Introductio
The skin forms a protective barrier between the
internal organs and the environment by preventing invasion of
pathogens, and fending off chemicals and physical assaults, as well
as preventing the unregulated loss of water and solutes (1,2). Skin is
arranged into three layers, including the epidermis, dermis and
hypodermis (from top to bottom). Cutaneous blood vessels and nerve
endings are presented in the dermis and hypodermis, while the
epidermis is avascular with no neural tissue. Therefore, the
cell-to-cell communications mediated by GJs provide a crucial
mechanism for the integrity of the epidermal barrier and dermal
supports (3).
GJ channels formed from Cxs are the predominant
intracellular connections that regulate cell permeability and
polarity. To date, 21 Cxs have been identified in humans and 20 Cxs
in mice (4,5). Cxs are named according to their
respective molecular weight, such as Cx26, Cx31, Cx43 and Cx57. The
structure differences between them lie in the cytoplasmic loop and
carboxyl (C) terminal region. The Cx subunit contains four
hydrophobic transmembrane domains, comprising two extracellular
loops, one cytoplasmic loop and one cytoplasmic N-terminal, as well
as a C-terminal region. When functioning, six Cx subunits form a
hemichannel in the plasma membrane that dock to another hemichannel
in the plasma membrane of an adjacent cell to assemble a complete
GJ channel (4). These channels allow
the exchange and diffusion of various compounds up to a molecular
mass of 1,000 Da, for example metabolites, ions, second messengers,
water and electrical impulses (6,7). The
half-life of Cxs is relatively short, ranging from 1.5 to 5 h in
the majority of tissue and cell types (8,9). However,
the mechanism of the balance between Cx synthesis and degradation
remains elusive. Abnormal Cx expression has been reported to be
associated with dysregulated cell proliferation, migration and
wound healing rates (10).
There are 10 Cxs in human skin, including Cx43,
Cx45, Cx40, Cx31, Cx26, Cx32, Cx30, Cx30.3, Cx41.8 and Cx39.3. Cxs
display distinct function in the epidermis and dermis with
overlapping expression (11). Among
these 10 Cxs, Cx43 is the most ubiquitously expressed in the skin.
It is produced by various different types of skin cell, such as
keratinocytes, fibroblasts, endothelial and basal cells,
melanocytes and dermal papilla cells (11,12).
Regulation of Cx43
Cx43 is encoded by gap junction α 1 gene (GJA1;
MIMno. 121014). The normal expression (10), proper location (13) and accurate connection with other Cxs
(14,15)
are crucial for its function.
Cx43 expression is regulated at the transcriptional
and post-transcriptional levels. One activator protein-1 (AP-1) and
two Sp1 transcription factor (SP1) binding sites exist in the
5′-flanking promoter of Cx43 (16).
Activated protein kinase C (PKC) and estrogen induce Cx43
transcription through AP-1 and SP1 sites (16,17). Wnt
signaling and protease-activated receptor-1 (PAR-1) also increase
the transcription level of Cx43 (18).
Post-transcriptional regulation of Cx43 predominantly relies on its
C-terminal region, which contains multiple phosphorylation sites
and acts as the functional domain of Cx43. Tumor growth promoting
factors, protein kinase and inflammatory mediators, such as PKC
(19), mitogen activated protein
kinase (20), Src kinase (21), casein kinase 1 (22) and PKA (23), may modulate phosphorylation of Cx43
through serine/tyrosine residues at the C-terminus and subsequently
regulate subcellular localization of Cx43 and GJ formation.
However, the effect of phosphorylation of Cx43 on GJ intracellular
communication remains uncertain. The ubiquitin (24) and small ubiquitin-related modifier
(25) system are important in
post-transcriptional regulation of Cx43 GJs. In addition to
post-transcriptional modifications, Cx43 interacts with a range of
cytoskeleton proteins, including zonula occludens-1 (ZO-1), ZO-2,
and α- and β-tubulin, to regulate cell adhesion and migration
(26–28).
Implications of Cx43 in skin system
Increasing evidence indicates that Cx43 directly
affects the proliferation and migration of keratinocytes and
fibroblasts (29). Cx43 also
participates in wound healing (30),
hyperkeratosis (31) and tumor
development of skin (32,33).
Cx43 in cutaneous wound healing. Normal skin
regenerates after wounding or damaging. Wound healing is a
complicated physiological process in which different types of cell,
containing various growth factors and chemokines, are involved
(34,35). There are four steps in the wound
healing process: Hemostasis, inflammation, migration and
proliferation, and remodeling (34,36). During
wound healing, the expression of Cx43 varies and influences cell
behaviors.
Numerous factors may regulate the expression levels
of Cx43 during repair processes (Fig.
1). Subsequent to wounding, a high concentration of cyclic
adenosine monophosphate at the wound site induces a reduction in
Cx43 expression levels and other junction proteins at the plasma
membrane, which subsequently destructs the cell junction and causes
cytoskeleton remodeling (30). The
activated AKT phosphorylates Cx43 at S373 and limits its
interaction with ZO-1, potentially leading to activation and
migration of keratinocytes in the skin (37).
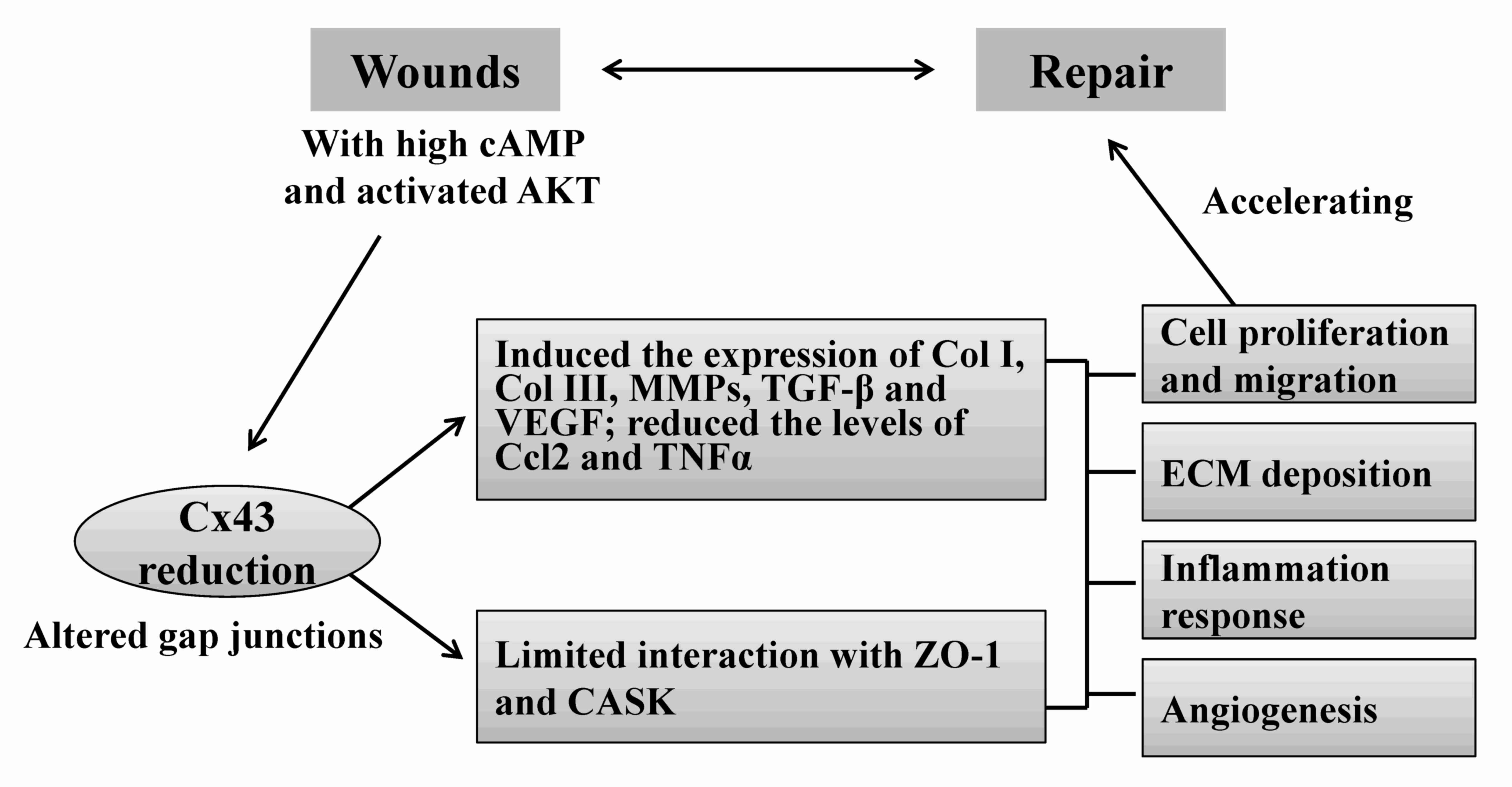 | Figure 1.Schematic of the regulation and
function of Cx43 in acute skin injury. Elevated cAMP and pAKT at
wound edges alter the expression and phosphorylation levels of
Cx43. By mediating the upregulation of Col I, Col III, MMP2, TGF-β1
and VEGF, and the downregulation of Ccl2 and TNFα, Cx43
participates in the important wound healing processes, including
ECM remodeling, epidermal/dermal cell proliferation and migration,
inflammation response and angiogenesis. In addition,
dysphosphorylation of Cx43 affects its interaction with other
proteins, such as CASK and ZO-1, and subsequently modulates cell
permeability and migration. Overall, Cx43 integrates multiple
pathogenic signals to regulate wound healing. Cx43, connexin 43;
cAMP, cyclic adenosine monophosphate; pAKT, phosphorylated AKT;
Col, collagen; MMP2, matrix metalloproteinase-2; TGF-1,
transforming growth factor; VEGF, vascular endothelial growth
factor; Ccl2, chemokine (C-C motif) ligand 2; TNFα, tumor necrosis
factor; ECM, extracellular matrix; CASK,
calcium/calmodulin-dependent serine protein kinase; ZO-1, zonula
occludens-1. |
The expression level of Cx43 changes dynamically
during the wound healing process. On days 1 and 2 subsequent to
injury, the mRNA and protein expression levels of Cx43 were
significantly decreased at the wound edge, and readjusted to normal
levels (like those in non-injured skin) from day 3 onwards in mice
(10). By contrast, the level of Cx43
expression remains at a low level throughout the entire healing
process in humans (29). Cx43
reduction has been shown to be associated with: i) Remodeling of
the extracellular matrix (ECM) (38);
ii) proliferation and migration of keratinocytes and fibroblasts
(10,39,40); and
iii) regulation of inflammatory responses through certain
cytokines, chemokines or growth factors (38,40).
Transient knockdown or artificial deficiency of Cx43 induces the
proliferation and migration of keratinocytes and dermal
fibroblasts, and enhances ECM production by upregulating collagen
type I, collagen type III, matrix metalloproteinase-2 and
transforming growth factor (TGF)-β1 (38). Additionally, Cx43 knockdown may
decrease the expression levels of chemokine (C-C motif) ligand 2
and tumor necrosis factor (TNF)α, and elevate the expression levels
of TGF-β1 and collagen α1, which affects the extravasations of
neutrophils and macrophages involved in the wound healing process
(40). Furthermore, the angiogenic
potential of endothelial cells would be impaired following Cx43
knockdown (41,42). Although the role of Cx43 reduction in
wound healing has been widely accepted, the underlying mechanism
and signaling pathway involved in its function require further
investigation to provide a solid theoretical basis for its clinical
application.
Cx43 is crucial in chronic wound healing. Chronic
wounds, such as diabetic foot ulcers, pressure ulcers, and venous
leg ulcers are an increasing issue worldwide (43). Diabetic ulcers, the most common
diabetic complication, represent a major concern for patients and
doctors with regard to quality of life and economics (44). Clinical and experimental evidence
suggests that chronic wounds do not follow an orderly progression
of wound healing (35). In the case of
diabetic ulcers, abnormal expression of Cx43 was reported (45). As mentioned above, the Cx43 expression
level is decreased at the wound borders during acute injury of
normal human skin. By contrast, its expression level elevates by
~10-fold in human chronic diabetic foot ulcers at the wound edge
(46). It was reported that the high
glucose level of diabetic cells induces Cx43 expression, and
subsequently represses filopodial extensions and fibroblast
migration rates (46). However, Vinnik
et al (47) observed a minor
increase of Cx43 expression at the wound borders in patients with
diabetes mellitus type II. In the study, the Cx43 expression levels
at the wound edge increased by ~1.9 times following ozone therapy;
however, the action mechanism of Cx43 in this case remains to be
elucidated (47). A finding by
Mendoza-Naranjo et al (48)
that is consistent with the above-mentioned observations
demonstrated an increased expression level of Cx43 in venous leg
ulcers, and increased healing rates following Cx43 shRNA treatment
(48).
The role of Cx43 is not simply to form GJ channels,
but also to stabilize a series of proteins, such as N-cadherin and
ZO-1, which are required for cell-to-cell adhesion and cell
migration (45,46,48), which
further illustrates that the sustained inhibition of Cx43 may be
efficient for rapid or chronic wound healing.
Cx43 in keratoderma. Cx43 is tightly associated with
keratinocyte behaviors. The human Cx43 gene, or GJA1, is located at
human chromosome 6q22-q23 within the candidate region for the
oculodentodigital dysplasia (ODDD) locus. A Cx43 mutation directly
causes the pleiotropic phenotype of ODDD (49). It is proposed that the gene mutations
(c.412G>A/p.Gly138Ser) (50) and
deletions (dinucleotide deletion 780_781delTG) (51) causing truncation of the Cx43 C-terminus
are necessary and sufficient for palmoplantar keratosis (PPK)
development in ODDD patients. More recently, other mutations of
Cx43 have been observed in various types of rare, inherited skin
disorder characterized by keratoderma or hypokeratosis with other
severe symptoms, including a heterozygous mutation (c.23G>T
[p.Gly8Val]) of GJA1 in a family with
keratoderma-hypotrichosis-leukonychia totalis syndrome (31) and de novo missense mutations (A44V and
E227D) of GJA1 in erythrokeratodermia variabilis et progressiva
(52). Mechanistically, GJA1 mutations
lead to disruption of Cx43 membrane localization and aggregation in
the Golgi, resulting in excessive opening of hemichannels and
cytoplasmic Ca2+ overload, and subsequent keratinocyte
apoptosis and hyperkeratosis.
In addition to the above-mentioned mutations, Cx43
participates in epidermal keratinization by interacting with other
members of the Cx family. For example, Cx26 mutations, G12R/N14Y or
H73R/S183F, directly caused keratitis-ichthyosis-deafness and PPK
syndrome, respectively. During these syndromes, Cx26 mutants may
interact with Cx43 more efficiently and exacerbate Cx43 hemichannel
activity, thus increasing cell membrane permeability and resulting
in ATP release and Ca2+ overload (14,15). These
studies further demonstrate the important role of Cx43 in genetic
skin disorders.
Cx43 in melanoma and non-melanoma skin cancer. Cx43
is closely associated with tumor initiation and development. In
skin cancer, Cx43 is overexpressed in malignant melanomas when
compared with the normal and benign nevi (32,33). The
upregulation of Cx43 was associated with an enhanced cell adhesion
and invasion of malignant tumor cells (53,54). By
acting as a downstream effecter of PAR-1, Cx43 also mediates
melanoma metastasis and intracellular communication between the
tumor microenvironment and the metastatic tumor cells (46). The roles of Cx43 in melanoma implicate
it as an oncogene; however, various independent groups obtained
opposing results. Schiffner et al (55) identified Cx43 as a downstream target of
nuclear RNA-binding protein p54nrb. Cx43 knockdown
mediated by p54nrb promotes cell proliferation and
migration in human melanoma cell lines. In mouse melanoma cell
lines, Cx43 reduction induced the expression of vascular
endothelial growth factor and tumor angiogenesis (56). Similarly, in human melanoma cell lines,
Cx43 overexpression reduced melanoma growth and metastasis, and
increased TNFα-induced cell apoptosis (57). These controversial results may be due
to differences between cell lines, experimental conditions and test
points in the studies.
Basal cell carcinoma (BCC) and squamous cell
carcinoma (SCC) are the major subtypes of non-melanoma skin cancer
(58). Stelkovics et al
(59) observed a higher expression
level of Cx43 in BCC than that in SCC, indicating that Cx43
prevented metastatic invasion of BCC (59). In a previous study, immunofluorescence
and immunoelectron microscopy were used, and the expression level
of Cx43 was relatively low in the basal layer of human normal skin;
but Cx43 was not detectable in BCC or SCC, which consequently led
to a small number of GJs in BCC and SCC (60). However, the underlying mechanisms of
Cx43 function in melanoma and non-melanoma skin cancers remain
poorly understood and require further study and discussion.
Cx43 in skin development. Cx43 contributes to
epidermal and follicular morphogenesis. During human fetal
epidermal development, Cx43 is expressed at the later stages (88
days) of estimated gestational age (61). In rat and mouse skin, Cx43 is expressed
in all of the epidermal layers in the early phase of development
(62,63), and is detected in hair follicles and
the arrector pili muscles (12,64). The
role and underlying mechanism of Cx43 in epidermal development
remains largely unknown. During mouse ovarian folliculogenesis,
Cx43-mediated GJs are required for coupling between granulose cells
and continued follicular growth (65).
In addition, Cx43 evolves in skin cell differentiation. Dyce et
al (66) compared stem cells from
the skin of wild-type and Cx43 knockout newborn mice. The authors
found reduced cell migration rates and decreased expression levels
of the pluripotency markers, octamer-binding transcription factor 4
and Nanog in Cx43-deficient stem cells, indicating the role of Cx43
in maintaining the multipotency of skin stem cells (66). The results are partially consistent
with a previous study, in which the expression level of Cx43 was
maintained at a higher level in an undifferentiated epidermis and
markedly decreased in the differentiation of the epidermis
(12).
Therapeutic modalities based on regulating Cx43
activity. Increasing evidence has identified a positive correlation
between Cx43 inhibition and wound healing rates (67,68).
Furthermore, Cx43 reduction relieves inflammation in chronic wounds
(69). Currently, drug design and
development based on Cx43 is an important research area. There are
two methods for artificially downregulating Cx43, which involve
antisense oligodeoxyribonucleotides (ODNs) and mimetic peptides
(Table I). Antisense ODNs may have
accelerated wound healing and reduced scar formation in normal
mouse skin and diabetic rat skin (40,45,70). Cx43 mimetic peptides include Gap 15
(71), Gap 18 (72), Gap 19 (73), Gap 26 (74), Gap 26M (Gap26 modified with acylation
to improve solubility and stability) (74), Gap 27 (74), Gap 35 (72) and Gap36 (72). Among these, the most evaluated peptides
in skin cells are Gap26, Gap26M and Gap27. Gap26 and Gap26M
directly interacted with amino acids 63–75 of the extracellular
loop 1 of Cx43 (VCYDKSFPISHVR); Gap27 mimicked amino acids 204–214
on the extracellular loop 2 of Cx43 (SRPTEKTIFII) (74). Gap26 and Gap26M are non-Cx43 specific,
whereas Gap27 is Cx43-selective (74).
Various studies have demonstrated that peptide treatment
significantly increases the migration rates of keratinocytes and
fibroblasts to wound edges (74,75). In
addition to the above-mentioned peptides, Ongstad et al
(76) designed a cell-permanent
α-connexin carboxyl-terminal (αCT1) peptide based on the C-terminus
of Cx43. The peptides encapsulated in pluronic F127 thermogel and
methylcellulose demonstrated a significant accelerating effect on
wound healing in pre-clinical animal models of the skin and heart.
An external application of αCT1 on wound healing is now set to
proceed into phase I and II clinical trials (76). Gap19 is currently applied for the
treatment of myocardial ischemia/reperfusion injury and, to the
best of our knowledge, there are no reports regarding its
application in wound healing (73).
Other peptides, including Gap15, Gap18, Gap35 and Gap36 were
reported as Cx43 selective blockers; however, the function of these
in cutaneous systems remain elusive (71,72). Various
chemicals, including octanol and 18β-glycyrrhetinic acid and its
water-soluble derivative, carbenoxolone, block intercellular
junctional communication by targeting Cxs (77–79).
 | Table I.Cx43 blockers and their functions in
the cutaneous system. |
Table I.
Cx43 blockers and their functions in
the cutaneous system.
| Author, year | Cx43 blockers | Type | Centerological
position | Oligos/peptide
sequence | Specificity | Function | (Refs.) |
|---|
| Mori et al,
2006; Wang et al, 2007; Qiu et al, 2003 |
Oligodeoxy-ribonucleotides |
Oligodeoxy-ribonucleotides |
| GTAATTGCGG
CAGGAGGAAT TGTTTCTGTC | Specific | Accelerates wound
healing with reduced scar formation in normal mouse skin and
diabetic rat skin | (40,45,70) |
| Boitano et
al, 1998 | Gap15 | Peptide | Intracellular
loop | EIKKFKYGIEEHC | Specific | N.R. | (71) |
| Oviedo-Orta et
al, 2000 | Gap18 | Peptide | Intracellular
N-terminal | MGDWSALGK
LLDKVQAC | Specific | N.R. | (72) |
| Wang et al,
2013 | Gap19 | Peptide | Intracellular
domain | KQIEIKKFK | Specific | N.R. | (73) |
|
| Gap26 | Peptide | Extracellular loop
1 | VCYDKSFPISHVR | Non-specific | Increases migration
rates of keratinocytes and fibroblasts |
|
|
| Gap26M | Peptide | Extracellular loop
1 | VCYDKSFPISHVR | Non-specific | Increases migration
rates of keratinocytes and fibroblasts |
|
|
| Gap27 | Peptide | Extracellular loop
2 | SRPTEKTIFII | Specific | Enhances wound
closure by inducing primary mouse keratinocytes |
|
| Oviedo-Orta et
al, 2000 | Gap35 | Peptide | Extracellular loop
1 | AWGDEQSAFR
CNTQQPGC | Specific | N.R. | (72) |
|
| Gap36 | Peptide | Extracellular loop
2 | KRDPCPHQV
DCFLSRPTEK | Specific | N.R. |
|
| Ongstad et
al, 2013 | αCT1 | Peptide | C terminus | N.R. | Specific | Enhances wound
healing in animal models of skin and heart | (76) |
| Goldberg et
al, 1996 Davidson and Baumgarten, 1988 | Octanol and
CBX | Chemicals | N.R. | N.R. | Non-specific | Blocks gap junction
communication in human fibroblasts at low concentration | (78,79) |
Notably, primary diabetic cells exhibited less
susceptibility to Cx43 inhibitors (80,81);
therefore, the clinical application of administering Cx43 peptides
for the treatment of chronic diabetic wounds requires further
critical consideration. As the mode-of-action of Cxs is not unique,
selective downregulation of one Cx rather than using broad-spectrum
inhibitors would be preferable. Furthermore, the functional
mechanism and effects of Cx43 mimetic peptides on other tissues
have yet to be determined.
Conclusion
Recent studies on Cx43 in the skin clearly
demonstrate that Cx43 is important in human skin biology and
pathology. The expression level and activity of Cx43 are strictly
regulated in certain situations. Downregulation of Cx43 following
tissue injury significantly reduces ECM deposition, inflammation
response and scar formation, and accelerates wound healing rates.
Currently, Cx43 expression levels and functions have been presented
in skin injury, skeletal muscle regeneration (82), ischemia/reperfusion injury (83) and cornea repair (84). The Cx-associated compounds are in
development and indicate promising therapeutic opportunities in
preclinical evaluation (85).
Non-toxic Cx43 specific inhibitors may also be effective for the
treatment of wounds, skin cancer or other skin associated
disorders. Therefore, it is considered urgent to investigate the
underlying mechanisms and clinical potential of Cx43 in the
skin.
Acknowledgements
The present study was supported by the Fundamental
Research Funds for the Central Universities (grant no. 2015IB004)
and the China Postdoctoral Science Foundation (grant no.
2015M582291).
References
|
1
|
Proksch E, Brandner JM and Jensen JM: The
skin: An indispensable barrier. Exp Dermatol. 17:1063–1072. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanitakis J: Anatomy, histology and
immunohistochemistry of normal human skin. Eur J Dermatol.
12:390–399; quiz 400–401. 2002.PubMed/NCBI
|
|
3
|
Martin PE, Easton JA, Hodgins MB and
Wright CS: Connexins: Sensors of epidermal integrity that are
therapeutic targets. FEBS Lett. 588:1304–1314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Söhl G and Willecke K: Gap junctions and
the connexin protein family. Cardiovasc Res. 62:228–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Söhl G and Willecke K: An update on
connexin genes and their nomenclature in mouse and man. Cell Commun
Adhes. 10:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar NM and Gilula NB: The gap junction
communication channel. Cell. 84:381–388. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alexander DB and Goldberg GS: Transfer of
biologically important molecules between cells through gap junction
channels. Curr Med Chem. 10:2045–2058. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hervé JC, Derangeon M, Bahbouhi B, Mesnil
M and Sarrouilhe D: The connexin turnover, an important modulating
factor of the level of cell-to-cell junctional communication:
Comparison with other integral membrane proteins. J Membr Biol.
217:21–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berthoud VM, Minogue PJ, Laing JG and
Beyer EC: Pathways for degradation of connexins and gap junctions.
Cardiovasc Res. 62:256–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kretz M, Euwens C, Hombach S, Eckardt D,
Teubner B, Traub O, Willecke K and Ott T: Altered connexin
expression and wound healing in the epidermis of connexin-deficient
mice. J Cell Sci. 116:3443–3452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di WL, Rugg EL, Leigh IM and Kelsell DP:
Multiple epidermal connexins are expressed in different
keratinocyte subpopulations including connexin 31. J Invest
Dermatol. 117:958–964. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Risek B, Klier FG and Gilula NB: Multiple
gap junction genes are utilized during rat skin and hair
development. Development. 116:639–651. 1992.PubMed/NCBI
|
|
13
|
Wu M, Moh MC and Schwarz H: HepaCAM
associates with connexin 43 and enhances its localization in
cellular junctions. Sci Rep. 6:362182016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garcia IE, Maripillán J, Jara O, Ceriani
R, Palacios-Muñoz A, Ramachandran J, Olivero P, Perez-Acle T,
González C, Sáez JC, et al: Keratitis-ichthyosis-deafness
syndrome-associated Cx26 mutants produce nonfunctional gap
junctions but hyperactive hemichannels when co-expressed with wild
type Cx43. J Invest Dermatol. 135:1338–1347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shuja Z, Li L, Gupta S, Mese G and White
TW: Connexin26 mutations causing palmoplantar keratoderma and
deafness interact with connexin43, modifying gap junction and
hemichannel properties. J Invest Dermatol. 136:225–235. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geimonen E, Jiang W, Ali M, Fishman GI,
Garfield RE and Andersen J: Activation of protein kinase C in human
uterine smooth muscle induces connexin-43 gene transcription
through an AP-1 site in the promoter sequence. J Biol Chem.
271:23667–23674. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Geimonen E, Boylston E, Royek A and
Andersen J: Elevated connexin-43 expression in term human
myometrium correlates with elevated c-Jun expression and is
independent of myometrial estrogen receptors. J Clin Endocrinol
Metab. 83:1177–1185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van der Heyden MA, Rook MB, Hermans MM,
Rijksen G, Boonstra J, Defize LH and Destrée OH: Identification of
connexin43 as a functional target for Wnt signalling. J Cell Sci.
111:1741–1749. 1998.PubMed/NCBI
|
|
19
|
Bao X, Lee SC, Reuss L and Altenberg GA:
Change in permeant size selectivity by phosphorylation of connexin
43 gap-junctional hemichannels by PKC. Proc Natl Acad Sci USA.
104:pp. 4919–4924. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riquelme MA, Burra S, Kar R, Lampe PD and
Jiang JX: Mitogen-activated protein kinase (MAPK) activated by
prostaglandin E2 phosphorylates connexin 43 and closes osteocytic
hemichannels in response to continuous flow shear stress. J Biol
Chem. 290:28321–28328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pahujaa M, Anikin M and Goldberg GS:
Phosphorylation of connexin43 induced by Src: Regulation of gap
junctional communication between transformed cells. Exp Cell Res.
313:4083–4090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cooper CD and Lampe PD: Casein kinase 1
regulates connexin-43 gap junction assembly. J Biol Chem.
277:44962–44968. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
TenBroek EM, Lampe PD, Solan JL, Reynhout
JK and Johnson RG: Ser364 of connexin43 and the upregulation of gap
junction assembly by cAMP. J Cell Biol. 155:1307–1318. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kjenseth A, Fykerud T, Rivedal E and
Leithe E: Regulation of gap junction intercellular communication by
the ubiquitin system. Cell Signal. 22:1267–1273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kjenseth A, Fykerud TA, Sirnes S, Bruun J,
Yohannes Z, Kolberg M, Omori Y, Rivedal E and Leithe E: The gap
junction channel protein connexin 43 is covalently modified and
regulated by SUMOylation. J Biol Chem. 287:15851–15861. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giepmans BN, Verlaan I and Moolenaar WH:
Connexin-43 interactions with ZO-1 and alpha- and beta-tubulin.
Cell Commun Adhes. 8:219–223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giepmans BN, Verlaan I, Hengeveld T,
Janssen H, Calafat J, Falk MM and Moolenaar WH: Gap junction
protein connexin-43 interacts directly with microtubules. Curr
Biol. 11:1364–1368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shaw RM, Fay AJ, Puthenveedu MA, von
Zastrow M, Jan YN and Jan LY: Microtubule plus-end-tracking
proteins target gap junctions directly from the cell interior to
adherens junctions. Cell. 128:547–560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Plante I, Stewart MK, Barr K, Allan AL and
Laird DW: Cx43 suppresses mammary tumor metastasis to the lung in a
Cx43 mutant mouse model of human disease. Oncogene. 30:1681–1692.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim MO, Ryu JM, Suh HN, Park SH, Oh YM,
Lee SH and Han HJ: cAMP promotes cell migration through cell
junctional complex dynamics and actin cytoskeleton remodeling:
Implications in skin wound healing. Stem Cells Dev. 24:2513–2524.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Cao X, Lin Z, Lee M, Jia X, Ren Y,
Dai L, Guan L, Zhang J, Lin X, et al: Exome sequencing reveals
mutation in GJA1 as a cause of
keratoderma-hypotrichosis-leukonychia totalis syndrome. Hum Mol
Genet. 24:243–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sargen MR, Gormley RH, Pasha TL, Yum S,
Acs G, Xu X and Zhang PJ: Melanocytic tumors express connexin 43
but not 26: Immunohistochemical analysis with potential
significance in melanocytic oncogenesis. Am J Dermatopathol.
35:813–817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rezze GG, Fregnani JH, Duprat J and
Landman G: Cell adhesion and communication proteins are
differentially expressed in melanoma progression model. Hum Pathol.
42:409–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shaw TJ and Martin P: Wound repair at a
glance. J Cell Sci. 122:3209–3213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Falanga V: Wound healing and its
impairment in the diabetic foot. Lancet. 366:1736–1743. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Janis JE, Kwon RK and Lalonde DH: A
practical guide to wound healing. Plast Reconstr Surg.
125:230e–244e. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dunn CA and Lampe PD: Injury-triggered Akt
phosphorylation of Cx43: A ZO-1-driven molecular switch that
regulates gap junction size. J Cell Sci. 127:455–464. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cogliati B, Vinken M, Silva TC, Araújo CM,
Aloia TP, Chaible LM, Mori CM and Dagli ML: Connexin 43 deficiency
accelerates skin wound healing and extracellular matrix remodeling
in mice. J Dermatol Sci. 79:50–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghatnekar GS, O'Quinn MP, Jourdan LJ,
Gurjarpadhye AA, Draughn RL and Gourdie RG: Connexin43
carboxyl-terminal peptides reduce scar progenitor and promote
regenerative healing following skin wounding. Regen Med. 4:205–223.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mori R, Power KT, Wang CM, Martin P and
Becker DL: Acute downregulation of connexin43 at wound sites leads
to a reduced inflammatory response, enhanced keratinocyte
proliferation and wound fibroblast migration. J Cell Sci.
119:5193–5203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang HH, Su CH, Wu YJ, Li JY, Tseng YM,
Lin YC, Hsieh CL, Tsai CH and Yeh HI: Reduction of connexin43 in
human endothelial progenitor cells impairs the angiogenic
potential. Angiogenesis. 16:553–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gartner C, Ziegelhöffer B, Kostelka M,
Stepan H, Mohr FW and Dhein S: Knock-down of endothelial connexins
impairs angiogenesis. Pharmacol Res. 65:347–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gottrup F: A specialized wound-healing
center concept: Importance of a multidisciplinary department
structure and surgical treatment facilities in the treatment of
chronic wounds. Am J Surg. 187 Suppl 5A:38S–43S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reiber GE, Lipsky BA and Gibbons GW: The
burden of diabetic foot ulcers. Am J Surg. 176 Suppl 2A:5S–10S.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang CM, Lincoln J, Cook JE and Becker DL:
Abnormal connexin expression underlies delayed wound healing in
diabetic skin. Diabetes. 56:2809–2817. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mendoza-Naranjo A, Cormie P, Serrano AE,
Wang CM, Thrasivoulou C, Sutcliffe JE, Gilmartin DJ, Tsui J, Serena
TE, Phillips AR and Becker DL: Overexpression of the gap junction
protein Cx43 as found in diabetic foot ulcers can retard fibroblast
migration. Cell Biol Int. 36:661–667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vinnik IuS, Salmina AB, Tepliakova OV,
Drobushevskaia AI, Malinovskaia NA, Pozhilenkova EA, Morgun AV and
Gitlina AG: Dynamics of local expression of connexin-43 and basic
fibroblast growth factor receptors in patients with skin and
soft-tissue infections against the background of diabetes mellitus
type II. Vestn Khir Im I I Grek. 173:47–52. 2014.(In Russian).
PubMed/NCBI
|
|
48
|
Mendoza-Naranjo A, Cormie P, Serrano AE,
Hu R, O'Neill S, Wang CM, Thrasivoulou C, Power KT, White A, Serena
T, et al: Targeting Cx43 and N-cadherin, which are abnormally
upregulated in venous leg ulcers, influences migration, adhesion
and activation of Rho GTPases. PLoS One. 7:e373742012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Paznekas WA, Boyadjiev SA, Shapiro RE,
Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C,
Hannibal MC and Jabs EW: Connexin 43 (GJA1) mutations cause the
pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum
Genet. 72:408–418. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kogame T, Dainichi T, Shimomura Y, Tanioka
M, Kabashima K and Miyachi Y: Palmoplantar keratosis in
oculodentodigital dysplasia with a GJA1 point mutation out of the
C-terminal region of connexin 43. J Dermatol. 41:1095–1097. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
van Steensel MA, Spruijt L, van der Burgt
I, Bladergroen RS, Vermeer M, Steijlen PM and van Geel M: A 2-bp
deletion in the GJA1 gene is associated with oculo-dento-digital
dysplasia with palmoplantar keratoderma. Am J Med Genet A.
132A:171–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Boyden LM, Craiglow BG, Zhou J, Hu R,
Loring EC, Morel KD, Lauren CT, Lifton RP and Bilguvar K: Yale
Center for Mendelian Genomics et al. Dominant de novo mutations in
GJA1 cause erythrokeratodermia variabilis et progressiva, without
features of oculodentodigital dysplasia. J Invest Dermatol.
135:1540–1547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mou YY, Zhao GQ, Lin JY, Zhao J, Lin H, Hu
LT, Xu Q, Wang Q and Sun WR: Expression of connexin 43 and
E-cadherin in choroidal melanoma. Int J Ophthalmol. 4:156–161.
2011.PubMed/NCBI
|
|
54
|
Lin JH, Takano T, Cotrina ML, Arcuino G,
Kang J, Liu S, Gao Q, Jiang L, Li F, Lichtenberg-Frate H, et al:
Connexin 43 enhances the adhesivity and mediates the invasion of
malignant glioma cells. J Neurosci. 22:4302–4311. 2002.PubMed/NCBI
|
|
55
|
Schiffner S, Zimara N, Schmid R and
Bosserhoff AK: p54nrb is a new regulator of progression of
malignant melanoma. Carcinogenesis. 32:1176–1182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang WK, Chen MC, Leong HF, Kuo YL, Kuo CY
and Lee CH: Connexin 43 suppresses tumor angiogenesis by
down-regulation of vascular endothelial growth factor via
hypoxic-induced factor-1α. Int J Mol Sci. 16:439–451. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tittarelli A, Guerrero I, Tempio F,
Gleisner MA, Avalos I, Sabanegh S, Ortíz C, Michea L, López MN,
Mendoza-Naranjo A and Salazar-Onfray F: Overexpression of connexin
43 reduces melanoma proliferative and metastatic capacity. Br J
Cancer. 113:259–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lomas A, Leonardi-Bee J and Bath-Hextall
F: A systematic review of worldwide incidence of nonmelanoma skin
cancer. Br J Dermatol. 166:1069–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stelkovics E, Kiszner G, Meggyeshazi N,
Korom I, Varga E, Nemeth I, Molnar J and Marczinovits I: Selective
in situ protein expression profiles correlate with distinct
phenotypes of basal cell carcinoma and squamous cell carcinoma of
the skin. Histol Histopathol. 28:941–954. 2013.PubMed/NCBI
|
|
60
|
Tada J and Hashimoto K: Ultrastructural
localization of gap junction protein connexin 43 in normal human
skin, basal cell carcinoma, and squamous cell carcinoma. J Cutan
Pathol. 24:628–635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Arita K, Akiyama M, Tsuji Y, McMillan JR,
Eady RA and Shimizu H: Changes in gap junction distribution and
connexin expression pattern during human fetal skin development. J
Histochem Cytochem. 50:1493–1500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Choudhry R, Pitts JD and Hodgins MB:
Changing patterns of gap junctional intercellular communication and
connexin distribution in mouse epidermis and hair follicles during
embryonic development. Dev Dyn. 210:417–430. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Goliger JA and Paul DL: Expression of gap
junction proteins Cx26, Cx31.1, Cx37, and Cx43 in developing and
mature rat epidermis. Dev Dyn. 200:1–13. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Butterweck A, Elfgang C, Willecke K and
Traub O: Differential expression of the gap junction proteins
connexin45,−43, −40, −31, and −26 in mouse skin. Eur J Cell Biol.
65:152–163. 1994.PubMed/NCBI
|
|
65
|
Ackert CL, Gittens JE, O'Brien MJ, Eppig
JJ and Kidder GM: Intercellular communication via connexin43 gap
junctions is required for ovarian folliculogenesis in the mouse.
Dev Biol. 233:258–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dyce PW, Li D, Barr KJ and Kidder GM:
Connexin43 is required for the maintenance of multipotency in
skin-derived stem cells. Stem Cells De. 23:1636–1646. 2014.
View Article : Google Scholar
|
|
67
|
Lorraine C, Wright CS and Martin PE:
Connexin43 plays diverse roles in co-ordinating cell migration and
wound closure events. Biochem Soc Trans. 43:482–488. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Moore K, Ghatnekar G, Gourdie RG and Potts
JD: Impact of the controlled release of a connexin 43 peptide on
corneal wound closure in an STZ model of type I diabetes. PLoS One.
9:e865702014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gilmartin DJ, Soon A, Thrasivoulou C,
Phillips AR, Jayasinghe SN and Becker DL: Sustained release of Cx43
antisense oligodeoxynucleotides from coated collagen scaffolds
promotes wound healing. Adv Healthc Mater. 5:1786–1799. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Qiu C, Coutinho P, Frank S, Franke S, Law
LY, Martin P, Green CR and Becker DL: Targeting connexin43
expression accelerates the rate of wound repair. Curr Biol.
13:1697–1703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Boitano S, Dirksen ER and Evans WH:
Sequence-specific antibodies to connexins block intercellular
calcium signaling through gap junctions. Cell Calcium. 23:1–9.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Oviedo-Orta E, Hoy T and Evans WH:
Intercellular communication in the immune system: Differential
expression of connexin40 and 43, and perturbation of gap junction
channel functions in peripheral blood and tonsil human lymphocyte
subpopulations. Immunology. 99:578–590. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang N, de Vuyst E, Ponsaerts R, Boengler
K, Palacios-Prado N, Wauman J, Lai CP, de Bock M, Decrock E, Bol M,
et al: Selective inhibition of Cx43 hemichannels by Gap19 and its
impact on myocardial ischemia/reperfusion injury. Basic Res
Cardiol. 108:3092013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wright CS, van Steensel MA, Hodgins MB and
Martin PE: Connexin mimetic peptides improve cell migration rates
of human epidermal keratinocytes and dermal fibroblasts in vitro.
Wound Repair Regen. 17:240–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kandyba EE, Hodgins MB and Martin PE: A
murine living skin equivalent amenable to live-cell imaging:
Analysis of the roles of connexins in the epidermis. J Invest
Dermatol. 128:1039–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ongstad EL, O'Quinn MP, Ghatnekar GS, Yost
MJ and Gourdie RG: A Connexin43 mimetic peptide promotes
regenerative healing and improves mechanical properties in skin and
heart. Adv Wound Care (New Rochelle). 2:55–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Contreras JE, Sanchez HA, Eugenin EA,
Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV and Sáez
JC: Metabolic inhibition induces opening of unapposed connexin 43
gap junction hemichannels and reduces gap junctional communication
in cortical astrocytes in culture. Proc Natl Acad Sci USA. 99:pp.
495–500. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Goldberg GS, Moreno AP, Bechberger JF,
Hearn SS, Shivers RR, MacPhee DJ, Zhang YC and Naus CC: Evidence
that disruption of connexon particle arrangements in gap junction
plaques is associated with inhibition of gap junctional
communication by a glycyrrhetinic acid derivative. Exp Cell Res.
222:48–53. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Davidson JS and Baumgarten IM:
Glycyrrhetinic acid derivatives: A novel class of inhibitors of
gap-junctional intercellular communication. Structure-activity
relationships. J Pharmacol Exp Ther. 246:1104–1107. 1988.PubMed/NCBI
|
|
80
|
Pollok S, Pfeiffer AC, Lobmann R, Wright
CS, Moll I, Martin PE and Brandner JM: Connexin 43 mimetic peptide
Gap27 reveals potential differences in the role of Cx43 in wound
repair between diabetic and non-diabetic cells. J Cell Mol Med.
15:861–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wright CS, Berends RF, Flint DJ and Martin
PE: Cell motility in models of wounded human skin is improved by
Gap27 despite raised glucose, insulin and IGFBP-5. Exp Cell Res.
319:390–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ishido M and Kasuga N: Characteristics of
the localization of connexin 43 in satellite cells during skeletal
muscle regeneration in vivo. Acta Histochem Cytochem. 48:53–60.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Schulz R, Görge PM, Gorbe A, Ferdinandy P,
Lampe PD and Leybaert L: Connexin 43 is an emerging therapeutic
target in ischemia/reperfusion injury, cardioprotection and
neuroprotection. Pharmacol Ther. 153:90–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Elbadawy HM, Mirabelli P, Xeroudaki M,
Parekh M, Bertolin M, Breda C, Cagini C, Ponzin D, Lagali N and
Ferrari S: Effect of Connexin 43 inhibition by the mimetic peptide
Gap27 on corneal wound healing, inflammation and
neovascularization. Br J Pharmacol. 173:2880–2893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Becker DL, Phillips AR, Duft BJ, Kim Y and
Green CR: Translating connexin biology into therapeutics. Semin
Cell Dev Biol. 50:49–58. 2016. View Article : Google Scholar : PubMed/NCBI
|















