Introduction
Cancer is one of the leading causes of mortality
worldwide (1,2). Despite numerous cancer studies and the
development of various anti-cancer therapeutic agents, cancer
remains dangerous. Anti-cancer therapeutic agents are chemically or
biologically produced, and their effects are well defined (3–7). However,
treatments continue to be associated with adverse effects and the
majority of patients have an aversion to them (8).
Herbal products have long been used to prevent or
treat diseases, including cancer (9–12).
Furthermore, certain anti-cancer therapeutic agents that are
chemically produced originate from herbal products and their
chemical characteristics are modified (7,12–14). Typically, patients prefer to take
herbal products (15–18); herbal products have historically been
used as traditional medicines, such as traditional Chinese and
Korean medicines, Kampo medicines and Ayurvedic medicine (13,14,19). Certain herbal products were
demonstrated to treat cancer and/or reduce the side effects of
cancer treatment (13,15–17,19–21).
Therefore, herbal products are considered to be promising for
cancer prevention and treatment.
Barley grass extract (Hordeum vulgare L.;
Bex) has long been used as a food product. Its biological effects
have also been addressed by various in vitro and in
vivo studies, although evidence there is limited evidence of
the efficacy of Bex against specific conditions (22). The effect of Bex on the immune system
was revealed in in vitro and in vivo experimental
sets (23–25). Accordingly, Bex inhibited atopic
dermatitis in NC/Nga mice by altering the expression levels of
cytokines (26). Similarly, Bex
repressed lipopolysaccharide-induced inflammation (27). Furthermore, its effect in type 2
diabetes was revealed in a genetically engineered mouse model and
patients (28,29). Therefore, the effects of Bex on
particular diseases have been demonstrated at least in experimental
systems. A previous study revealed that Bex caused apoptosis of
leukemia and lymphoma cell lines (30); however, its effect in cancer remains
unclear.
The present study examined the effect of Bex in
different cancer cell lines, including breast cancer MDA-MB-231
cells and prostate cancer DU145 cells. Bex induced apoptotic cell
death in MDA-MB-231 and DU145 cells. Furthermore, its effect
resulted from an increased intracellular reactive oxygen species
(ROS) level. Thus, the current study suggests that Bex may be
useful for treating cancer, particularly breast and prostate
cancer.
Materials and methods
Cell culture and herbal extract
MDA-MB-231 and DU-145 cells (American Type Culture
Collection, Manassas, VA, USA) were cultured in Dulbecco's modified
Eagle's medium with 10% fetal bovine serum and 1%
penicillin-streptomycin (all Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Barley grass extract (Bex) was obtained from
Chungbuk Agricultural Cooperation (Jecheon, South Korea). Bex was
solubilized in water containing 0.01% dimethyl sulfoxide (DMSO).
Therefore, all control groups in the experiment were treated with
0.01% DMSO.
Cell viability assay
Cell viability was examined using an EZ-CYTOX cell
viability/cytotoxicity assay kit (cat. no. EZ-000, Daeil Lab
Service, Seoul, South Korea) according to the manufacturer's
instructions. Briefly, 100,000 cells per well were cultured in
96-well plates and treated with different doses (0, 0.01, 0.1, 1,
10, 100, 250 and 500 µg/ml) of Bex for 72 h. Cell viability at 24,
48 and 72 h was measured using a microplate reader at a wavelength
of 450 nm. Experiments were performed in triplicate and repeated
three times independently.
Apoptosis assay
Cells (3×106) were treated with different
concentrations (0, 0.01, 0.1, 1, 10, 100, 250 and 500 µg/ml) of Bex
for 24 h and stained with Annexin V-fluorescein isothiocyanate
(FITC) and 7-aminoactinomycin D (7-AAD). Apoptotic cell death was
determined using BD FACSCalibur flow cytometry with BD MultiSET
software (BD Biosciences, San Jose, CA, USA). For western blot
analysis, 1×106 cells were treated with 100 µg/ml Bex
for 24 h and lysed with RIPA buffer. Protein (30 µg) was loaded
onto SDS-PAGE and transferred to the membrane. After blocking with
5% milk, the membrane was incubated with an appropriate primary
antibody for 1 h at room temperature. Anti-poly(ADP-ribose)
polymerases (PARP) (cat. no. 9542), anti-cleaved caspase 8 (cat.
no. 9496), anti-cleaved caspase 9 (cat. no. 7237), anti-cleaved
caspase-3 (cat. no. 9664) and anti-β-tubulin (cat. no. 2146)
antibodies were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Intracellular ROS detection assay
ROS levels were measured using 10 µM
2′,7′-dichlorofluorescin diacetate (H2DCF-DA; Molecular Probes;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells
(1×106) were treated with 100 µg/ml Bex for 5 min and
treated with H2DCF-DA for a further 24 h. The flow cytometry
experiments were conducted in triplicate and repeated three times
independently. Sigma-Aldrich N-acetyl-L-cystein (NAC; Merck
KGaA, Darmstadt, Germany) at 10 mM was used to inhibit ROS
induction. Cells (1×106) were pretreated with 10 mM NAC
for 1 h before being treated with 100 µg/ml Bex for 24 h.
Statistical analysis
All experiments were performed in triplicate and
repeated three times independently. Statistical significance was
evaluated using Student's t-test and analysis was conducted using
SPSS version 24.0 software (IBM Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Bex inhibits cancer cell
viability
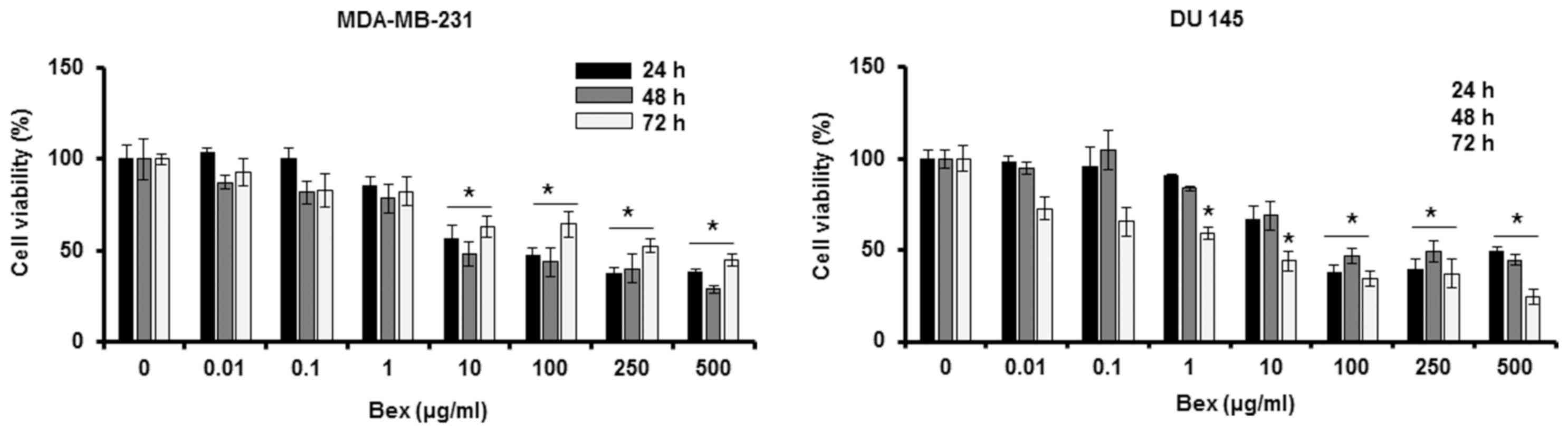
To examine the effect of Bex on cancer cell
viability, MDA-MB-231 breast cancer cells and DU-145 prostate
cancer cells were treated with different concentrations (0, 0.01,
0.1, 1, 10, 100, 250 and 500 µg/ml) of Bex for 24, 48 and 72 h. Bex
reduced the viability of those cancer cells in a dose-dependent
manner (Fig. 1). Thus, the MTT assay
data indicates that Bex inhibits cancer cell viability.
Bex causes apoptosis of cancer
cells
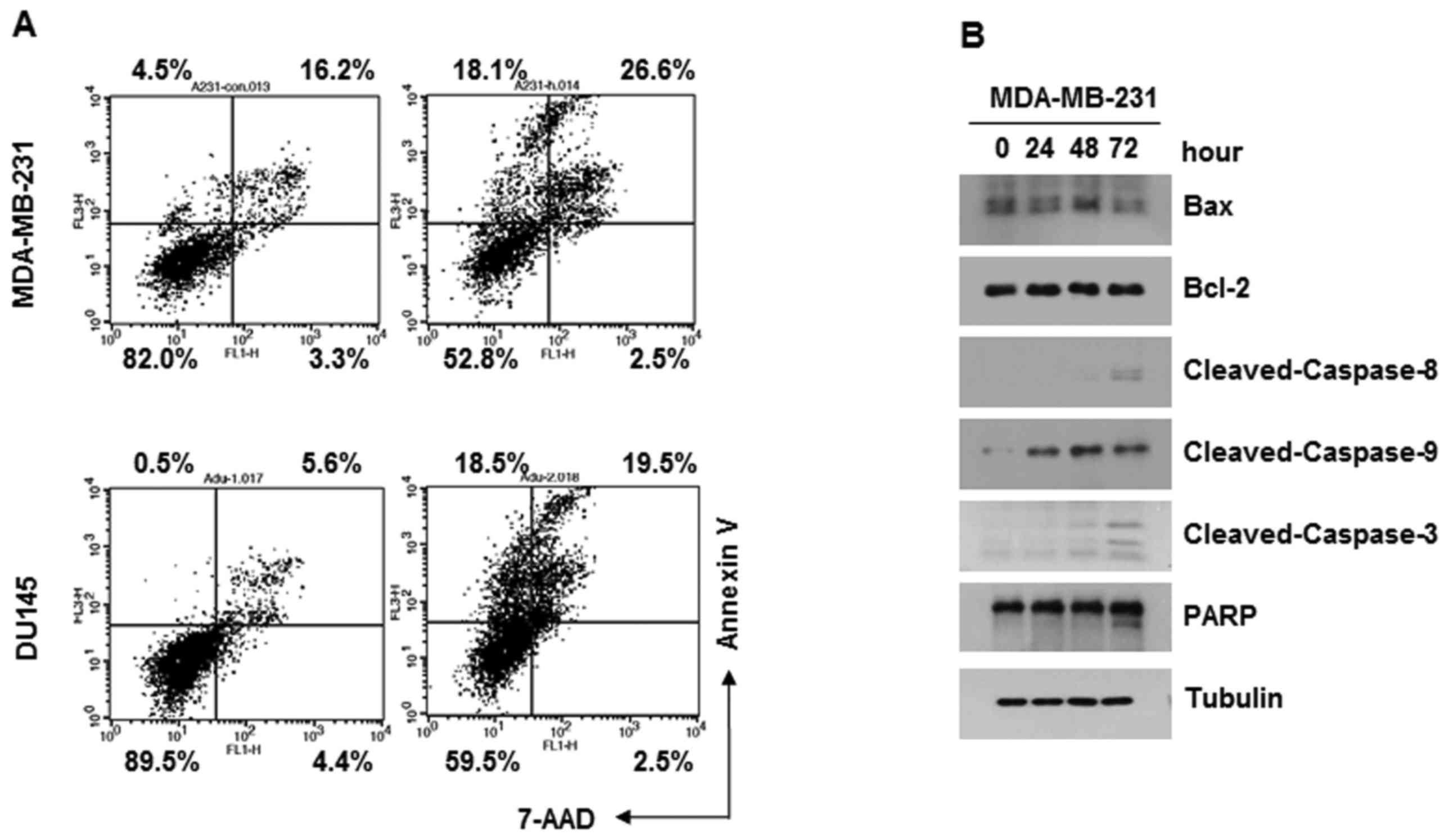
Annexin V assays were performed to examine whether
Bex induces apoptosis of cancer cells. MDA-MB-231 or DU-145 cells
were treated with 100 µg/ml Bex for 24 h, followed by Annexin
V-FITC and 7-AAD. Flow cytometry data indicated that Bex induced
apoptosis of the two types of cancer cell (Fig. 2A). Consistently, Bex induced PARP
cleavage and caspase activation in the MDA-MB-231 cells (Fig. 2B; data for DU-145 not shown). Thus, the
current data indicates that Bex causes apoptosis of cancer
cells.
Bex reduces the level of intracellular
ROS
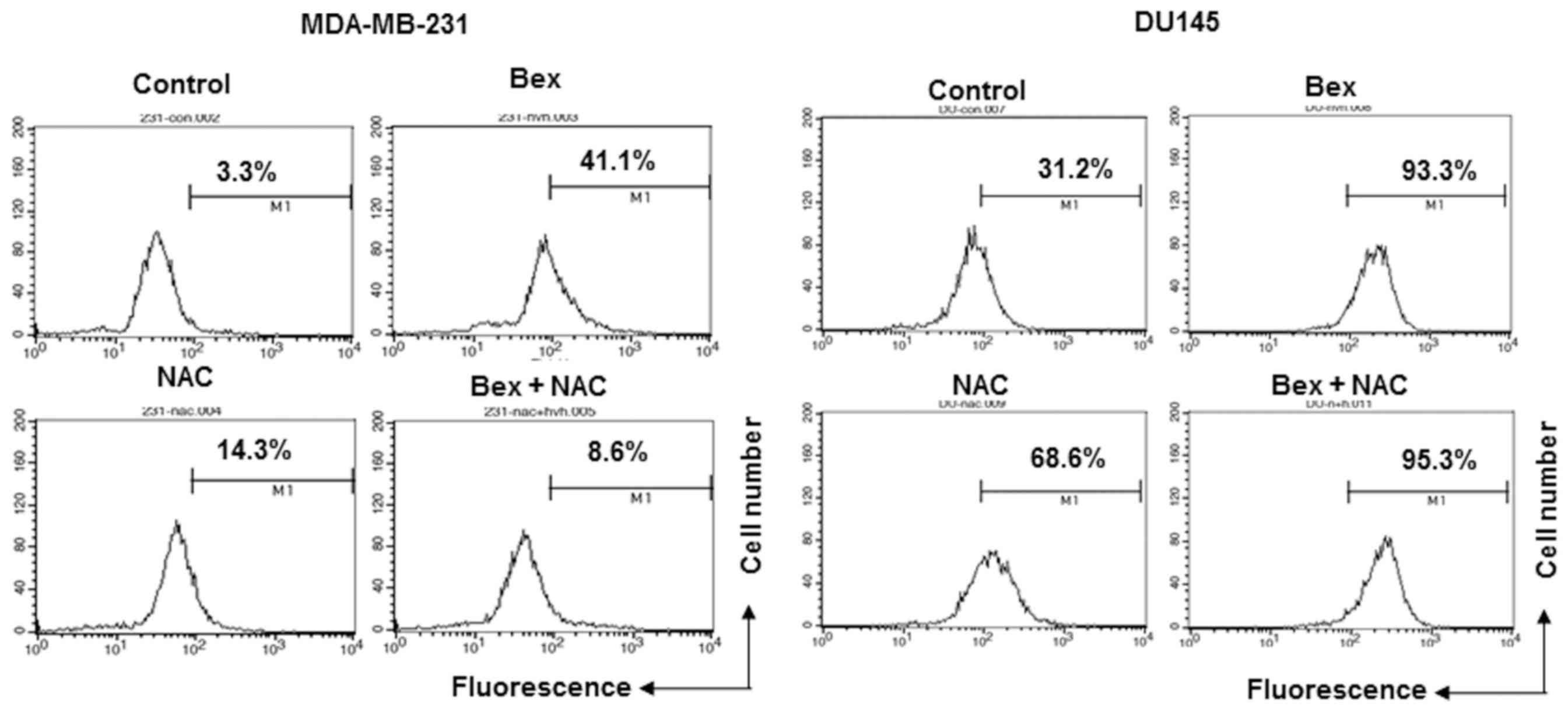
To examine whether Bex affects the production of
intracellular ROS, MDA-MB-231 and DU-145 cells were treated with
100 µg/ml Bex for 1 h, and the intracellular ROS level was measured
by detecting H2DCF-DA fluorescence using flow cytometry. Bex
increased the intracellular ROS level in MDA-MB-231 breast and
DU-145 prostate cancer cells (Fig.
3).
Bex-mediated increase of intracellular
ROS level is critical for apoptosis
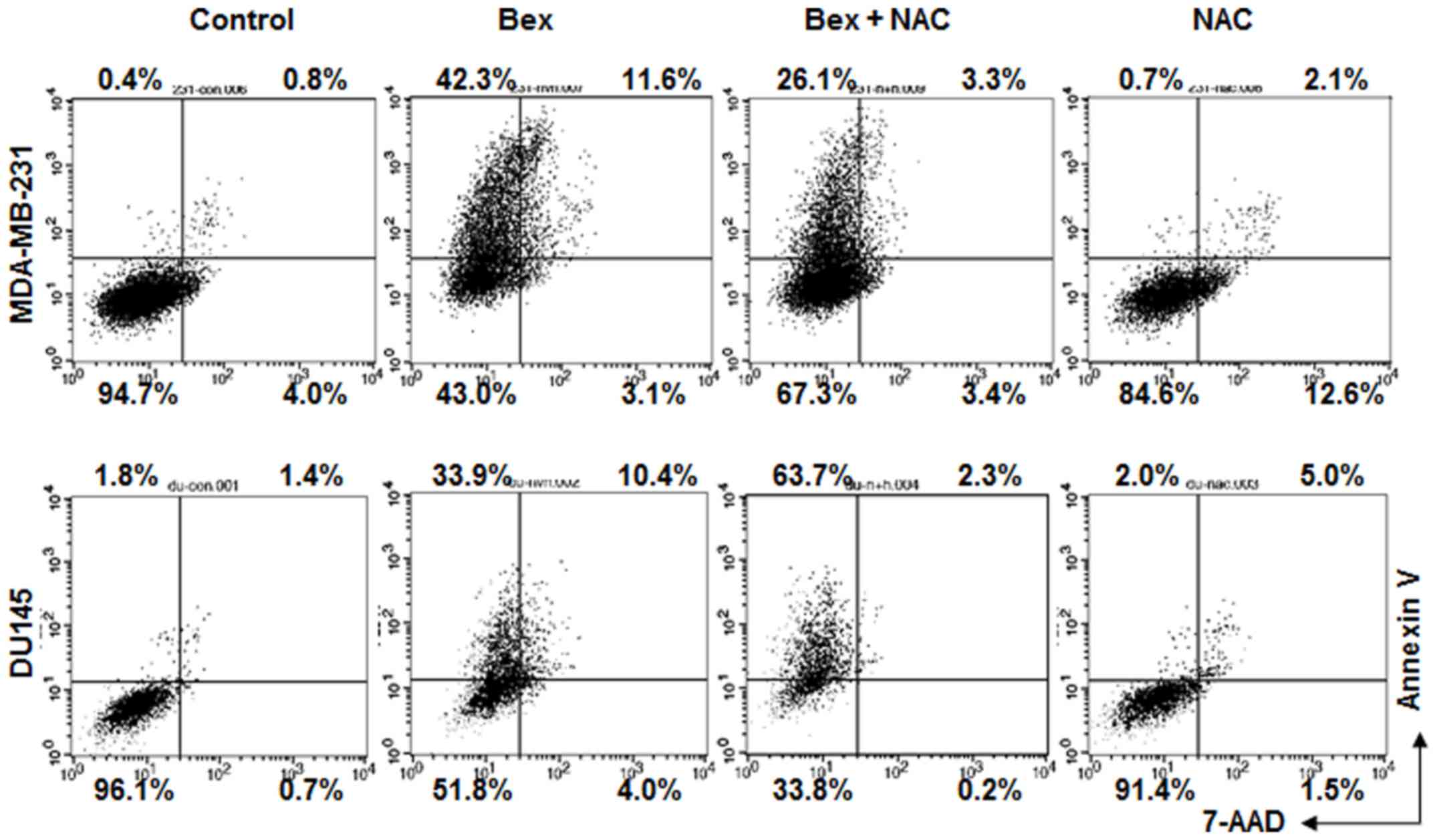
Whether Bex-induced apoptosis required an increase
of intracellular ROS level was subsequently examined. Bex-induced
apoptosis was blocked in cells treated with NAC (Fig. 4). The data indicate that Bex-induced
ROS accumulation is important for apoptosis.
Discussion
Bex has long been incorporated into diets for
disease prevention. However, to the best of our knowledge, its
effects in cancer are yet to be investigated. In the present study,
Bex caused apoptosis of breast and prostate cancer cells by
increasing the intracellular ROS level. The present data indicate
that cancer could be, in part, treated using natural products in
food. Bex is widely used in food. Therefore, the present study
demonstrates that foods containing Bex may be useful for cancer
treatment during therapeutic interventions.
A recent study demonstrated that Bex induced
apoptosis of leukemia and lymphoma cell lines (30). While not shown in the present study,
the data demonstrated no apoptotic effect of Bex in Jurkat T cells
(data not shown). It is possible that the experimental conditions,
such as the extraction method and concentration, may have
influenced the controversial results. In the present study, Bex
induced apoptotic cell death of highly metastatic MDA-MB-231 breast
cancer cells and DU-145 prostate cancer cells. Thus, the
anti-cancer effect of Bex is not limited to blood cancer. This is
consistent with results obtained using Bex-treated B16 melanoma
cells or HepG2 hepatoma cells (31,32). Bex is
one of the ingredients in cereal and the anti-cancer effect of
peptides from cereal has previously been demonstrated (33). Furthermore, meta-analyses indicated
that cereal reduces cancer risk (34,35). Thus,
the present study provides evidence that dietary components are
beneficial for cancer prevention and treatment.
In conclusion, Bex induction of ROS was crucial for
apoptotic cell death. While the chemical components to produce ROS
and induce apoptotic cell death in those breast and prostate cancer
cells require further investigation, this is the first study, to
the best of our knowledge, that shows the role of Bex in cancer
cell death.
Acknowledgements
The present study was supported by the Korean
National University of Transportation 2016.
References
|
1
|
de Martel C, Ferlay J, Franceschi S,
Vignat J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: A review and synthetic
analysis. Lancet Oncol. 13:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hare JI, Lammers T, Ashford MB, Puri S,
Storm G and Barry ST: Challenges and strategies in anti-cancer
nanomedicine development: An industry perspective. Adv Drug Deliv
Rev. 1:25–38. 2017. View Article : Google Scholar
|
|
4
|
Dawidczyk CM, Kim C, Park JH, Russell LM,
Lee KH, Pomper MG and Searson PC: State-of-the-art in design rules
for drug delivery platforms: Lessons learned from FDA-approved
nanomedicines. J Control Release. 187:133–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellou S, Pentheroudakis G, Murphy C and
Fotsis T: Anti-angiogenesis in cancer therapy: Hercules and hydra.
Cancer Lett. 338:219–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ali I, Wani WA, Saleem K and Haque A:
Platinum compounds: A hope for future cancer chemotherapy.
Anticancer Agents Med Chem. 13:296–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fauzee NJ: Taxanes: Promising anti-cancer
drugs. Asian Pac J Cancer Prev. 12:837–851. 2011.PubMed/NCBI
|
|
8
|
Vera-Badillo FE, Al-Mubarak M, Templeton
AJ and Amir E: Benefit and harms of new anti-cancer drugs. Curr
Oncol Rep. 15:270–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghorbani A: Clinical and experimental
studies on polyherbal formulations for diabetes: Current status and
future prospective. J Integr Med. 12:336–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang M, Yang J, Zhang C, Liu B, Chan K,
Cao H and Lu A: Clinical studies with traditional Chinese medicine
in the past decade and future research and development. Planta Med.
76:2048–2064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ilyas U, Katare DP, Aeri V and Naseef PP:
A review on hepatoprotective and immunomodulatory herbal plants.
Pharmacogn Rev. 10:66–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bahmani M, Shirzad H, Shahinfard N,
Sheivandi L and Rafieian-Kopaei M: Cancer phytotherapy: Recent
views on the role of antioxidant and angiogenesis activities. J
Evid Based Complementary Altern Med. 2156587215625157. 2016.
|
|
13
|
Xu H, Zhao X, Liu X, Xu P, Zhang K and Lin
X: Antitumor effects of traditional Chinese medicine targeting the
cellular apoptotic pathway. Drug Des Devel Ther. 9:2735–2744.
2015.PubMed/NCBI
|
|
14
|
Ichikawa H, Nakamura Y, Kashiwada Y and
Aggarwal BB: Anticancer drugs designed by mother nature: Ancient
drugs but modern targets. Curr Pharm Des. 13:3400–3416. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poonthananiwatkul B, Howard RL, Williamson
EM and Lim RH: Cancer patients taking herbal medicines: A review of
clinical purposes, associated factors, and perceptions of benefit
or harm. J Ethnopharmacol. 175:58–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JW, Lee WB, Kim W, Min BI, Lee H and
Cho SH: Traditional herbal medicine for cancer pain: A systematic
review and meta-analysis. Complement Ther Med. 23:265–274. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leggett S, Koczwara B and Miller M: The
impact of complementary and alternative medicines on cancer
symptoms, treatment side effects, quality of life, and survival in
women with breast cancer - a systematic review. Nutr Cancer.
67:373–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao Y, Kong X, Yang L, Liu R, Shi Z, Li W,
Hua B and Hou W: Complementary and alternative medicine for cancer
pain: An overview of systematic reviews. eCAM.
2014.170396https://doi.org/10.1155/2014/170396PubMed/NCBI
|
|
19
|
Wang CY, Bai XY and Wang CH: Traditional
Chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohnishi S and Takeda H: Herbal medicines
for the treatment of cancer chemotherapy-induced side effects.
Front Pharmacol. 6:142015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park B, Jun JH, Jung J, You S and Lee MS:
Herbal medicines for cancer cachexia: Protocol for a systematic
review. BMJ Open. 4:e0050162014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lahouar L, El-Bok S and Achour L:
Therapeutic potential of young green barley leaves in prevention
and treatment of chronic diseases: An overview. Am J Chin Med.
43:1311–1329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyazaki Y, Tokunaga Y, Takagaki K,
Tsusaki S, Tachibana H and Yamada K: Effect of dietary cabbage
fermentation extract and young barley leaf powder on immune
function of Sprague-Dawley rats. J Nutr Sci Vitaminol (Tokyo).
47:253–257. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cremer L, Herold A, Avram D and Szegli G:
Inhibitory capacity of some fractions isolated from a green barley
extract upon TNF alpha production by the cells of the THP-1 human
monocytes line. Roum Arch Microbiol Immunol. 55:285–294.
1996.PubMed/NCBI
|
|
25
|
Cremer L, Herold A, Avram D and Szegli G:
A purified green barley extract with modulatory properties upon TNF
alpha and ROS released by human specialised cells isolated from RA
patients. Roum Arch Microbiol Immunol. 57:231–242. 1998.PubMed/NCBI
|
|
26
|
Iguchi T, Kawata A, Watanabe T, Mazumder
TK and Tanabe S: Fermented barley extract suppresses the
development of atopic dermatitis-like skin lesions in NC/Nga mice,
probably by inhibiting inflammatory cytokines. Biosci Biotechnol
Biochem. 73:489–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi KC, Hwang JM, Bang SJ, Son YO, Kim
BT, Kim DH, Lee SA, Chae M, Kim DH and Lee JC: Methanol extract of
the aerial parts of barley (Hordeum vulgare) suppresses
lipopolysaccharide-induced inflammatory responses in vitro and in
vivo. Pharm Biol. 51:1066–1076. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong H and Jai Maeng W: Effects of malted
barley extract and banaba extract on blood glucose levels in
genetically diabetic mice. J Med Food. 7:487–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu YM, Chang WC, Chang CT, Hsieh CL and
Tsai CE: Effects of young barley leaf extract and antioxidative
vitamins on LDL oxidation and free radical scavenging activities in
type 2 diabetes. Diabetes Metab. 28:107–114. 2002.PubMed/NCBI
|
|
30
|
Robles-Escajeda E, Lerma D, Nyakeriga AM,
Ross JA, Kirken RA, Aguilera RJ and Varela-Ramirez A: Searching in
mother nature for anti-cancer activity: Anti-proliferative and
pro-apoptotic effect elicited by green barley on leukemia/lymphoma
cells. PLoS One. 8:e735082013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghavami L, Goliaei B, Taghizadeh B and
Nikoofar A: Effects of barley β-glucan on radiation damage in the
human hepatoma cell line HepG2. Mutat Res Genet Toxicol Environ
Mutagen. 775:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng TX, Irino N and Kondo R: Melanin
biosynthesis inhibitory activity of a compound isolated from young
green barley (Hordeum vulgare L.) in B16 melanoma cells. J Nat Med.
69:427–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ortiz-Martinez M, Winkler R and
García-Lara S: Preventive and therapeutic potential of peptides
from cereals against cancer. J Proteomics. 111:165–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lei Q, Zheng H, Bi J, Wang X, Jiang T, Gao
X, Tian F, Xu M, Wu C, Zhang L, et al: Whole grain intake reduces
pancreatic cancer risk: A meta-analysis of observational studies.
Medicine (Baltimore). 95:e27472016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aune D, Keum N, Giovannucci E, Fadnes LT,
Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E and Norat
T: Whole grain consumption and risk of cardiovascular disease,
cancer, and all cause and cause specific mortality: Systematic
review and dose-response meta-analysis of prospective studies. BMJ.
353:i27162016. View Article : Google Scholar : PubMed/NCBI
|