Introduction
Widely used in the clinic, hyperbaric oxygen (HBO)
treatment has be shown to be an effective adjunct to management of
brain injury (1), diabetic ulcers
(2) and chronic wounds (3). However, the roles of HBO as an adjunctive
therapy for tumors remains controversial. Some previous studies
suggest that HBO therapy may improve postoperative outcome and
outcome of radiotherapy and chemotherapy, especially proving
beneficial for the treatment of radiotherapy (4,5). A
combinatorial approach using chemotherapeutic drugs with HBO may
enhance sensitivity of tumor cells to chemotherapeutic agents
(6,7) and
the application of HBO in chemotherapy for malignant lymphoma,
brain tumor, lung cancer, gastric cancer and breast cancer may
increase chemotherapeutic efficacy while decreasing treatment
related toxicity (8). However, HBO
treatment alone may stimulate the proliferation of tumor tissues
(9). Other studies indicate that
cancer cells in hypoxic conditions are more likely to metastasize
and thus be more deadly (10–12). Meanwhile, hypoxic cells undergo a high
rate of mutation to become a treatment-resistant genotype, and HBO
treatment may improve it (13). The
current study is to explore the effects of hyperbaric oxygen
treatment alone on proliferation, autophagy and oxidative stress
response of gastric cancer SGC7901 cells.
Materials and methods
Cell culture
Gastric cancer SGC7901 cell lines were purchased
from the American Type Culture Collection (Manassas, VA, USA),
inoculated in Dulbecco's modified Eagle's (high glucose) culture
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 10% fetal bovine serum (Clark Bioscience, Richmond, VA, USA)
and cultured in a 5% CO2 incubator at 37°C.
HBO treatment
Prior to the experiment, the hyperbaric chamber was
disinfected using UV techniques for 20 min, and cleaned using pure
oxygen for 10 min. The cells were placed flat in the chamber under
aseptic conditions for 90 min each time. The pressure of HBO was
increased slowly to 0.2 MPa within 15 min and, after 60 min, the
pressure was decreased to normal pressure within 15 min. Then, the
cells were taken out of the chamber and cultured in the
CO2 incubator. During the experiment, the HBO chamber
was kept ventilated with 95% oxygen at a flow rate of 2 l/min.
Cell grouping
The cells were split into two groups. HBO group:
Cells at log phase were subjected to HBO treatment once a day.
Control group: SGC7901 cells at a log phase of their growth were
cultured in the CO2 incubator without HBO treatment.
When cells of HBO group were receiving HBO treatment, this group of
cells were removed from the incubator and maintained at room
temperature.
SGC7901 cell proliferation measured
using an MTT assay following HBO treatment
Following trypsinization, cells at log phase were
resuspended and seeded in a 96-well cell culture plate at a density
of 5×103 cells/well. Following treating the cells with
HBO, 20 µl MTT (5 mg/ml, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) solution was added into each well after 48 h. The cells
were cultured for a further 4 h in the incubator. Then, the
supernatant was abandoned and 100 µl DMSO (Sigma-Aldrich; Merck
KGaA) was added to each well. Following oscillation in a constant
temperature culture vibration machine at 37°C for 10 min until the
crystal was completely dissolved, the absorbance value (A value) at
the wavelength of 490 nm was measured at a enzyme-linked
immunosorbent assay reader (ELX800; BioTek Instruments, Inc.,
Winooski, VT, USA). The experiments were performed in triplicate
and results are represented as the average value of three
independent experiments.
Expression levels of cell autophagy
and of oxidative stress associated proteins measured by western
blot analysis
Following trypsinization, cells at log phase were
resuspended and seeded in a 6-well cell culture plate at a density
of 3×105 cells per well. At 48 h following HBO
treatment, radioimmunoprecipitation assay buffer (25 mM HEPES, 1.5%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 0.5 M NaCl, 5 mM
EDTA, 50 mM NaF, 0.1 mM sodium vanadate, 1 mM phenylmethylsulfonyl
fluoride and 0.1 g/l leupeptin, pH 7.8) was used for cell lysis and
total protein extraction. The quantitative determination of total
protein concentration of each group was made by the bicinchoninic
acid assay method (cat. no. P0009; Beyotime, Institute of
Biotechnology, Haimen, China). The same amount of protein (50 µg)
of each group was loaded onto 12.5% SDS-PAGE gels. Electrophoresis
separated proteins were then transferred onto polyvinylidene
difluoride membranes. Following blocking with 5% skimmed milk,
membranes were incubated overnight with the primary antibody
against LC3-phosphatidylethanolamine conjugate (dilution, 1:500;
cat. no. sc-134226; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), binding immunoglobulin protein (BiP; dilution, 1:400; cat.
no. sc-1051; Santa Cruz Biotechnology, Inc.) or
CCAAT-enhancer-binding protein homologous protein (CHOP; dilution,
1:500; cat. no. sc-7351; Santa Cruz Biotechnology, Inc.) at 4°C.
Membranes were then washed at room temperature and incubated for 2
h with IgG-horseradish peroxidase conjugate (dilution, 1:10,000;
goat anti-mouse IgG; cat. no. AP124P), goat anti-rabbit IgG
(dilution, 1:10,000; cat. no. AP132P) and rabbit anti-goat IgG
(dilution, 1:10,000; cat. no. AP106P) all obtained from EMD
Millipore (Billerica, MA, USA). Following another washing step (any
unbound secondary antibody was removed by washing), membranes were
treated with ECL visualization reagents (Thermo Fisher Scientific,
Inc.) in the dark room. β-actin was used as the internal control in
the experiment. Protein band intensities were determined by using
the Quantity One software (version, 4.6.2; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The experiment was repeated three times
and results were averaged. The ratio of the target protein band
intensity to that of the internal control in each group was also
calculated.
Statistical analysis
All statistical analysis was calculated using SPSS
16.0 statistical analysis software (SPSS, Inc., Chicago, IL, USA).
The difference between control group and treatment group was
evaluated using Student's t-test. P<0.05 was considered to be
statistically significant.
Results
Effects of HBO treatment on SGC7901
cell proliferation
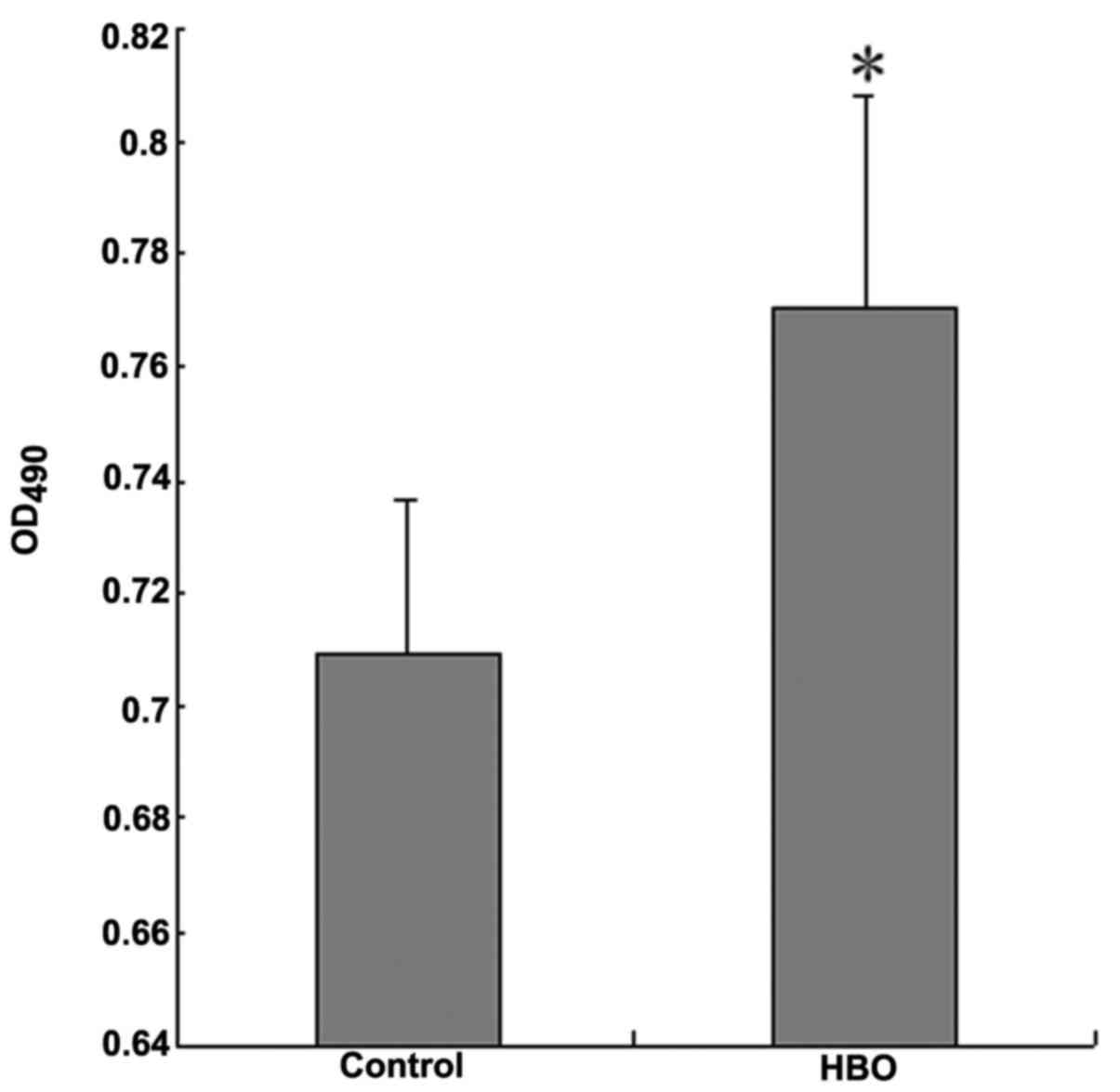
An MTT assay was used for measuring cell
proliferation following HBO treatment. The result indicated that
the OD490 value of SGC7901 cells following HBO treatment
was significantly higher than that of control group with
statistical difference (Fig. 1;
P<0.05), which suggested that HBO treatment may promote the
proliferation of gastric cancer SGC7901 cells.
Effects of HBO treatment on autophagy
of SGC7901 cells
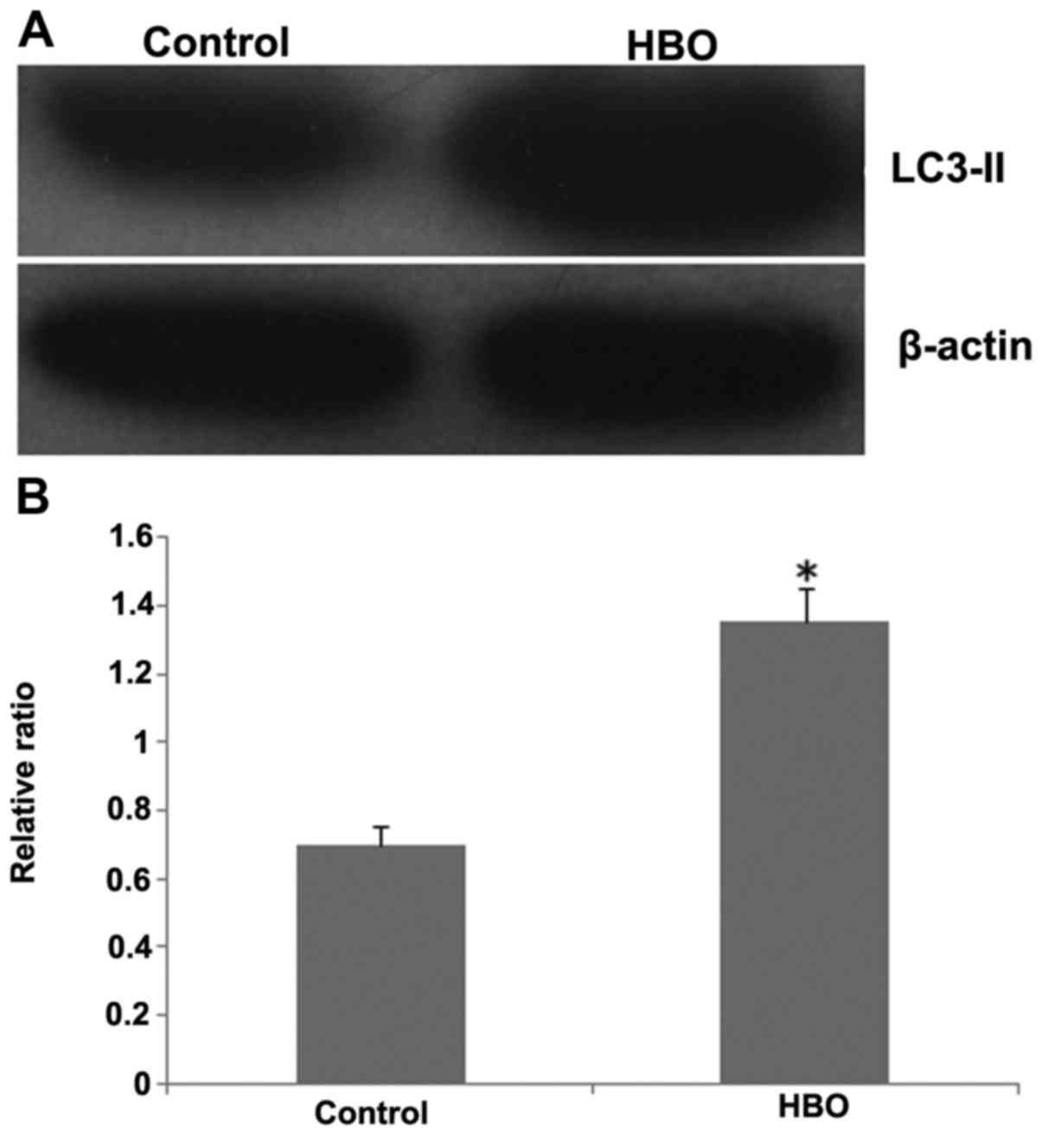
As presented in Fig. 2,
the expression level of autophagosome marker protein LC3-II
following HBO treatment was significantly increased when compared
with the control group (Fig. 2B;
P<0.05). The result demonstrated that HBO treatment may
significantly stimulate the autophagy of gastric cancer cells
SGC7901.
Effects of HBO treatment on the
expression levels of oxidative stress-associated proteins, BiP and
CHOP
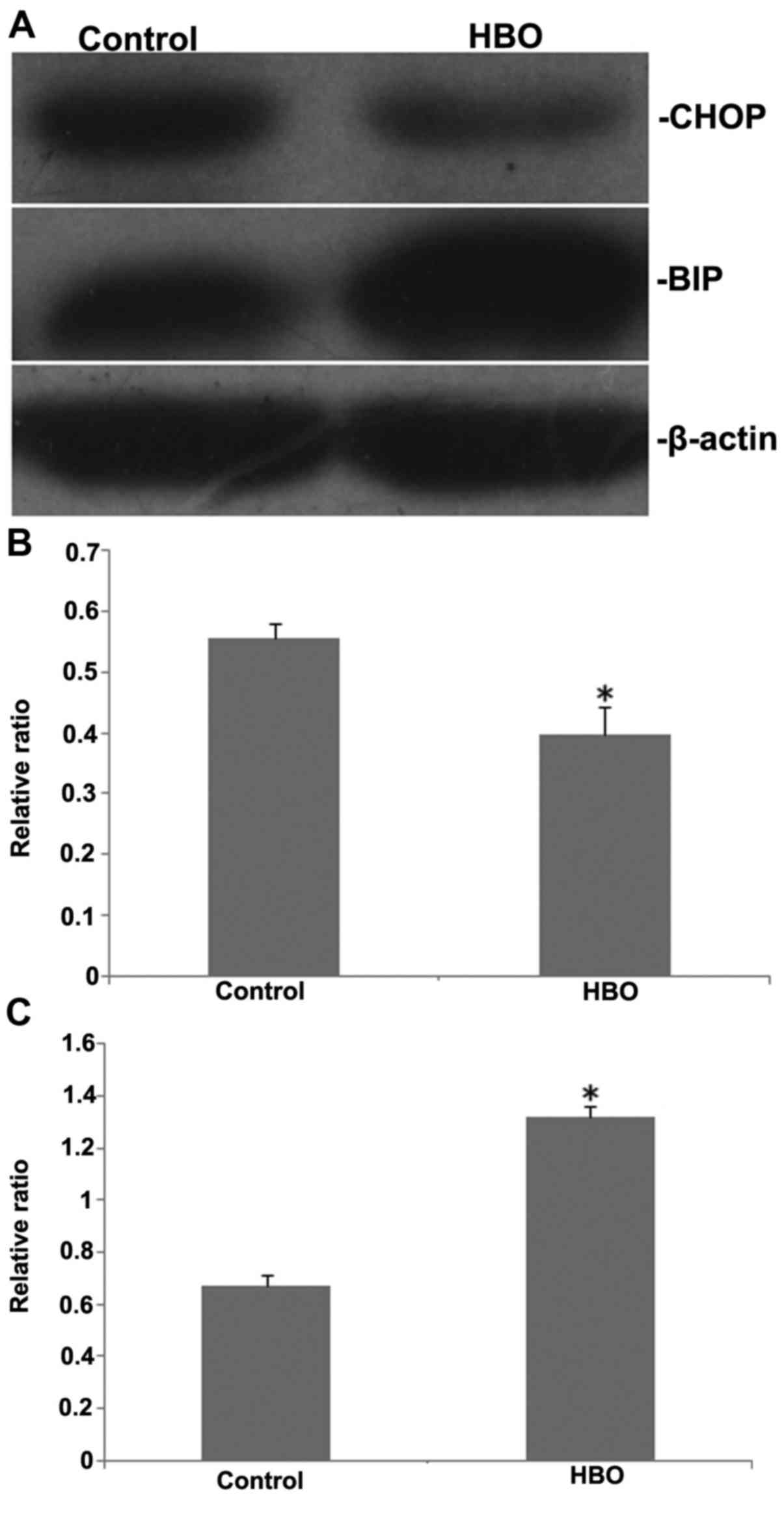
Western blot analysis was used to evaluate the
expression levels of oxidative stress-associated proteins BiP and
CHOP following HBO treatment. The results are presented in Fig. 3. The expression level of BiP was
significantly increased when compared with the control group
(P<0.05). Conversely, the level of CHOP was significantly
decreased (P<0.05). These data suggested that HBO treatment may
promote SGC7901 cell survival and inhibit cell apoptosis caused by
oxidative stress.
Discussion
HBO therapy provides 100% oxygen in a chamber with
increased pressure. This treatment provides extra oxygen to support
the growth of new blood vessels at the hypoperfusion area and is
beneficial to help treat conditions such as wounds, carbon monoxide
poisoning and soft tissue infections (14). Typically, tumor cells are less
well-oxygenated than normal tissues, and tumor hypoxia often leads
to rapid tumor growth as well as resistance to radiotherapy and
anticancer chemotherapy (15).
Therefore, HBO may theoretically be used as an effective therapy
for tumor treatment by reversing tissue hypoxia (16), as tumor cells can be stimulated
resulting in an increased sensitivity to chemo- and radiotherapy,
and the effect of chemo- and radiotherapy can be enhanced by HBO
(17–20). However, the effect of hyperbaric oxygen
treatment alone on tumor treatment remains controversial (21). Some studies suggested that HBO may
possess tumor-inhibitory effects (22–25), while
others indicated that HBO treatment alone may stimulate tumor
growth and metastasize (8,26,27).
In addition to the mitochondrial apoptosis pathway,
previous findings have demonstrated that autophagy and endoplasmic
reticulum (ER) stress can be also involved in apoptosis (28,29). As a
highly conserved self-digestion process, autophagy serves as a
‘battery’ to promote cell survival in response to nutrient
starvation, hypoxia and other metabolic stresses until the stress
subsides. However, excessive or sustained autophagy has the
potential to induce cell death (autophagic cell death), which makes
autophagy a double-edged sword that could be either protective or
detrimental to cells (30–32). LC3 can be used as a reliable
autophagosome marker for monitoring autophagy. During autophagy, a
cytosolic form of LC3 (LC3-I) is conjugated to
phosphatidylethanolamine to form LC3-phosphatidylethanolamine
conjugate (LC3-II), which is recruited to autophagosomal membranes
(33). ER stress-induced apoptosis can
be mediated by the expression/activation of apoptosis-related
molecules (such as CHOP and caspase-12) or pro-survival molecules
(such as glutamate decarboxylase and BiP) (34,35). ER
stress and autophagy can both be seen as compensatory roles
important for tumor cells under chronic metabolic stress to survive
in the harsh environment and there are findings that certain
anticancer drugs can induce autophagy and ER stress at the same
time (36,37).
The present study sought to explore the effects of
HBO treatment alone on proliferation, autophagy and ER stress of
gastric cancer cell line SGC7901. The results indicated that,
following HBO treatment, the increase in SGC7901 cell proliferation
was significant compared with that in the control group, and in
addition, there was a significant increase level in autophagosome
marker LC3-II, as well as prosurvival molecule BiP level. However,
there was a significant decrease in the levels of apoptosis-related
molecule, CHOP. Changes in expression levels of CHOP and BiP
suggest cells adaptation to stress conditions following HBO
treatment. These factors have indicated that HBO treatment may
induce both autophagy and ER stress, and promote cell survival by
regulating cell autophagy. Due to vigorous cell proliferation
situations, it can be concluded that HBO treatment alone in
vitro may promote tumor cell proliferation and enhance cell
survival. However, it does not mean that tumor growth and
metastasize would be stimulated if tumor patients receive HBO
treatment alone, as cultured tumor cells in vitro are not
deprived of oxygen, this is different from the hypoxic conditions
in vivo. Therefore, the fact of tumor cell proliferation and
inhibition of apoptosis in vitro after HBO does not suggest
the same results on tumor patients who receive HBO treatment. For
further research in the future, the authors intend to mimic the
in vivo hypoxic conditions of tumor cells in vitro
and investigate hypoxic cell survival effect following HBO
treatment to further evaluate the promotion or inhibition effect of
HBO treatment on gastric cancer and provide experimental evidence
for the clinical treatment of gastric cancer.
Acknowledgements
The present study was supported by the Fund for
Young Talents in College of Anhui Province (grant no. 2012SQRL067),
the National Natural Science Foundation of China (grant nos.
81201907 and 81272399) and the Research Fund for Doctor in Anhui
Medical University (grant no. XJ201229).
References
|
1
|
Sahni T, Jain M, Prasad R, Sogani SK and
Singh VP: Use of hyperbaric oxygen in traumatic brain injury:
Retrospective analysis of data of 20 patients treated at a tertiary
care centre. Br J Neurosurg. 26:202–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gottrup F and Apelqvist J: Present and new
techniques and devices in the treatment of DFU: A critical review
of evidence. Diabetes Metab Res Rev. 28:64–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kranke P, Bennett MH, Martyn-St James M,
Schnabel A and Debus SE: Hyperbaric oxygen therapy for chronic
wounds. Cochrane Database Syst Rev. 18:CD0041232012.
|
|
4
|
Valadão J, Pearl J, Verma S, Helms A and
Whelan H: Hyperbaric oxygen treatment for post-radiation central
nervous system injury: A retrospective case series. Undersea Hyperb
Med. 41:87–96. 2014.PubMed/NCBI
|
|
5
|
Hoggan BL and Cameron AL: Systematic
review of hyperbaric oxygen therapy for the treatment of
non-neurological soft tissue radiation-related injuries. Support
Care Cancer. 22:1715–1726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng HS, Liao MB, Zhang MY, Xie Y, Xu L,
Zhang YJ, Zheng XF, Wang HY and Chen YF: Synergistic inhibitory
effect of hyperbaric oxygen combined with sorafenib on hepatoma
cells. PLoS One. 9:e1008142014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gendimenico GJ and Haugaard N: Adverse
effects of hyperbaric oxygen on [3H]uridine incorporation and
uridine kinase activity in B104 rat neuroblastoma cells. Mol Cell
Biochem. 95:71–76. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moen I and Stuhr LE: Hyperbaric oxygen
therapy and cancer-a review. Target Oncol. 7:233–242. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paniello RC, Fraley PL and O'Bert R:
Effect of hyperbaric oxygen therapy on a murine squamous cell
carcinoma model. Head Neck. 6:1743–1746. 2014. View Article : Google Scholar
|
|
10
|
He C, Wang L, Zhang J and Xu H:
Hypoxia-inducible microRNA-224 promotes the cell growth, migration
and invasion by directly targeting RASSF8 in gastric cancer. Mol
Cancer. 16:352017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang P, Wan WW, Xiong SL, Feng H and Wu N:
Cancer stem-like cells can be induced through dedifferentiation
under hypoxic conditions in glioma, hepatoma and lung cancer. Cell
Death Discov. 3:161052017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenza GL: Oxygen sensing,
hypoxia-inducible factors, and disease pathophysiology. Annu Rev
Pathol. 9:47–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeClerck K and Elble RC: The role of
hypoxia and acidosis in promoting metastasis and resistance to
chemotherapy. Front Biosci (Landmark Ed). 15:213–225. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carney AY: Hyperbaric oxygen therapy: An
introduction. Crit Care Nurs Q. 36:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ackerman D and Simon MC: Hypoxia, lipids,
and cancer: Surviving the harsh tumor microenvironment. Trends Cell
Biol. 24:472–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiche J, Ilc K, Laferrière J, Trottier E,
Dayan F, Mazure NM, Brahimi-Horn MC and Pouysségur J:
Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell
growth by counteracting acidosis through the regulation of the
intracellular pH. Cancer Res. 69:358–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogawa K, Kohshi K, Ishiuchi S, Matsushita
M, Yoshimi N and Murayama S: Old but new methods in radiation
oncology: Hyperbaric oxygen therapy. Int J Clin Oncol. 18:364–370.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moen I, Tronstad KJ, Kolmannskog O,
Salvesen GS, Reed RK and Stuhr LE: Hyperoxia increases the uptake
of 5-fluorouracil in mammary tumors independently of changes in
interstitial fluid pressure and tumor stroma. BMC Cancer.
9:4462009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu XY, Cao K, Li QY, Yuan ZC and Lu PS:
The synergistic therapeutic effect of temozolomide and hyperbaric
oxygen on glioma U251 cell lines is accompanied by alterations in
vascular endothelial growth factor and multidrug
resistance-associated protein-1 levels. J Int Med Res. 40:995–1004.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng ZR, Zhong WH, Liu J and Xiao PT:
Effects of the combination of hyperbaric oxygen and 5-fluorouracil
on proliferation and metastasis ofhuman nasopharyngeal carcinoma
CNE-2Z cells. Undersea Hyperb Med. 37:141–150. 2010.PubMed/NCBI
|
|
21
|
Wenwu L, Xuejun S, Hengyi T and Kan L:
Hyperbaric oxygen and cancer: More complex than we expected. Target
Oncol. 8:79–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Granowitz EV, Tonomura N, Benson RM, Katz
DM, Band V, Makari-Judson GP and Osborne BA: Hyperbaric oxygen
inhibits benign and malignant human mammary epithelial cell
proliferation. Anticancer Res. 25:3833–3842. 2005.PubMed/NCBI
|
|
23
|
Chen YC, Chen SY, Ho PS, Lin CH, Cheng YY,
Wang JK and Sytwu HK: Apoptosis of T-leukemia and B-myeloma cancer
cells induced by hyperbaric oxygen increased phosphorylation of p38
MAPK. Leuk Res. 31:805–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raa A, Stansberg C, Steen VM, Bjerkvig R,
Reed RK and Stuhr LE: Hyperoxia retards growth and induces
apoptosis and loss of glands and blood vessels in DMBA-induced rat
mammary tumors. BMC Cancer. 7:232007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stuhr LE, Raa A, Oyan AM, Kalland KH,
Sakariassen PO, Petersen K, Bjerkvig R and Reed RK: Hyperoxia
retards growth and induces apoptosis, changes in vascular density
and gene expression in transplanted gliomas in nude rats. J
Neurooncol. 85:191–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding JB, Chen JR, Xu HZ and Qin ZY: Effect
of hyperbaric oxygen on the growth of intracranial glioma in rats.
Chin Med J (Engl). 128:3197–3203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moen I, Øyan AM, Kalland KH, Tronstad KJ,
Akslen LA, Chekenya M, Sakariassen PØ, Reed RK and Stuhr LE:
Hyperoxic treatment induces mesenchymal-to-epithelial transition in
a rat adenocarcinoma model. PLoS One. 4:e63812009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cerella C, Teiten MH, Radogna F, Dicato M
and Diederich M: From nature to bedside: Pro-survival and cell
death mechanisms as therapeutic targets in cancer treatment.
Biotechnol Adv. 32:1111–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Logue SE, Cleary P, Saveljeva S and Samali
A: New directions in ER stress-induced cell death. Apoptosis.
18:537–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dalby KN, Tekedereli I, Lopez-Berestein G
and Ozpolat B: Targeting the prodeath and prosurvival functions of
autophagy as novel therapeutic strategies in cancer. Autophagy.
6:322–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roy S and Debnath J: Autophagy and
tumorigenesis. Semin Immunopathol. 32:383–396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levine B and Klionsky DJ: Development by
serf-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 4:463–477. 2004. View Article : Google Scholar
|
|
33
|
Mizushima N: Methods for monitoring
autophagy. Int J Biochem Cell Biol. 36:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schönthal AH: Pharmacological targeting of
endoplasmic reticulum stress signaling in cancer. Biochem
Pharmacol. 85:653–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harding HP, Novoa I, Zhang Y, Zeng H, Wek
R, Schapira M and Ron D: Regulated translation initiation controls
stress induced gene expression in mammalian cells. Mol Cell.
6:1099–1108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moenner M, Pluquet O, Bouchecareilh M and
Chevet E: Integrated endoplasmic reticulum stress responses in
cancer. Cancer Res. 67:10631–10634. 2007. View Article : Google Scholar : PubMed/NCBI
|