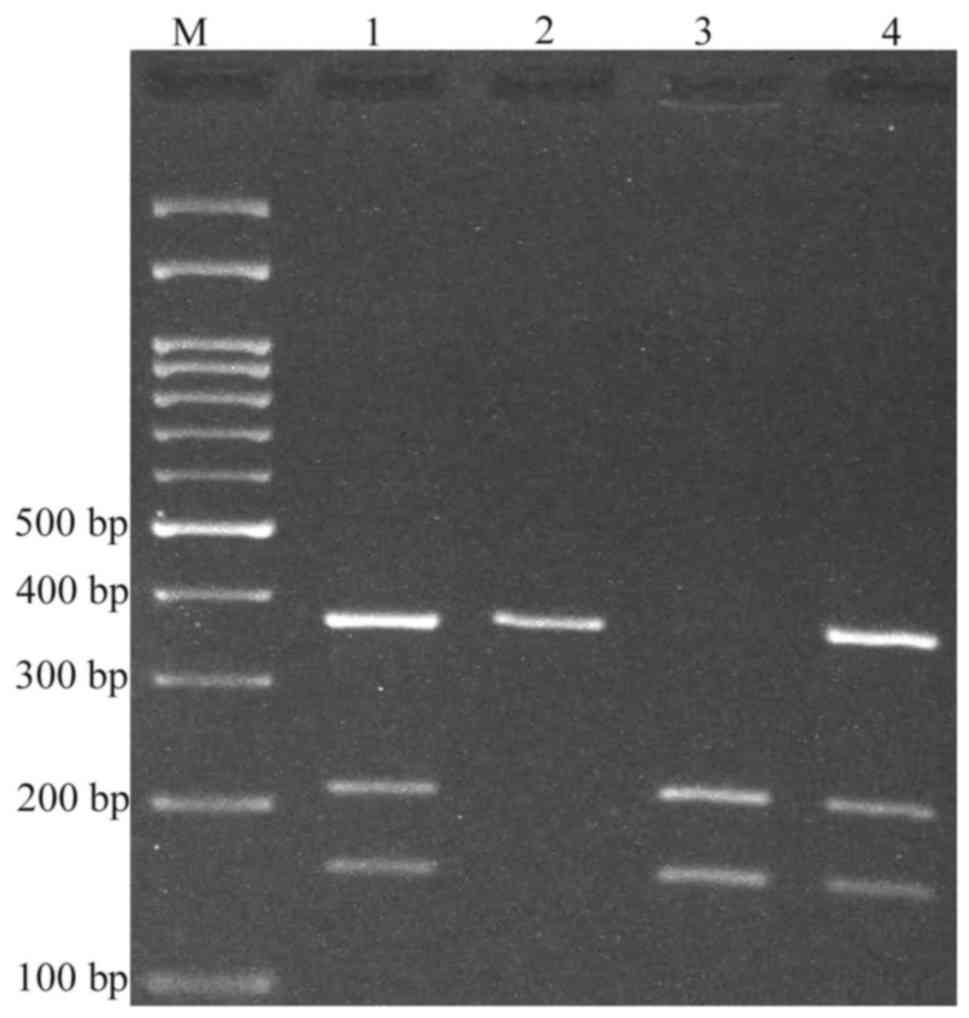
(and presumed 26 bp fragment not visible on agarose gel; Fig. 2). For rs6726151, the T allele was
digested and produced 211 and 160 bp fragments while the G allele
was undigested (371 bp; Fig. 3). The
lengths of all fragments following restriction digestion are
summarized in Table I.
Association between the variants and
childhood ALL risk
The genotype and allele distributions of PAX8-AS1
polymorphisms in pediatric patients with ALL and healthy controls
are presented in Table II. The
findings suggested that the rs4848320 variant was associated with
risk of ALL in codominant (CT vs. CC: OR=2.13, 95% CI=1.16–3.90,
P=0.014; and TT vs. CC: OR=2.21, 95% CI=1.03–4.74, P=0.041),
dominant (CT+TT vs. CC: OR=2.15, 95% CI=1.22–3.81, P=0.009,) and
allele (T vs. C: OR=1.55, 95% CI=1.07–2.25, P=0.024,) inheritance
models. For the rs6726151 variant, the findings indicated that this
variant significantly increased the risk of ALL in codominant (GT
vs. GG: OR=1.88, 95% CI=1.08–3.27, P=0.036) and overdominant (GT
vs. GG+TT: OR=2.08, 95% CI=1.23–3.53, P=0.008) inheritance models.
No significant association was observed between the rs1110839
G>T variant and disease risk/protection in childhood ALL.
 | Table II.Association of paired-box gene 8
antisense RNA 1 polymorphisms and risk of acute lymphoblastic
leukemia. |
Table II.
Association of paired-box gene 8
antisense RNA 1 polymorphisms and risk of acute lymphoblastic
leukemia.
| Polymorphism | Cases, n (%) | Controls, n
(%) | OR (95% CI) | P-value |
|---|
| rs4848320 |
|
|
|
|
|
Codominant |
|
|
|
|
|
CC | 26
(23.6) | 48
(40.0) | 1.00 | – |
|
CT | 60
(54.6) | 52
(43.3) | 2.13
(1.16–3.90) | 0.014 |
|
TT | 24
(21.8) | 20
(16.7) | 2.21
(1.03–4.74) | 0.041 |
|
Dominant |
|
|
|
|
|
CC | 26
(23.6) | 48
(40.0) | 1.00 | – |
|
CT+TT | 84
(76.4) | 72
(60.0) | 2.15
(1.22–3.81) | 0.009 |
|
Recessive |
|
|
|
|
|
CC+CT | 86
(78.2) | 100 (83.3) | 1.00 | – |
|
TT | 24
(21.8) | 20
(16.7) | 1.39
(0.72–2.70) | 0.322 |
|
Overdominant |
|
|
|
|
|
CC+TT | 50
(45.4) | 68
(56.7) | 1.00 | – |
|
CT | 60
(54.6) | 52
(43.3) | 1.57
(0.93–2.64) | 0.090 |
|
Allele |
|
|
|
|
|
C | 112 (50.9) | 148 (61.7) | 1.00 | – |
|
T | 108 (49.1) | 92
(38.3) | 1.55
(1.07–2.25) | 0.024 |
| rs1110839 |
|
|
|
|
|
Codominant |
|
|
|
|
|
TT | 43
(39.1) | 54
(45.0) | 1.00 | – |
|
TG | 43
(39.1) | 34
(28.3) | 1.59
(0.87–2.90) | 0.132 |
|
GG | 24
(21.8) | 32
(26.7) | 0.94
(0.48–1.83) | 0.860 |
|
Dominant |
|
|
|
|
|
TT | 43
(39.1) | 54
(45.0) | 1.00 | – |
|
TG+GG | 67
(60.9) | 66
(55.0) | 1.27
(0.75–2.16) | 0.365 |
|
Recessive |
|
|
|
|
|
TT+TG | 86
(78.2) | 88
(73.3) | 1.00 | – |
|
GG | 24
(21.8) | 32
(26.7) | 0.77
(0.42–1.41) | 0.393 |
|
Overdominant |
|
|
|
|
|
TT+GG | 67
(60.9) | 86
(71.7) | 1.00 | – |
|
TG | 43
(39.1) | 34
(28.3) | 1.62
(0.93–2.82) | 0.085 |
|
Allele |
|
|
|
|
|
T | 129 (58.6) | 142 (59.2) | 1.00 | – |
|
G | 91
(60.4) | 98
(40.8) | 1.02
(0.70–1.48) | 0.924 |
| rs6726151 |
|
|
|
|
|
Codominant |
|
|
|
|
|
GG | 40
(36.3) | 56
(46.6) | 1.00 | – |
|
GT | 63
(57.3) | 47
(39.2) | 1.88
(1.08–3.27) | 0.036 |
|
TT | 7
(6.4) | 17
(14.2) | 0.58
(0.22–1.52) | 0.576 |
|
Dominant |
|
|
|
|
|
GG | 40
(36.4) | 56
(46.6) | 1.00 | – |
|
GT+TT | 70
(63.7) | 64
(53.4) | 1.53
(0.90–2.60) | 0.141 |
|
Recessive |
|
|
|
|
|
GG+GT | 103 (93.6) | 103 (85.8) | 1.00 | – |
|
TT | 7
(6.4) | 17
(14.2) | 0.41
(0.16–1.04) | 0.082 |
|
Overdominant |
|
|
|
|
|
GG+TT | 47
(42.7) | 73
(60.8) | 1.00 | – |
|
GT | 63
(57.3) | 47
(39.2) | 2.08
(1.23–3.53) | 0.008 |
|
Allele |
|
|
|
|
|
G | 143 (65.0) | 159 (72.3) | 1.00 | – |
|
T | 77
(35.0) | 81
(27.7) | 1.06
(0.72–1.55) | 0.844 |
Results of the haplotype analysis of the three
variants are presented in Table
III. The findings did not support an association between
haplotype and risk of childhood ALL. Associations between the
PAX8-AS1 polymorphisms and patient clinical characteristics were
also estimated. As depicted in Table
IV, a significant association between rs4848320 and sex was
observed [χ2=8.45, degrees of freedom (df)=2, P=0.015].
Notably, the CT genotype significantly decreased the risk of ALL in
females (OR=0.32, 95% CI=0.13–0.84, P=0.0355; data not shown). For
rs6726151, the findings indicated that this variant was associated
with organomegaly (χ2=8.21, df=2, P=0.017) and
lymphadenopathy (χ2=11.48, df=2, P=0.003; Table IV). No significant associations were
identified between rs1110839 and patient clinical
characteristics.
 | Table III.Association of paired-box gene 8
antisense RNA 1 haplotypes and risk of acute lymphoblastic
leukemia. |
Table III.
Association of paired-box gene 8
antisense RNA 1 haplotypes and risk of acute lymphoblastic
leukemia.
| Polymorphism |
|
|
|
|
|---|
|
|
|
|
|
|---|
| rs4848320 | rs1110839 | rs6726151 | Cases,
frequency | Controls,
frequency | OR (95% CI) | P-value |
|---|
| C | T | T | 0.2025 | 0.2216 | 1.00 [ref.] | – |
| T | G | G | 0.2010 | 0.1680 | 1.32
(0.71–2.47) | 0.390 |
| T | T | G | 0.2177 | 0.1472 | 1.63
(0.83–3.19) | 0.160 |
| C | T | G | 0.1402 | 0.2042 | 0.77
(0.38–1.58) | 0.480 |
| C | G | G | 0.0910 | 0.1431 | 0.68
(0.33–1.42) | 0.310 |
| C | G | T | 0.0754 | 0.0478 | 1.87
(0.55–6.43) | 0.320 |
| T | G | T | 0.0463 | 0.0495 | 1.09
(0.38–3.11) | 0.880 |
| T | T | T | 0.0259 | 0.0186 | 1.35
(0.20–9.20) | 0.760 |
 | Table IV.Association of paired-box gene 8
antisense RNA 1 polymorphisms with demographic and clinical
features of patients. |
Table IV.
Association of paired-box gene 8
antisense RNA 1 polymorphisms with demographic and clinical
features of patients.
|
| rs4848320 |
| rs1110839 |
| rs6726151 |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Factor | CC | CT | TT | P-value | TT | TG | GG | P-value | GG | GT | TT | P-value |
|---|
| Sex, n |
|
|
| 0.015 |
|
|
| 0.583 |
|
|
| 0.073 |
|
Male | 19 | 28 | 18 |
| 28 | 24 | 13 |
| 22 | 36 | 7 |
|
|
Female | 7 | 32 | 6 |
| 15 | 19 | 11 |
| 18 | 27 | 0 |
|
| Age at diagnosis,
years | 5.2±3.1 | 6.5±3.9 | 5.7±4.5 | 0.355 | 5.9±3.7 | 5.5±3.9 | 7.2±4.1 | 0.231 | 5.6±4.0 | 6.2±3.9 | 6.3±3.8 | 0.757 |
| WBC,
×106/ml, mean ± SD | 31.3±49.6 | 40.8±55.3 | 40.9±46.8 | 0.715 | 33.8±42.9 | 44.0±66.2 | 37.5±36.4 | 0.656 | 42.1±50.4 | 39.5±54.9 | 10.1±15.5 | 0.318 |
| Hemoglobin, g/dl,
mean ± SD | 7.2±2.1 | 7.0±2.3 | 7.8±2.3 | 0.362 | 7.1±2.3 | 7.1±2.4 | 7.6±2.0 | 0.607 | 7.6±2.
3 | 7.9±2.2 | 8.0±1.6 | 0.172 |
| Platelet,
×106/ml, mean ± SD | 71.9±64.0 | 52.3±45.4 | 49.1±38.0 | 0.173 | 54.4±44.0 | 60.8±61.1 | 56.2±49.4 | 0.874 | 61.6±50.6 | 54.3±50.5 |
42.9±430.0 | 0.587 |
| Organomegaly |
|
|
| 0.152 |
|
|
| 0.163 |
|
|
| 0.017 |
|
Positive | 24 | 52 | 24 |
| 39 | 37 | 24 |
| 40 | 55 | 5 |
|
|
Negative | 2 | 8 | 0 |
| 4 | 6 | 0 |
| 0 | 8 | 2 |
|
|
Lymphadenopathy |
|
|
| 0.105 |
|
|
| 0.649 |
|
|
| 0.003 |
|
Positive | 14 | 44 | 19 |
| 31 | 28 | 18 |
| 31 | 45 | 1 |
|
|
Negative | 12 | 16 | 5 |
| 12 | 15 | 6 |
| 9 | 18 | 6 |
|
| Cerebrospinal fluid
involvement |
|
|
| 0.536 |
|
|
| 0.498 |
|
|
| 0.669 |
|
Positive | 1 | 5 | 3 |
| 5 | 2 | 2 |
| 4 | 5 | 0 |
|
|
Negative | 25 | 55 | 21 |
| 38 | 41 | 22 |
| 36 | 58 | 7 |
|
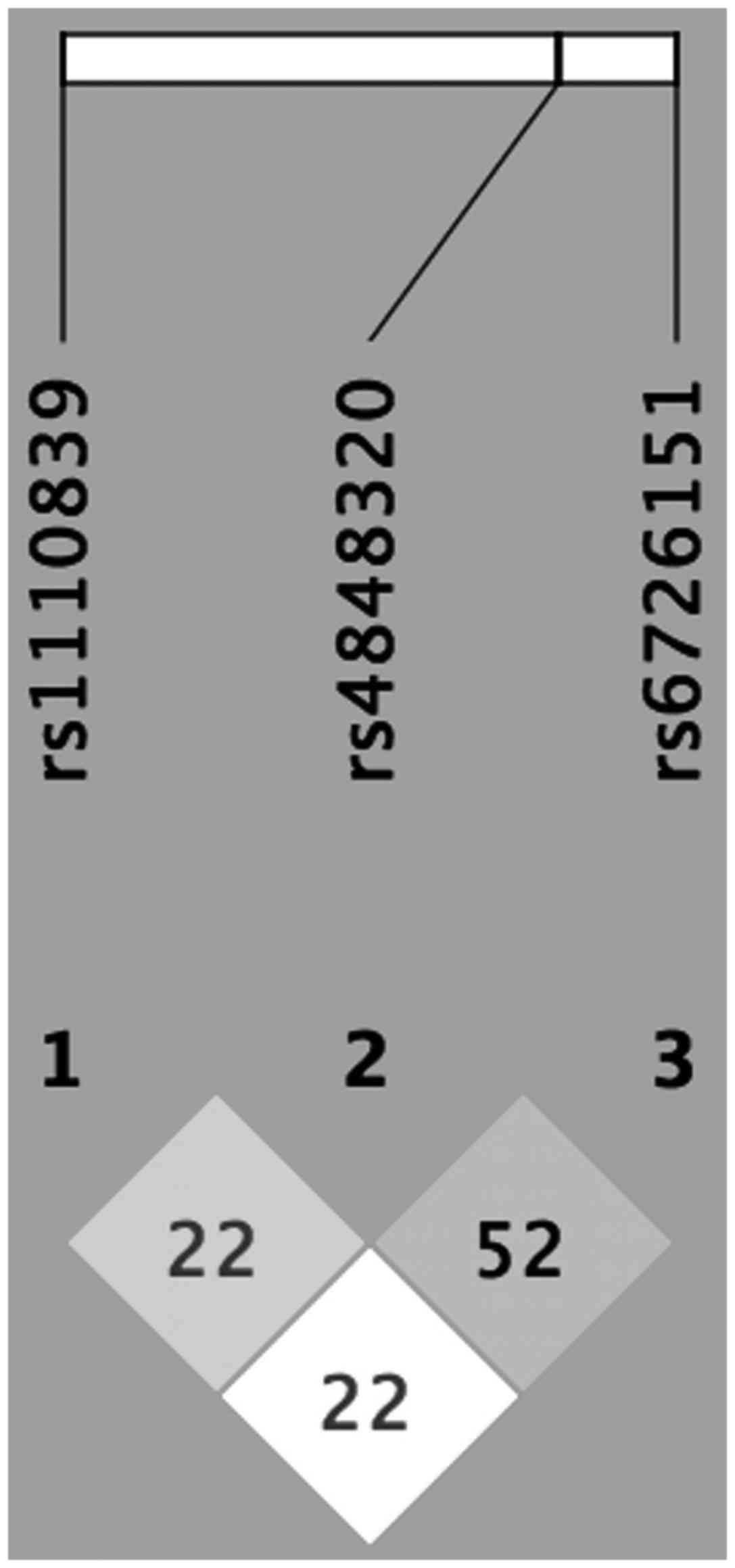
Furthermore, linkage disequilibrium was observed
between rs4848320 and rs1110839 (D'=0.2242, r2=0.0455); rs4848320
and rs6726151 (D'=0.5268, r2=0.1117); and rs1110839 and rs6726151
(D'=0.2279, r2=0.0189; Fig. 4).
Discussion
In the present study, the possible association
between PAX8-AS1 polymorphisms and risk of childhood ALL in a
Southeast Iranian population was investigated. The findings
indicated that the rs4848320 and rs6726151 variants of PAX8-AS1
significantly increased the risk of developing childhood ALL, while
there was no association between rs1110839 and disease
risk/protection. By contrast, the haplotype analysis did not
identify a significant association of any of the variants with risk
of childhood ALL. However, stratification of the variants according
to the clinical characteristics of patients indicated that the
rs4848320 variant was associated with sex while the rs6726151
variant was associated with organomegaly and lymphadenopathy.
Previous results have indicated that non-coding
transcripts in the human genome serve crucial and diverse
biological roles (29). The findings
of macromolecular interactions have revealed that tissue-specific
lncRNAs form base-pairing interactions with numerous mRNAs
associated with tissue-differentiation, indicating that tissue
specificity is an critical factor in controlling human lncRNA-mRNA
interactions (30). LncRNAs have
tissue-specific expression and serve an important role in the human
transcriptome by regulating normal tissue differentiation as well
as cancer development (30). Notably,
a number of previous studies have implicated a role of lncRNA
dysregulation, of transcripts including leukemia-induced non-coding
activator RNA, B-ALL-associated long RNA (BALR)-6 and −2,
NOTCH1-associated lncRNA in T-ALL and CCDC26, in tumorigenicity in
leukemias (31–34). SNPs may significantly influence gene
expression and function. Altered expression of lncRNAs in various
cancers indicates the potential tumor suppressor or oncogenic
functions of the lncRNAs (35–39).
Recently, an association between the rs2147578 polymorphism of
lnc-LAMC2-1 and risk of childhood ALL has been demonstrated
(6).
There is limited information on the impact of
PAX8-AS1 polymorphisms on cancer risk (19,24). Han
et al (19) demonstrated that
PAX8-AS1 rs4848320 and rs1110839 polymorphisms decreased the risk
of cervical cancer, while Ma et al (24) reported that rs4848320 and rs1110839
were associated with prognosis of HCC in a Chinese population. It
has been proposed that the PAX genes act as oncogenes, and that PAX
overexpression facilitates malignant development through effects on
apoptotic resistance, tumor cell proliferation and migration, and
repression of terminal differentiation (40). As PAX8-AS1 is a potential regulator of
PAX8, polymorphisms in the PAX8-AS1 may affect its function and
alter the expression of PAX8.
There are a number of limitations to the present
study. First, a relatively small sample size was used. Second,
there was a lack of data regarding the response of patients to
treatment; therefore, it was not possible to analyze the
association between the variants and response to treatment.
In conclusion, the present results suggested that
PAX8-AS1 polymorphisms significantly increased the risk of
childhood ALL in a Southeast Iranian population. As this, to the
best of our knowledge, was the first study to examine the
association of polymorphisms in PAX8-AS1 with risk of childhood
ALL, future studies with larger sample sizes and different
ethnicities are required to confirm the findings.
Acknowledgements
The present study was financially supported by a
research grant from the Deputy for Research of Zahedan University
of Medical Sciences, (Zahedan, Iran; grant no. 8224).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bahari G, Hashemi M, Naderi M and Taheri
M: IKZF1 gene polymorphisms increased the risk of childhood acute
lymphoblastic leukemia in an Iranian population. Tumour Biol.
37:9579–9586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bahari G, Hashemi M, Naderi M,
Sadeghi-Bojd S and Taheri M: Association of SHMT1 gene
polymorphisms with the risk of childhood acute lymphoblastic
leukemia in a sample of Iranian population. Cell Mol Biol
(Noisy-le-grand). 62:45–51. 2016.PubMed/NCBI
|
|
4
|
Hasani SS, Hashemi M, Eskandari-Nasab E,
Naderi M, Omrani M and Sheybani-Nasab M: A functional polymorphism
in the miR-146a gene is associated with the risk of childhood acute
lymphoblastic leukemia: A preliminary report. Tumour Biol.
35:219–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tong N, Chu H, Wang M, Xue Y, Du M, Lu L,
Zhang H, Wang F, Fang Y, Li J, et al: Pri-miR-34b/c rs4938723
polymorphism contributes to acute lymphoblastic leukemia
susceptibility in Chinese children. Leuk Lymphoma. 57:1436–1441.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashemi M, Bahari G, Naderi M, Bojd S
Sadeghi and Taheri M: Association of lnc-LAMC2-1:1 rs2147578 and
CASC8 rs10505477 polymorphisms with risk of childhood acute
lymphoblastic leukemia. Asian Pac J Cancer Prev. 17:4985–4989.
2016.PubMed/NCBI
|
|
7
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hashemi M, Bahari G, Naderi M,
Sadeghi-Bojd S and Taheri M: Pri-miR-34b/c rs4938723 polymorphism
is associated with the risk of childhood acute lymphoblastic
leukemia. Cancer Genet. 209:493–496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tripathi V, Ellis JD, Shen Z, Song DY, Pan
Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, et al: The
nuclear-retained noncoding RNA MALAT1 regulates alternative
splicing by modulating SR splicing factor phosphorylation. Mol
Cell. 39:925–938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi P and Du X: The long non-coding RNAs, a
new cancer diagnostic and therapeutic gold mine. Mod Pathol.
26:155–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poleev A, Fickenscher H, Mundlos S,
Winterpacht A, Zabel B, Fidler A, Gruss P and Plachov D: PAX8, a
human paired box gene: Isolation and expression in developing
thyroid, kidney and Wilms' tumors. Development. 116:611–623.
1992.PubMed/NCBI
|
|
13
|
Ordóñez NG: Value of PAX 8 immunostaining
in tumor diagnosis: A review and update. Adv Anat Pathol.
19:140–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nonaka D, Chiriboga L and Soslow RA:
Expression of pax8 as a useful marker in distinguishing ovarian
carcinomas from mammary carcinomas. Am J Surg Pathol. 32:1566–1571.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong GX, Weeden EM, Hamele-Bena D, Huan Y,
Unger P, Memeo L and O'Toole K: Expression of PAX8 in nephrogenic
adenoma and clear cell adenocarcinoma of the lower urinary tract:
Evidence of related histogenesis? Am J Surg Pathol. 32:1380–1387.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tong GX, Yu WM, Beaubier NT, Weeden EM,
Hamele-Bena D, Mansukhani MM and O'Toole KM: Expression of PAX8 in
normal and neoplastic renal tissues: An immunohistochemical study.
Mod Pathol. 22:1218–1227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Becker N, Chernock RD, Nussenbaum B and
Lewis JS Jr: Prognostic significance of β-human chorionic
gonadotropin and PAX8 expression in anaplastic thyroid carcinoma.
Thyroid. 24:319–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lang D, Powell SK, Plummer RS, Young KP
and Ruggeri BA: PAX genes: Roles in development, pathophysiology,
and cancer. Biochem Pharmacol. 73:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han J, Zhou W, Jia M, Wen J, Jiang J, Shi
J, Zhang K, Ma H, Liu J, Ren J, et al: Expression quantitative
trait loci in long non-coding RNA PAX8-AS1 are associated with
decreased risk of cervical cancer. Mol Genet Genomics.
291:1743–1748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nica AC and Dermitzakis ET: Expression
quantitative trait loci: Present and future. Philos Trans R Soc
Lond B Biol Sci. 368:201203622013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boyle AP, Hong EL, Hariharan M, Cheng Y,
Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, et
al: Annotation of functional variation in personal genomes using
RegulomeDB. Genome Res. 22:1790–1797. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stranger BE, Nica AC, Forrest MS, Dimas A,
Bird CP, Beazley C, Ingle CE, Dunning M, Flicek P, Koller D, et al:
Population genomics of human gene expression. Nat Genet.
39:1217–1224. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Veyrieras JB, Kudaravalli S, Kim SY,
Dermitzakis ET, Gilad Y, Stephens M and Pritchard JK:
High-resolution mapping of expression-QTLs yields insight into
human gene regulation. PLoS Genet. 4:e10002142008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma S, Yang J, Song C, Ge Z, Zhou J, Zhang
G and Hu Z: Expression quantitative trait loci for PAX8 contributes
to the prognosis of hepatocellular carcinoma. PLoS One.
12:e01737002017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sherry ST, Ward MH, Kholodov M, Baker J,
Phan L, Smigielski EM and Sirotkin K: dbSNP: The NCBI database of
genetic variation. Nucleic Acids Res. 29:308–311. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashemi M, Bojd H Hanafi, Nasab E
Eskandari, Bahari A, Hashemzehi NA, Shafieipour S, Narouie B,
Taheri M and Ghavami S: Association of adiponectin rs1501299 and
rs266729 gene polymorphisms with nonalcoholic fatty kiver disease.
Hepat Mon. 13:e95272013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Solé X, Guinó E, Valls J, Iniesta R and
Moreno V: SNPStats: A web tool for the analysis of association
studies. Bioinformatics. 22:1928–1929. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barrett JC, Fry B, Maller J and Daly MJ:
Haploview: Analysis and visualization of LD and haplotype maps.
Bioinformatics. 21:263–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iwakiri J, Terai G and Hamada M:
Computational prediction of lncRNA-mRNA interactionsby integrating
tissue specificity in human transcriptome. Biol Direct. 12:152017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rodríguez-Malavé NI, Fernando TR, Patel
PC, Contreras JR, Palanichamy JK, Tran TM, Anguiano J, Davoren MJ,
Alberti MO, Pioli KT, et al: BALR-6 regulates cell growth and cell
survival in B-lymphoblastic leukemia. Mol Cancer. 14:2142015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Wu P, Lin R, Rong L, Xue Y and
Fang Y: LncRNA NALT interaction with NOTCH1 promoted cell
proliferation in pediatric T cell acute lymphoblastic leukemia. Sci
Rep. 5:137492015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trimarchi T, Bilal E, Ntziachristos P,
Fabbri G, Dalla-Favera R, Tsirigos A and Aifantis I: Genome-wide
mapping and characterization of Notch-regulated long noncoding RNAs
in acute leukemia. Cell. 158:593–606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirano T, Yoshikawa R, Harada H, Harada Y,
Ishida A and Yamazaki T: Long noncoding RNA CCDC26, controls
myeloid leukemia cell growth through regulation of KIT expression.
Mol Cancer. 14:902015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun QL, Zhao CP, Wang TY, Hao XB, Wang XY,
Zhang X and Li YC: Expression profile analysis of long non-coding
RNA associated with vincristine resistance in colon cancer cells by
next-generation sequencing. Gene. 572:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang X, Song JH, Cheng Y, Wu W, Bhagat T,
Yu Y, Abraham JM, Ibrahim S, Ravich W, Roland BC, et al: Long
non-coding RNA HNF1A-AS1 regulates proliferation and migration in
oesophageal adenocarcinoma cells. Gut. 63:881–890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xing CY, Hu XQ, Xie FY, Yu ZJ, Li HY,
Bin-Zhou, Wu JB, Tang LY and Gao SM: Long non-coding RNA HOTAIR
modulates c-KIT expression through sponging miR-193a in acute
myeloid leukemia. FEBS Lett. 589:1981–1987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morlando M, Ballarino M and Fatica A: Long
non-coding RNAs: New players in hematopoiesis and leukemia. Front
Med (Lausanne). 2:232015.PubMed/NCBI
|
|
39
|
Emmrich S, Streltsov A, Schmidt F,
Thangapandi VR, Reinhardt D and Klusmann JH: LincRNAs MONC and
MIR100HG act as oncogenes in acute megakaryoblastic leukemia. Mol
Cancer. 13:1712014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Muratovska A, Zhou C, He S, Goodyer P and
Eccles MR: Paired-box genes are frequently expressed in cancer and
often required for cancer cell survival. Oncogene. 22:7989–7997.
2003. View Article : Google Scholar : PubMed/NCBI
|