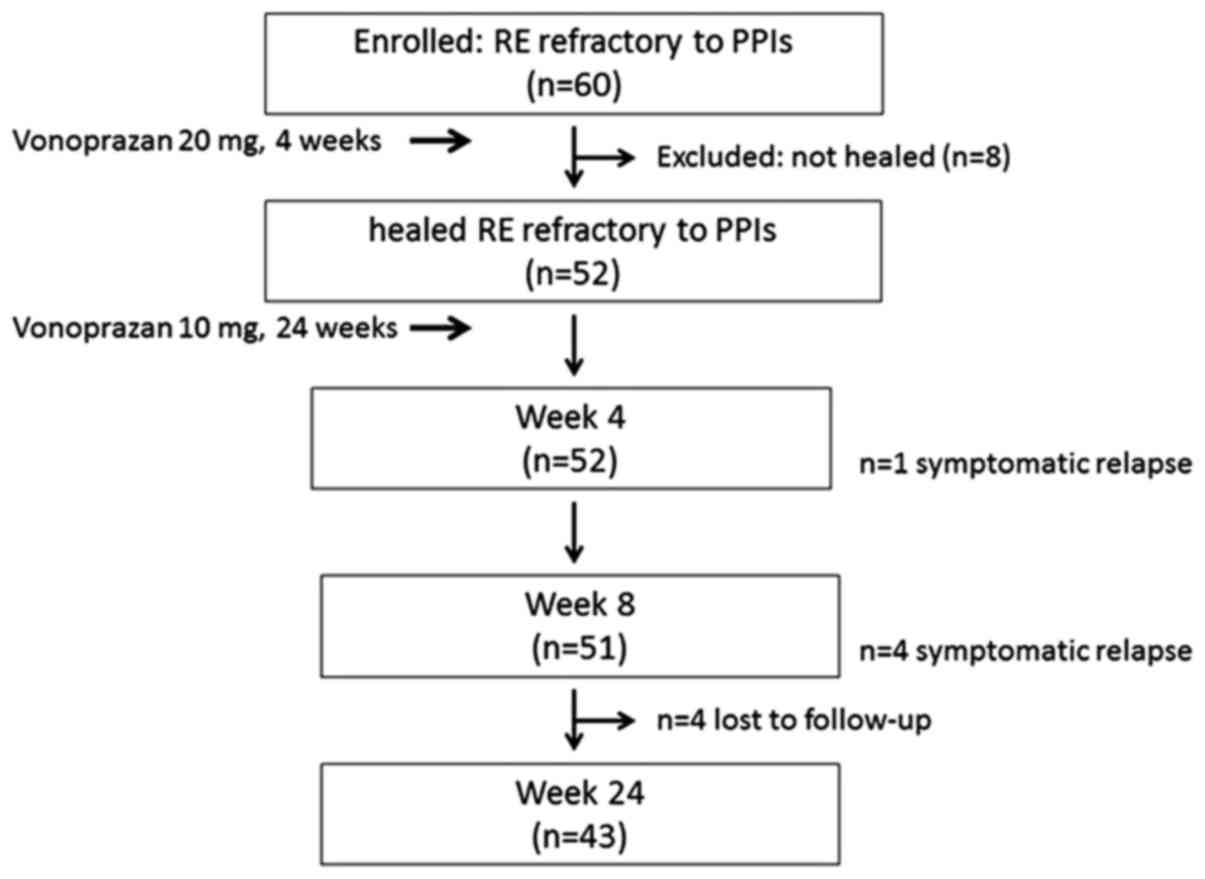
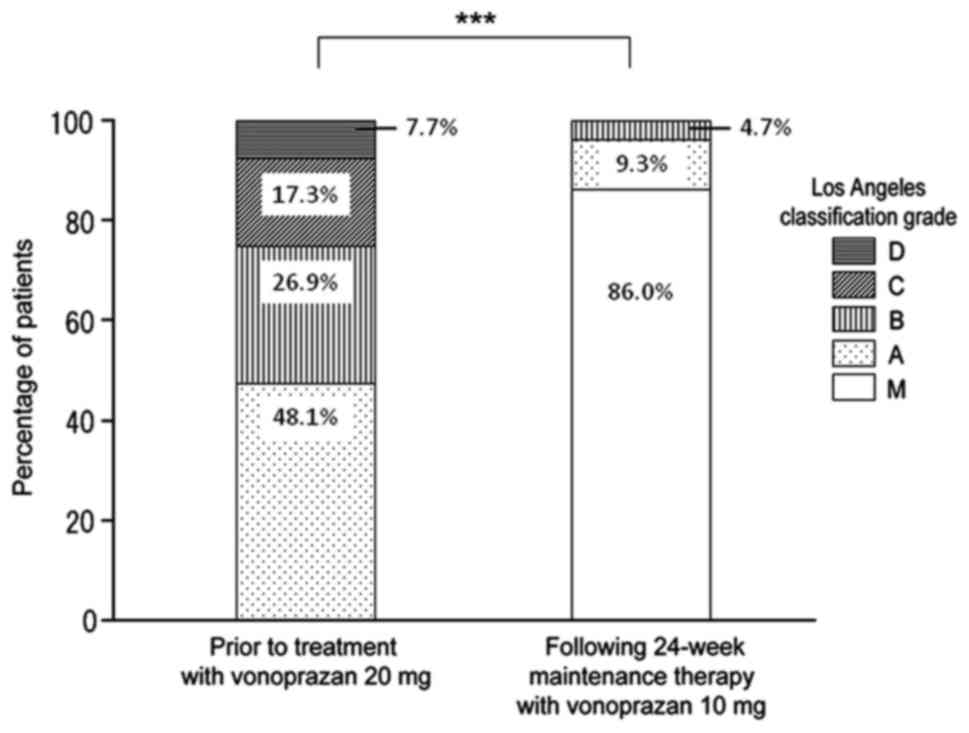
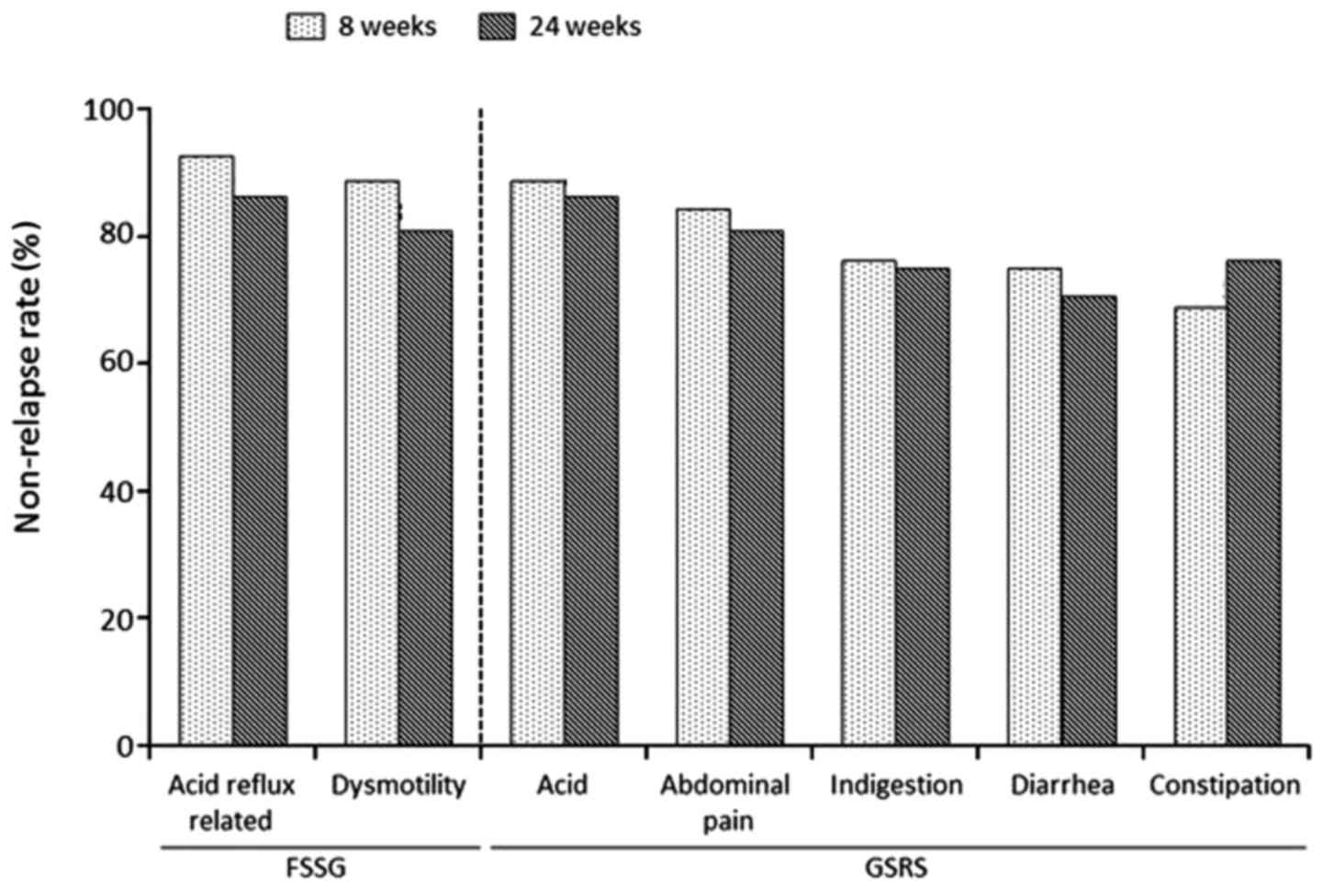
|
1
|
Iwakiri K, Kinoshita Y, Habu Y, Oshima T,
Manabe N, Fujiwara Y, Nagahara A, Kawamura O, Iwakiri R, Ozawa S,
et al: Evidence-based clinical practice guidelines for
gastroesophageal reflux disease 2015. J Gastroenterol. 51:751–767.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujiwara Y and Arakawa T: Epidemiology and
clinical characteristics of GERD in the Japanese population. J
Gastroenterol. 44:518–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miwa H, Yokoyama T, Hori K, Sakagami T,
Oshima T, Tomita T, Fujiwara Y, Saita H, Itou T, Ogawa H, et al:
Interobserver agreement in endoscopic evaluation of reflux
esophagitis using a modified Los Angeles classification
incorporating grades N and M: A validation study in a cohort of
Japanese endoscopists. Dis Esophagus. 21:355–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kahrilas PJ, Shaheen NJ and Vaezi MF;
American Gastroenterological Association Institute, ; Clinical
Practice and Quality Management Committee, : American
Gastroenterological Association Institute technical review on the
management of gastroesophageal reflux disease. Gastroenterology.
135:1392–1413, 1413.e1-1413.e5. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joh T, Miwa H, Higuchi K, Shimatani T,
Manabe N, Adachi K, Wada T, Sasaki M, Fujiwara Y, Hongo M, et al
ARS Research Group, : Validity of endoscopic classification of
nonerosive reflux disease. J Gastroenterol. 42:444–449. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pace F, Negrini C, Wiklund I, Rossi C and
Savarino V; ITALIAN ONE INVESTIGATORS STUDY GROUP, : Quality of
life in acute and maintenance treatment of non-erosive and mild
erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther.
22:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wada T, Sasaki M, Kataoka H, Tanida S,
Itoh K, Ogasawara N, Oshima T, Togawa S, Kubota E, Yamada T, et al:
Efficacy of famotidine and omeprazole in healing symptoms of
non-erosive gastro-oesophageal reflux disease:
Randomized-controlled study of gastro-oesophageal reflux disease.
Aliment Pharmacol Ther. 21 Suppl 2:2–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fass R: Erosive esophagitis and nonerosive
reflux disease (NERD): Comparison of epidemiologic, physiologic,
and therapeutic characteristics. J Clin Gastroenterol. 41:131–137.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fass R, Shapiro M, Dekel R and Sewell J:
Systematic review: Proton-pump inhibitor failure in
gastro-oesophageal reflux disease - where next? Aliment Pharmacol
Ther. 22:79–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizuno H, Matsuhashi N, Sakaguchi M, Inoue
S, Nakada K, Higuchi K, Haruma K and Joh T: Recent effectiveness of
proton pump inhibitors for severe reflux esophagitis: The first
multicenter prospective study in Japan. J Clin Biochem Nutr.
57:233–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersson K and Carlsson E:
Potassium-competitive acid blockade: A new therapeutic strategy in
acid-related diseases. Pharmacol Ther. 108:294–307. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hori Y, Imanishi A, Matsukawa J, Tsukimi
Y, Nishida H, Arikawa Y, Hirase K, Kajino M and Inatomi N:
1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine
monofumarate (TAK-438), a novel and potent potassium-competitive
acid blocker for the treatment of acid-related diseases. J
Pharmacol Exp Ther. 335:231–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashida K, Sakurai Y, Nishimura A, Kudou K,
Hiramatsu N, Umegaki E, Iwakiri K and Chiba T: Randomised clinical
trial: A dose-ranging study of vonoprazan, a novel
potassium-competitive acid blocker, vs. lansoprazole for the
treatment of erosive oesophagitis. Aliment Pharmacol Ther.
42:685–695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jenkins H, Sakurai Y, Nishimura A, Okamoto
H, Hibberd M, Jenkins R, Yoneyama T, Ashida K, Ogama Y and
Warrington S: Randomised clinical trial: Safety, tolerability,
pharmacokinetics and pharmacodynamics of repeated doses of TAK-438
(vonoprazan), a novel potassium-competitive acid blocker, in
healthy male subjects. Aliment Pharmacol Ther. 41:636–648. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakurai Y, Mori Y, Okamoto H, Nishimura A,
Komura E, Araki T and Shiramoto M: Acid-inhibitory effect of
vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10
mg in healthy adult male subjects - a randomised open-label
cross-over study. Aliment Pharmacol Ther. 42:719–730. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashida K, Sakurai Y, Hori T, Kudou K,
Nishimura A, Hiramatsu N, Umegaki E and Iwakiri K: Randomised
clinical trial: Vonoprazan, a novel potassium-competitive acid
blocker, vs. lansoprazole for the healing of erosive oesophagitis.
Aliment Pharmacol Ther. 43:240–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoshino S, Kawami N, Takenouchi N, Umezawa
M, Hanada Y, Hoshikawa Y, Kawagoe T, Sano H, Hoshihara Y, Nomura T,
et al: Efficacy of Vonoprazan for Proton Pump Inhibitor-Resistant
Reflux Esophagitis. Digestion. 95:156–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okuyama M, Nakahara K, Iwakura N, Hasegawa
T, Oyama M, Inoue A, Ishizu H, Satoh H and Fujiwara Y: Factors
associated with potassium-competitive acid blocker non-response in
patients with proton pump inhibitor-refractory gastroesophageal
reflux disease. Digestion. 95:281–287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwakiri K, Sakurai Y, Shiino M, Okamoto H,
Kudou K, Nishimura A, Hiramatsu N, Umegaki E and Ashida K: A
randomized, double-blind study to evaluate the acid-inhibitory
effect of vonoprazan (20 mg and 40 mg) in patients with proton-pump
inhibitor-resistant erosive esophagitis. Therap Adv Gastroenterol.
10:439–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kusano M, Shimoyama Y, Sugimoto S,
Kawamura O, Maeda M, Minashi K, Kuribayashi S, Higuchi T, Zai H,
Ino K, et al: Development and evaluation of FSSG: Frequency scale
for the symptoms of GERD. J Gastroenterol. 39:888–891. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lundell LR, Dent J, Bennett JR, Blum AL,
Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler
SJ, et al: Endoscopic assessment of oesophagitis: Clinical and
functional correlates and further validation of the Los Angeles
classification. Gut. 45:172–180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinoshita Y and Adachi K: Hiatal hernia
and gastroesophageal flap valve as diagnostic indicators in
patients with gastroesophageal reflux disease. J Gastroenterol.
41:720–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 1:87–97. 1969. View Article : Google Scholar
|
|
24
|
Hongo M, Fukuhara S and Green J:
Gastrointestinal-related QOL-QOL assessment using the Japanese
version of GSRS. Diagn Treat. 87:731–736. 1999.
|
|
25
|
Drossman DA: The functional
gastrointestinal disorders and the Rome III process.
Gastroenterology. 130:1377–1390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dent J, Brun J, Fendrick A, Fendrick M,
Janssens J, Kahrilas P, Lauritsen K, Reynolds J, Shaw M, et al: An
evidence-based appraisal of reflux disease management - the Genval
Workshop Report. Gut. 44 Suppl 2:S1–S16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DeVault KR and Castell DO; American
College of Gastroenterology, : Updated guidelines for the diagnosis
and treatment of gastroesophageal reflux disease. Am J
Gastroenterol. 100:190–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Röhss K, Lind T and Wilder-Smith C:
Esomeprazole 40 mg provides more effective intragastric acid
control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40
mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux
symptoms. Eur J Clin Pharmacol. 60:531–539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lauritsen K, Devière J, Bigard MA,
Bayerdörffer E, Mózsik G, Murray F, Kristjánsdóttir S, Savarino V,
Vetvik K, De Freitas D, et al: Metropole study results:
Esomeprazole 20 mg and lansoprazole 15 mg in maintaining healed
reflux oesophagitis: Metropole study results. Aliment Pharmacol
Ther. 17:333–341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Labenz J, Armstrong D, Lauritsen K,
Katelaris P, Schmidt S, Schütze K, Wallner G, Juergens H,
Preiksaitis H, Keeling N, et al: Esomeprazole 20 mg vs.
pantoprazole 20 mg for maintenance therapy of healed erosive
oesophagitis: Results from the EXPO study. Aliment Pharmacol Ther.
22:803–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Devault KR, Johanson JF, Johnson DA, Liu S
and Sostek MB: Maintenance of healed erosive esophagitis: A
randomized six-month comparison of esomeprazole twenty milligrams
with lansoprazole fifteen milligrams. Clin Gastroenterol Hepatol.
4:852–859. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vakil NB, Shaker R, Johnson DA, Kovacs T,
Baerg RD, Hwang C, D'Amico D and Hamelin B: The new proton pump
inhibitor esomeprazole is effective as a maintenance therapy in
GERD patients with healed erosive oesophagitis: A 6-month,
randomized, double-blind, placebo-controlled study of efficacy and
safety. Aliment Pharmacol Ther. 15:927–935. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Atug O, Giral A, Kalayci C, Dolar E,
Isitan F, Oguz D, Ovunc O, Ozgur O, Soykan I, Simsek I, et al
Turkish HEMANEX Study Group, : Esomeprazole in acute and
maintenance treatment of reflux oesophagitis: A multicentre
prospective study. Adv Ther. 25:552–566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Piche T and Galmiche JP: Pharmacological
targets in gastro-oesophageal reflux disease. Basic Clin Pharmacol
Toxicol. 97:333–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sachs G, Shin JM and Howden CW: Review
article: The clinical pharmacology of proton pump inhibitors.
Aliment Pharmacol Ther. 23 Suppl 2:2–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimatani T, Inoue M, Kuroiwa T, Xu J,
Mieno H, Nakamura M and Tazuma S: Acid-suppressive effects of
rabeprazole, omeprazole, and lansoprazole at reduced and standard
doses: A crossover comparative study in homozygous extensive
metabolizers of cytochrome P450 2C19. Clin Pharmacol Ther.
79:144–152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Norris V, Baisley K, Dunn K, Warrington S
and Morocutti A: Combined analysis of three crossover clinical
pharmacology studies of effects of rabeprazole and esomeprazole on
24-h intragastric pH in healthy volunteers. Aliment Pharmacol Ther.
25:501–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamasaki H, Kawaguchi N, Nonaka M,
Takahashi J, Morohashi A, Hirabayashi H, Moriwaki T and Asahi S: In
vitro metabolism of TAK-438, vonoprazan fumarate, a novel
potassium-competitive acid blocker. Xenobiotica. 47:1027–1034.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kusunoki H, Kusaka M, Kido S, Yamauchi R,
Fujimura Y, Watanabe Y, Kobori M, Miwa H, Tomita T, Kin Y, et al:
Comparison of the effects of omeprazole and famotidine in treatment
of upper abdominal symptoms in patients with reflux esophagitis. J
Gastroenterol. 44:261–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hori K, Matsumoto T and Miwa H: Analysis
of the gastrointestinal symptoms of uninvestigated dyspepsia and
irritable bowel syndrome. Gut Liver. 3:192–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Locke GR III, Zinsmeister AR, Fett SL,
Melton LJ III and Talley NJ: Overlap of gastrointestinal symptom
complexes in a US community. Neurogastroenterol Motil. 17:29–34.
2005.Soleceperum esequi ut reperum autem es sequat. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Quat Nimusa: Umquid maios rerrore mporum
evenis eaque sequam, nissitiae rem quam quuntusdae sequo te pro
corionsequid magnisint quis duntis ellicae prehent estrume ndanda
nonet, ut millace aquatur aspedi dolorro conessimust, a dis im
fuga. Itatemq uissusc illatem sernatu saerum qui omniam, sinum
fugitium alit facescient.
|