Introduction
Rheumatoid arthritis (RA) is associated with T cells
and immune cell activation, resulting in joint synovitis. Dendritic
cells (DCs) are antigen-presenting cells (APCs) specialized in
activating T cells with a positive or negative immune response
(1,2).
DCs play different functional roles at different maturation states.
The antigen-presenting ability of mature DCs promotes immune
response by the activation of T cells, while that of immature DCs
inhibits the activation of T lymphocytes and activates regulatory T
cells (Tregs) (3,4). Immature DCs mediate immune tolerance.
Tumor necrosis factor receptor-associated factor 6 (TRAF6)
activates TAK1 and NF-κB molecules in Toll-like receptor
(TLR)-signaling pathways, which enter into the nucleus (5,6). To the best
of our knowledge, no report has yet established any association
between TRAF6 and DC maturation, and that TRAF6 promotes a
DC-mediated immune response. We induced DCs at different maturation
states to detect TRAF6 expression by cytokines to analyze the
correlation between TRAF6 and DCs. Thus, this experiment mainly
studied the effect of TRAF6-specific expression on immature
dendritic cell (iDC) maturation and the immune tolerance
re-establishment.
Materials and methods
The study was approved by the Ethics Committee of
the Guilin Medical University.
Preparation of the collagen-induced
arthritis (CIA) animal model
Fifty 8-week-old male C57 mice, weighing
approximately 30 g were obtained from the Guilin Medical College
Experimental Animal Center. Prior to the experiment, chicken type
II collagen (including acetic acid with a concentration of 2 mg/ml)
was added slowly to the same volume of complete Freund's adjuvant
(CFA), prepared in sufficient emulsification on ice and the final
concentration of the mixture was 1 mg/ml. The C57 mice were
immunized intradermally twice at the base of the tail and the back
at intervals of 4 weeks, while the normal controls were
intragastrically administered with saline. The mice were monitored
daily for swelling of limbs for at least 28 days and serum samples
were collected at day 7 or 14 after boosting immunity. After the
building model was established, the mice that scored >6 points
in the arthritis index (AI) scoring were used for further
testing.
Generation and activation of bone
marrow-derived DCs (BMDCs)
BMDCs were obtained from the femurs and tibias of
the CIA mice, and the red cells were treated with a lysis buffer
solution (4.15 g of ammonium chloride per 500 ml of 0.01 M Tris-HCl
buffer). The cells were then washed and cultured in culture flasks
at 1×109 cells/ml in a complete RPMI-1640 culture medium
supplemented with 10% fetal bovine serum (FBS), IL-4 (5,000 U/ml),
and GM-CSF (1,000 U/ml). The culture medium was changed on culture
days 3 and 5. New medium and cytokines (GM-CSF and IL-4) were added
after rinsing the non-adherent cells. On day 6, any loosely
adherent clustered cells were defined as immature BMDCs.
Different maturation stimuli on
DCs
DCs were harvested on culture day 6. Briefly, BMDCs
(1×106/well) were seeded in 24-well plates in 1 ml of
culture medium and treated with recombinant granulocyte-macrophage
colony-stimulating factor (rmGM-CSF). IDCs were induced to DCs at
different maturation states according to different methods. The
cells were divided into four groups, as follows: group A, the
control group was treated with rmGM-CSF and recombinant
interleukin-4 (rmIL-4) (rmGM-CSF, rmIL-4 group). Group B, this
group was treated with TNF-α (1×106 U/l) (TNF-α group).
Group C, this group was treated with LPS (1 mg/l) (LPS group).
Group D, this group was treated with rmGM-CSF (10 ng/ml), FK506 (10
ng/ml), and LPS (1 mg/l) (FK506 group). Each group was then
incubated for 24 h.
Flow cytometric analysis of DC
phenotypes
The cells were collected on day 7, and washed with
PBS. The cells were stained with fluorescence-labeled antibodies
(anti-mouse CD11c-APC, CD80-PE, CD86-PE, and MHC-II-PE) for 30 min
on ice, washed with FBS, and analyzed via flow cytometry. Data
analyses were performed using BD FACSDiva software
(Becton-Dickinson, Mountain View, CA, USA). The initially gated
cells were further analyzed for CD11c, CD80, CD86, and MHC-II
expression.
Different levels of IL-12 secretion by
enzyme-linked immunosorbent assay (ELISA)
A commercial ELISA kit (PBL Biomedical Laboratories,
Piscataway, NJ, USA) was used to measure the serum levels of IL-12
secretion according to the manufacturer's instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Cells in the logarithmic phase were collected, and
then total RNA was extracted from the cells using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). RNA was quantified using UV
absorbance at 260 and 280 nm (A260/280) by NanoDrop 2000
spectrophotometry (Thermo Fisher Scientific, Waltham, MA, USA). RNA
was reverse-transcribed using the BU-Script RT kit (Tiangen Biotech
Co., Ltd., Beijing, China) according to the manufacturer's
instructions. Primers TRAF6-F (5′-GCC GAA ATG GAA GCA CAG-3′) and
TRAF6-R (5′-GGG CTA TGG ATG ACA ACA GG-3′) were used in
conventional PCR detection (Tiangen Biotech Co., Ltd.). The
procedures were performed as described elsewhere (7).
Expression of TRAF6 protein by western
blot analysis
Total protein from cell lysates was separated by
SDS-PAGE and transferred to a nitrocellulose membrane, which was
incubated with the 1:200 diluted specific primary antibodies TRAF6
(ab33915, rabbit polyclonal antibody; Abcam, Cambridge, UK)
overnight at 4°C. After incubation with the 1:1,000 diluted
HRP-labeled secondary antibody (horseradish peroxidase conjugated
goat anti-rabbit immunoglobulin; Abcam) at room temperature for 1
h, the membranes were developed with enhanced chemiluminescence
(ECL) detection system (Gel Logic 2200 PRO; Kodak, Tokyo,
Japan).
Statistical analysis
SPSS 17.0 statistical software (SPSS Inc., Chicago,
IL, USA) was used to conduct analyses. Data were presented as means
± SD to indicate single factor variance. Comparisons were made
using one-way ANOVA. The differences were considered significant
when P<0.05.
Results
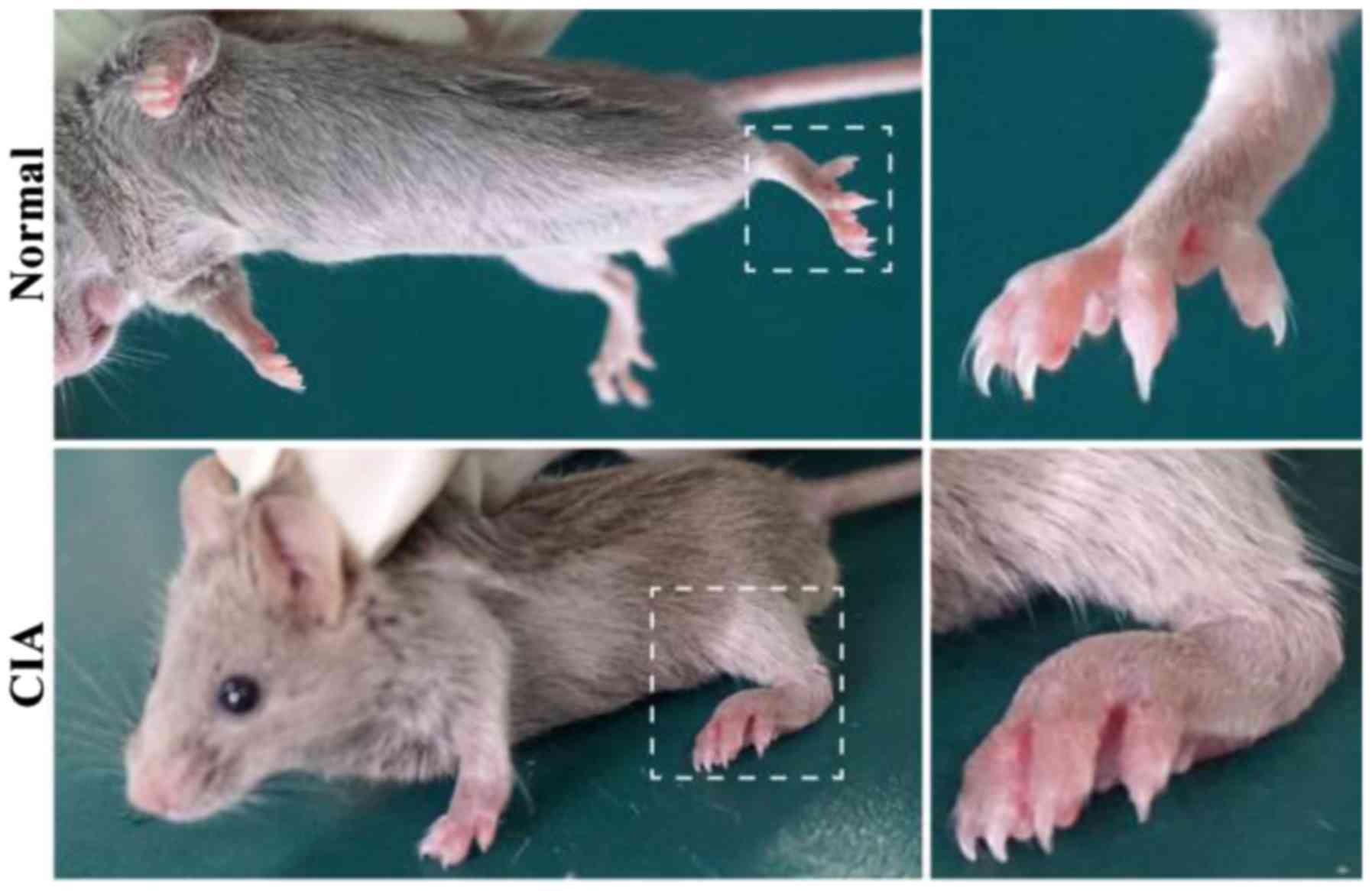
CIA induction
A boost injection of equal collagen-IFA suspension
was given in the same manner on day 21. The experimental group with
50 C57 mice had 40 mice successfully modeling CIA (4/5). Clinical
symptoms of CIA started to occur around day 28 after the primary
immunization, including joint swelling and joint deformity
(Fig. 1).
DC cultures
Bone marrow cells of the C57 mice were induced to
differentiate the BMDCs by cytokines rmGM-CSF and rmIL-4. Flow
cytometric identification of CD11c DCs was >70%.
Changes in the expression of DC
maturation
To analyze the expression of the co-stimulatory
molecules (i.e., CD80, CD86, and MHC-II) from differentially
matured DCs in the experimental groups, the results show that there
is an increasing trend in the expression of the co-stimulatory
molecules among the four groups (Table
I), and the difference was statistically significant
(P<0.05). The expression of CD86 in group D was higher than that
in the other three groups (mean 69.55±2.26). The results suggested
that group D demonstrated highly increased levels of DC
maturation.
 | Table I.Expression of the co-stimulatory
molecules in the four groups of DCs. |
Table I.
Expression of the co-stimulatory
molecules in the four groups of DCs.
| Group | CD80 | CD86 | MHC-II |
|---|
| A |
9.06±0.58 | 12.32±0.62 |
8.36±0.62 |
| B | 11.06±0.68 | 18.65±0.83 |
9.23±0.61 |
| C | 14.67±0.74 | 30.37±1.37 | 14.28±0.60 |
| D | 19.01±0.92 | 69.55±0.96 | 18.19±0.96 |
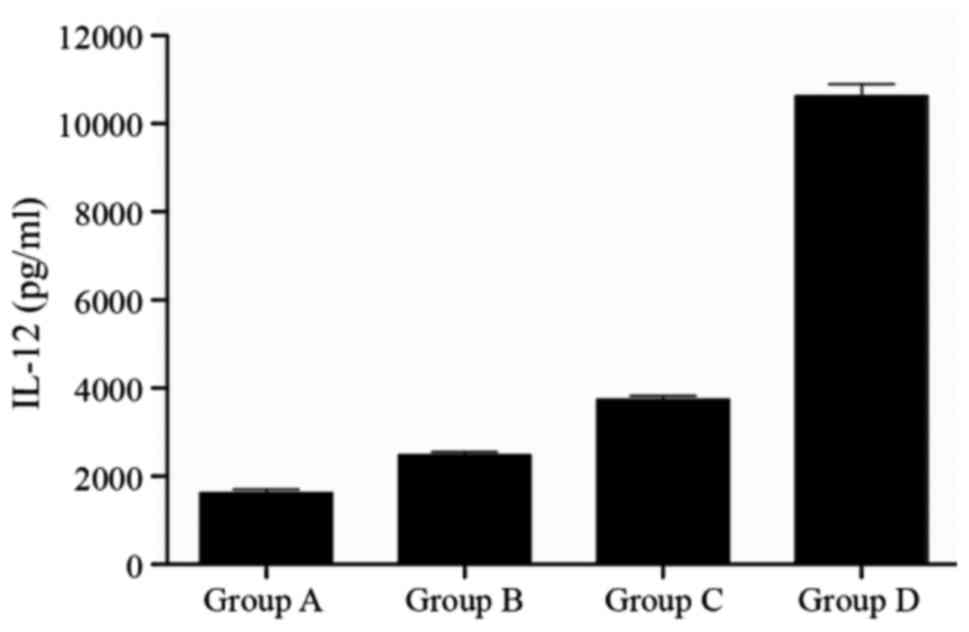
IL-12 expression by DCs at different
matured states
The supernatants from the four groups were harvested
and analyzed by ELISA. The concentration of each group was
calculated from OD readings using a standard curve. The values were
as follows: group A, 1,623.35±83.32 pg/ml; group B, 2,486.14±65.26
pg/ml; group C, 3,742.58±87.16 pg/ml; and group D, 10,620.73±276.73
pg/ml (Fig. 2). There was a
significant difference in each group (P<0.05).
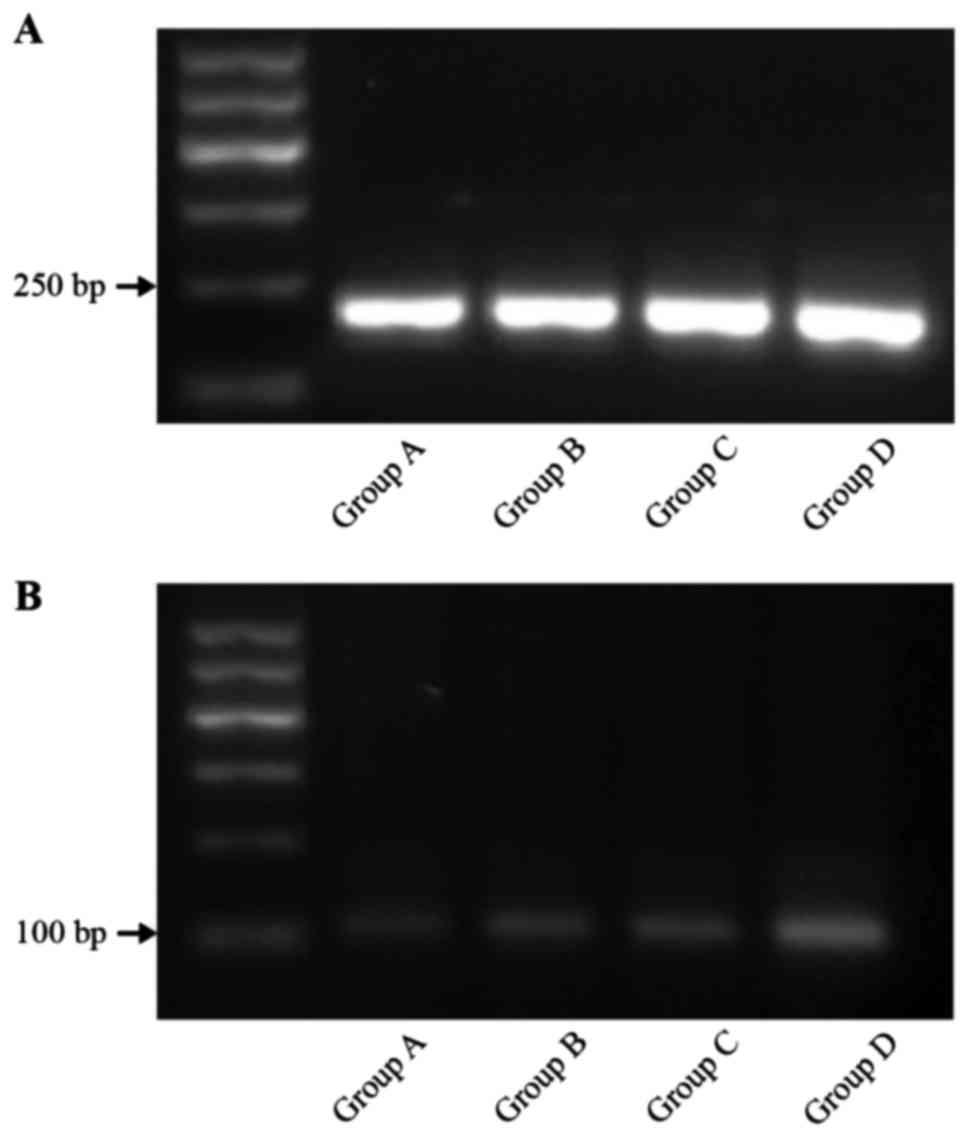
mRNA-TRAF6 by pulsed-field gel
electrophoresis (PFGE)
The expression of mRNA-TRAF6 in group D was superior
to that of the three other groups (P<0.01). The level of
mRNA-TRAF6 expression in groups B and C was higher than that in the
control group (P<0.01). The results were as follows: group A,
0.046±0.007; group B, 0.063±0.006; group C, 0.062±0.006; and group
D, 0.151±0.08 (Fig. 3).
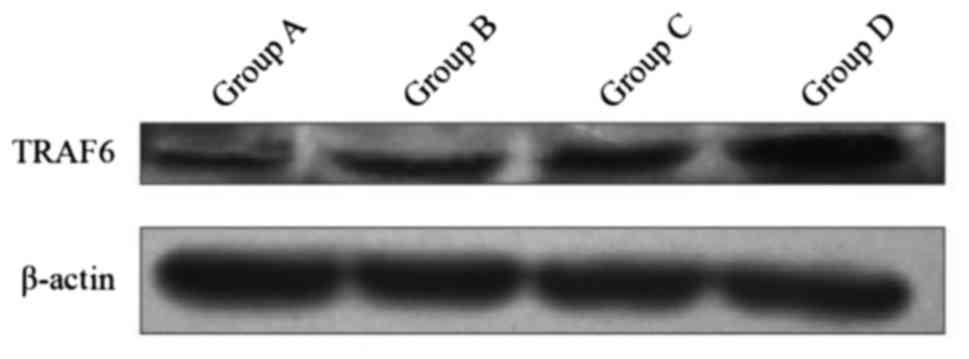
Expression of protein TRAF6 in
DCs
The expression of protein TRAF6 in group D was
superior to the three other groups by inducing DC at different
maturation states. The differences were statistically significant
(P<0.01). The difference between groups B and C, and group A was
statistically significant (P<0.01) (Fig. 4). The results in the four groups were
as follows: group A, 0.49±0.006; group B, 0.65±0.005; group C,
0.75±0.005; and group D, 1.15±0.003.
Discussion
RA is a systemic, inflammatory, autoimmune disorder.
However, the exact mechanism has yet to be fully clarified. Most
scholars believe that the pathogenesis of RA may be caused by
chronic inflammation of synovial tissue. During the onset of RA, an
acute inflammation of the synovial lining (synovitis) leads to an
extensive expansion of the corresponding cells (i.e., pannus
formation and inflammatory cytokines), massive infiltration of
leukocytes of the innate immune system (i.e., T and B cells,
plasmocytes, and macrophages), and synovial fibroblast
proliferation (8–10). The latter are regarded as effector
cells responsible for cartilage and bone destruction in RA. Then,
RA leads to deformity and evenutally to significant functional
decline (11,12).
Following in-depth study of the pathogenesis of RA,
most scholars agree that the abnormalities of autoreactive T cells
induced by antigen-presenting cells (APCs) are the central link in
the pathogenesis of RA (13–15). DCs are the most powerful APCs and play
an important role in the abnormal activation of T cells and the
occurrence and development of RA. DCs present a two-way immune
regulation: i) Ability to capture, process, and present exogenous
antigens leading to T-cell differentiation and immune response; and
ⅱ) ability of DC to trigger an effective T-cell response and
production of self- and foreign antigens. Thus, DCs are important
in sustaining the central and peripheral immune tolerance (16–19).
Recent studies found that TLRs are crucial cellular
sentinels used to detect ‘danger’ signals released in DCs and may
be important initiators of DC maturation and activation. TRAF6 is a
key to TLR activation and intersecting signaling pathways activated
the NF-κB and mitogen-activated protein kinase pathways. TRAF6
assists in the maturity of DC-mediated signal transduction and is a
key to DC formation, activation, and maturation (20–22). Thus,
to explore specific interventions of TRAF6 expression is essential,
which inhibits DC maturation, reconstructs RA immune tolerance, and
plays a therapeutic role (23,24).
We found that DCs derived from mouse bone marrow and
different methods stimulated differentiation and maturation of DCs.
Expression of co-stimulatory molecules (i.e., CD80 and CD86)
increased in the mature DC, enhanced the ability of antigen
presentation, and decreased antigen uptake. The highest maturity
degree of DCs is CD86 expression, while in the experiments IL-12 is
produced mainly by DCs, macrophages, and B lymphocytes. The
increased secretion of IL-12 into the supernatants was altered with
the increase of maturity degree, which is consistent with previous
literature (25,26). The present findings have shown that the
TRAF6 gene and protein expression were correlated with DC
maturity, which showed an increasing trend. Among the most obvious
was the cocktail method. Some studies found that under appropriate
stimulation conditions, DCs with a TRAF6 gene defect failed
to upregulate co-stimulatory molecules (i.e., CD80 and CD86), MHC,
and release of pro-inflammatory cytokines and did not activate T
cell-mediated immune responses (27).
Those results are consistent with our findings, which show that
TRAF6 plays an important role in DC differentiation and
maturation.
Previous results showed that no or a low expression
of co-stimulatory molecules was detected in immature DCs by
mediating immune clearance, immune incompetent, and Treg cell
induction. Thus, the iDCs induced peripheral immune tolerance,
maintained immune balance, and inhibited autoimmunity disease. The
study found that there was an increasing trend for DCs in different
maturation states with increasing TRAF6 expression Thus, we
inhibited the expression of TRAF6 in DCs, which reduced the
Toll-TRAF6 NF-κB signaling pathway, maintained DCs in an immature
state, and decreased the release of pro-inflammatory cytokines
(i.e., IL-1, IL-6, IL-12, and TNF-α). It is expected that the
immune tolerance may be re-established through the intervention of
TRAF6 expression. This study has clearly shown that TRAF6 regulates
the differentiation and maturation of DCs in animal experiments.
Thus, TRAF6 should be evaluated as a potential target for clinical
therapy.
Acknowledgements
Te present study was supported by grants from the
Chinese National Natural Science Foundation (no. 81360462) and the
Guangxi Natural Science Foundation (no. 2013GXNSFAA019111).
References
|
1
|
Church LD, Filer AD, Hidalgo E, Howlett
KA, Thomas AM, Rapecki S, Scheel-Toellner D, Buckley CD and Raza K:
Rheumatoid synovial fluid interleukin-17-producing CD4 T cells have
abundant tumor necrosis factor-alpha co-expression, but little
interleukin-22 and interleukin-23R expression. Arthritis Res Ther.
12:R1842010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xq E, Meng HX, Cao Y, Zhang SQ, Bi ZG and
Yamakawa M: Distribution of regulatory T cells and interaction with
dendritic cells in the synovium of rheumatoid arthritis. Scand J
Rheumatol. 41:413–420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee HW, Kim TS, Kang YJ, Kim JY, Lee S,
Lee WJ and Sohn Y: Up-regulated S100 calcium binding protein A8 in
Plasmodium-infected patients correlates with
CD4+CD25+Foxp3 regulatory T cell generation.
Malar J. 14:3852015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferraccioli G and Zizzo G: The potential
role of Th17 in mediating the transition from acute to chronic
autoimmune inflammation: Rheumatoid arthritis as a model. Discov
Med. 11:413–424. 2011.PubMed/NCBI
|
|
5
|
Guo M, James AW, Kwak JH, Shen J, Yokoyama
KK, Ting K, Soo CB and Chiu RH: Cyclophilin A (CypA) plays dual
roles in regulation of bone anabolism and resorption. Sci Rep.
6:223782016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hontelez S, Ansems M, Karthaus N,
Zuidscherwoude M, Looman MW, Triantis V and Adema GJ: Dendritic
cell-specific transcript: Dendritic cell marker and regulator of
TLR-induced cytokine production. J Immunol. 189:138–145. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu M, Mo H, Li D, Luo X and Zhang L:
Th17/Treg imbalanceinduced by increased incidence of
atherosclerosis in patients with Systemic Lupus Erythematosus
(SLE). Clinical Rheumatology. 32:1045–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Xu Y, Shen J, He F, Zhang D, Chen
Z, Duan Y and Sun J: Feasibility study of DCs/CIKs combined with
thoracic radiotherapy for patients with locally advanced or
metastatic non-small-cell lung cancer. Radiat Oncol. 11:602016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salmon H, Idoyaga J, Rahman A, Leboeuf M,
Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J,
Tung N, et al: Expansion and activation of CD103(+) dendritic cell
progenitors at the tumor site enhances tumor responses to
therapeutic PD-L1 and BRAF inhibition. Immunity. 44:924–938. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kao JY, Zhang M, Miller MJ, Mills JC, Wang
B, Liu M, Eaton KA, Zou W, Berndt BE, Cole TS, et al: Helicobacter
pylori immune escape is mediated by dendritic cell-induced Treg
skewing and Th17 suppression in mice. Gastroenterology.
138:1046–1054. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jalil SF, Arshad M, Bhatti A, Ahmad J,
Akbar F, Ali S and John P: Rheumatoid arthritis: What have we
learned about the causing factors? Pak J Pharm Sci. 29:629–645.
2016.PubMed/NCBI
|
|
12
|
Wehmeyer C, Frank S, Beckmann D, Böttcher
M, Cromme C, König U, Fennen M, Held A, Paruzel P, Hartmann C, et
al: Sclerostin inhibition promotes TNF-dependent inflammatory joint
destruction. Sci Transl Med. 8:330ra35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simmons DP, Wearsch PA, Canaday DH,
Meyerson HJ, Liu YC, Wang Y, Boom WH and Harding CV: Type I IFN
drives a distinctive dendritic cell maturation phenotype that
allows continued class II MHC synthesis and antigen processing. J
Immunol. 188:3116–3126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Popa C, van Lieshout AW, Roelofs MF,
Geurts-Moespot A, van Riel PL, Calandra T, Sweep FC and Radstake
TR: MIF production by dendritic cells is differentially regulated
by Toll-like receptors and increased during rheumatoid arthritis.
Cytokine. 36:51–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sabatté J, Maggini J, Nahmod K, Amaral MM,
Martínez D, Salamone G, Ceballos A, Giordano M, Vermeulen M and
Geffner J: Interplay of pathogens, cytokines and other stress
signals in the regulation of dendritic cell function. Cytokine
Growth Factor Rev. 18:5–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Snir O, Rieck M, Gebe JA, Yue BB, Rawlings
CA, Nepom G, Malmström V and Buckner JH: Identification and
functional characterization of T cells reactive to citrullinated
vimentin in HLA-DRB1*0401-positive humanized mice and rheumatoid
arthritis patients. Arthritis Rheum. 63:2873–2883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu H, Chen J, Song S, Yuan P, Liu L, Zhang
Y, Zhou A, Chang Y, Zhang L and Wei W: β2-adrenoceptor signaling
reduction in dendritic cells is involved in the inflammatory
response in adjuvant-induced arthritic rats. Sci Rep. 6:245482016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tucci M, Stucci S, Savonarola A,
Ciavarella S, Cafforio P, Dammacco F and Silvestris F: Immature
dendritic cells in multiple myeloma are prone to osteoclast-like
differentiation through interleukin-17A stimulation. Br J Haematol.
161:821–831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaisho T, Hoshino K, Iwabe T, Takeuchi O,
Yasui T and Akira S: Endotoxin can induce MyD88-deficient dendritic
cells to support T(h)2 cell differentiation. Int Immunol.
14:695–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamaki Y, Takakubo Y, Hirayama T,
Konttinen YT, Goodman SB, Yamakawa M and Takagi M: Expression of
Toll-like receptors and their signaling pathways in rheumatoid
synovitis. J Rheumatol. 38:810–820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kobayashi T, Walsh PT, Walsh MC, Speirs
KM, Chiffoleau E, King CG, Hancock WW, Caamano JH, Hunter CA, Scott
P, et al: TRAF6 is a critical factor for dendritic cell maturation
and development. Immunity. 19:353–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, Wang J, Fang Y, Gong S, Li M, Wu M,
Lai X, Zeng G, Wang Y, Yang K, et al: MicroRNA-146a promotes
mycobacterial survival in macrophages through suppressing nitric
oxide production. Sci Rep. 6:233512016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Varney ME, Niederkorn M, Konno H,
Matsumura T, Gohda J, Yoshida N, Akiyama T, Christie S, Fang J,
Miller D, et al: Loss of Tifab, a del(5q) MDS gene, alters
hematopoiesis through derepression of Toll-like receptor-TRAF6
signaling. J Exp Med. 212:1967–1985. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Chen W, Wang L, Li F, Zhang C and
Xu L: Tumor necrosis factor receptor-associated factor 6 promotes
migration of rheumatoid arthritis fibroblast-like synoviocytes. Mol
Med Rep. 11:2761–2766. 2015.PubMed/NCBI
|
|
25
|
Arellano-Orden E, Calero-Acuña C,
Moreno-Mata N, Gómez-Izquierdo L, Sánchez-López V, López-Ramírez C,
Tobar D, López-Villalobos JL, Gutiérrez C, Blanco-Orozco A, et al:
Cigarette smoke decreases the maturation of lung myeloid dendritic
cells. PLoS One. 11:e01527372016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colletti NJ, Liu H, Gower AC, Alekseyev
YO, Arendt CW and Shaw MH: TLR3 signaling promotes the induction of
unique human BDCA-3 dendritic cell populations. Front Immunol.
7:882016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walsh MC, Lee JE and Choi Y: Tumor
necrosis factor receptorassociated factor 6 (TRAF6) regulation of
development, function, and homeostasis of the immune system.
Immunological Reviews. 266:72–92. 2015. View Article : Google Scholar : PubMed/NCBI
|