Introduction
Although the incidence and mortality of gastric
cancer have both declined in most areas of the world, gastric
cancer is still the fourth most frequent cancer occurred and the
second leading cause of cancer related death worldwide, and is
especially higher among East Asian countries (1). Thus, identification of possible risk
factors is especially essential for the prevention of gastric
cancer. It is suggested that gastric cancer is relevance to many
factors, including gastric precursor lesions, Helicobacter
pylori infection and genetic polymorphisms. Among all
polymorphisms, the variants of pro- and anti-inflammatory cytokines
such as interleukin (2) and tumor
necrosis factors (TNFs) (3) were most
extensively investigated. TNF-α is a cytokine initially
taken as a serum factor causing necrosis of transplanted tumors and
it serves an important role in host defense against infectious
diseases, whereas excessive expression product may lead to organ
failure and a strong inflammatory response which may modify gastric
cancer risk (4).
Previously, identification of polymorphisms of
TNF-α gave some suggestions on understanding the genetic
predisposition of gastric and colorectal cancers (5). The expression level of TNF-α was proved
to be obviously affected by polymorphisms in its promoter region,
and previous studies have identified that such polymorphisms at 238
(rs361525), 308 (rs1800629), 857 (rs1799724) and 1031 (rs1799964)
positions may influence production of TNF-α (6–9). However,
the hypotheses that TNF-α polymorphisms may be associated
with gastric cancers are still in controversial and the results of
previous association studies have been largely inconclusive.
Most of the published studies about TNF-α
polymorphisms just refer to a small or modest sample size, and none
of them were able to get a reliable conclusion. Therefore, the
authors conducted a new meta-analysis to review studies that have
examined those polymorphisms, to further investigate the relevance
between polymorphisms of TNF-α and the risk of gastric
cancer.
Materials and methods
Data sources
The authors searched for all articles that had been
published about the relevance between TNF-α polymorphisms
and the inclination of gastric cancer, applying the following
topics in the MEDLINE, PubMed, EMBASE and the Cochrane library:
[‘Tumor Necrosis Factor-alpha’ (MeSH) OR (Tumor Necrosis) OR TNF]
AND [‘Polymorphism, Genetic’ (MeSH) OR polymorphism OR
polymorphisms OR risk] AND (gastric cancer). All articles were
updated on July 15, 2013. References of all primary studies and
review articles were reviewed for additional references. The search
was carried out by two individual researchers to confirm that no
published papers were missed.
Criteria for inclusion and
exclusion
Subjects enrolled in the study must meet the
following criteria: i) Case-control studies about the relevance
between TNF-α polymorphisms and gastric cancer; ii)
available genotype frequencies in cases and controls provided; and
iii) self-reported results and risk assessment and/or displayed
data necessary for evaluating OR with 95% CI. The authors
eliminated studies that crossing with other studies or reported
with data from the same authors.
The process of data extraction
The data was picked up independently by two
scientists. Related information of the author's last name,
publication year, country of origin, study population origin,
genotypes and the number of cases and controls were recorded. The
number of studies on TNF-α 308, TNF-α 238, TNF-α
857, TNF-α 1031, TNF-α 863 and gastric cancer
were 33, 16, 8, 6, 5, respectively. More than half of the studies
took frequency-matched controls to cases by age and sex.
Statistical data analysis
The authors used the Hardy-Weinberg equilibrium to
compare the observed genotype frequencies with expected genotype
frequencies in controls of all studies. ORs and 95% CIs were
adopted to evaluate the robust relationship between TNF-α
polymorphisms and inclination of gastric cancer under homozygote
comparison and dominant genetic model comparison. Random-effects
models were taken to compute overall summary ORs and 95% CIs. Study
populations were divided into western (Europe and America) or
eastern (China, Korea, India and Iran).
The importance of the overall ORs was assessed by
the Z-test, in which two-sided P<0.05 was considered to indicate
a statistically significant difference. The Q-statistic was adopted
to evaluate the heterogeneity among studies, and P<0.1 was
considered as a significant gap. The I2-statistic
can also be taken to check test heterogeneity efficiently, with
I2<25%, 25–75% and >75% considered to
display low, moderate and high degree of inconsistency,
respectively. Begg's funnel plot was taken to gauging the
underlying publication bias (10). As
for the sensitivity analysis, relatively smaller studies were
rejected and the overall ORs (95% CIs) were checked again. All data
analyses were carried out by STATA software (version 12.0; STATA
Corporation, College Station, TX, USA).
Results
Characteristics of studies
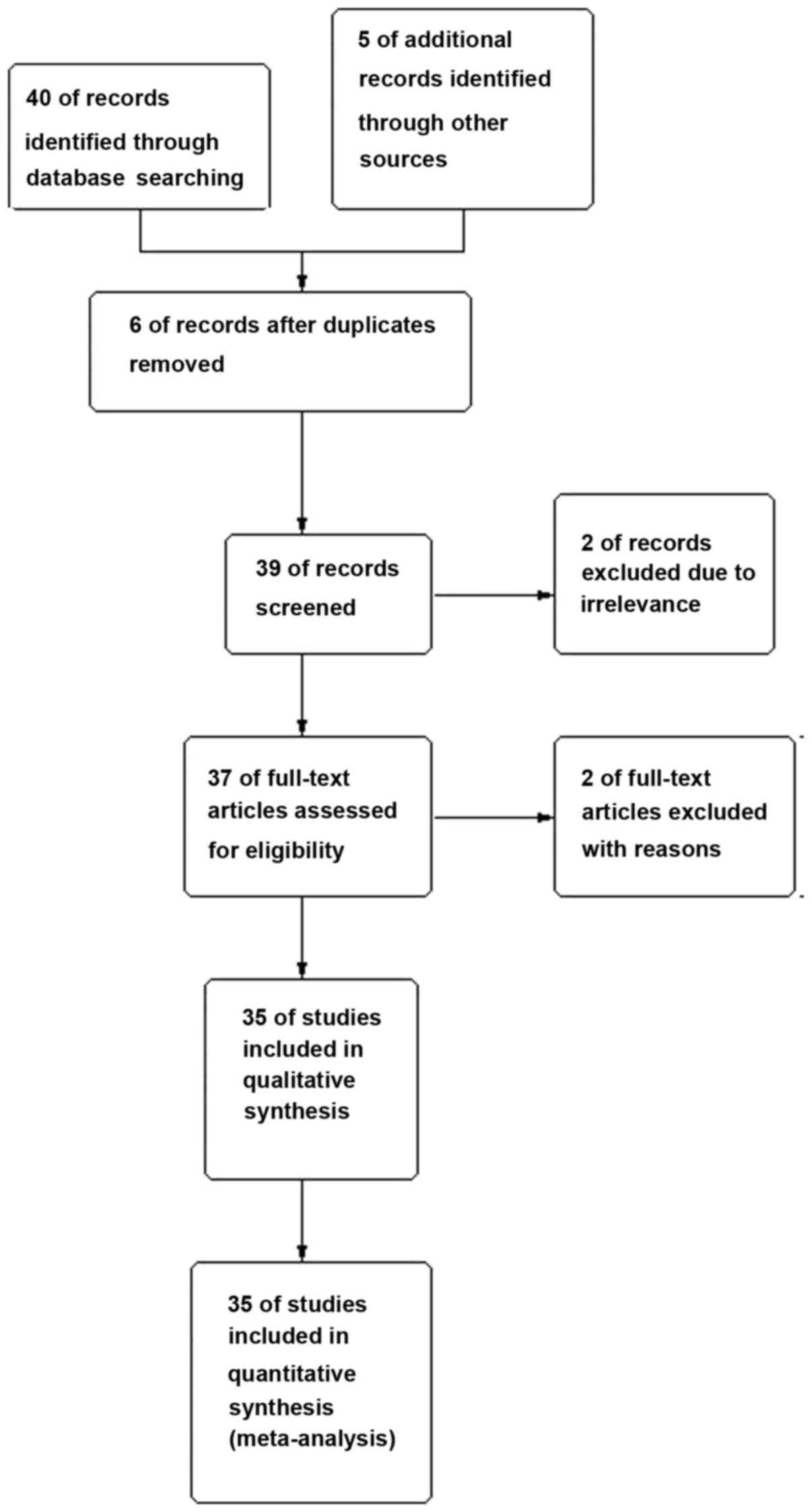
The authors searched 355 records. Following
elimination of duplicated and irrelevant records by checking the
titles and abstracts, 35 full-text articles were picked up for
intensive study. The process of selection process was shown in
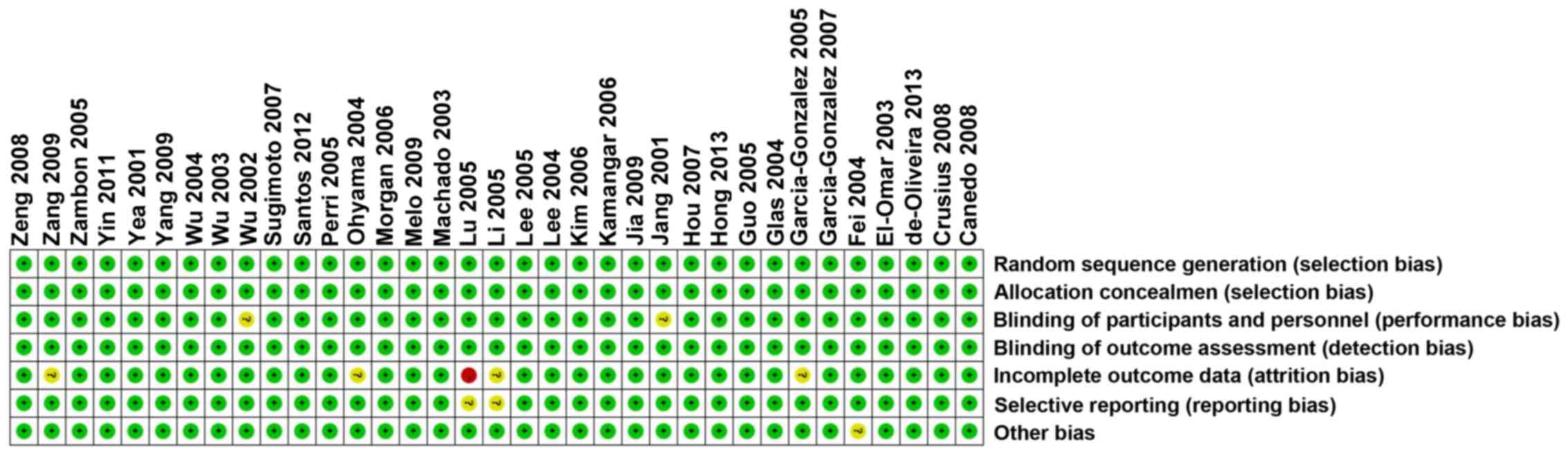
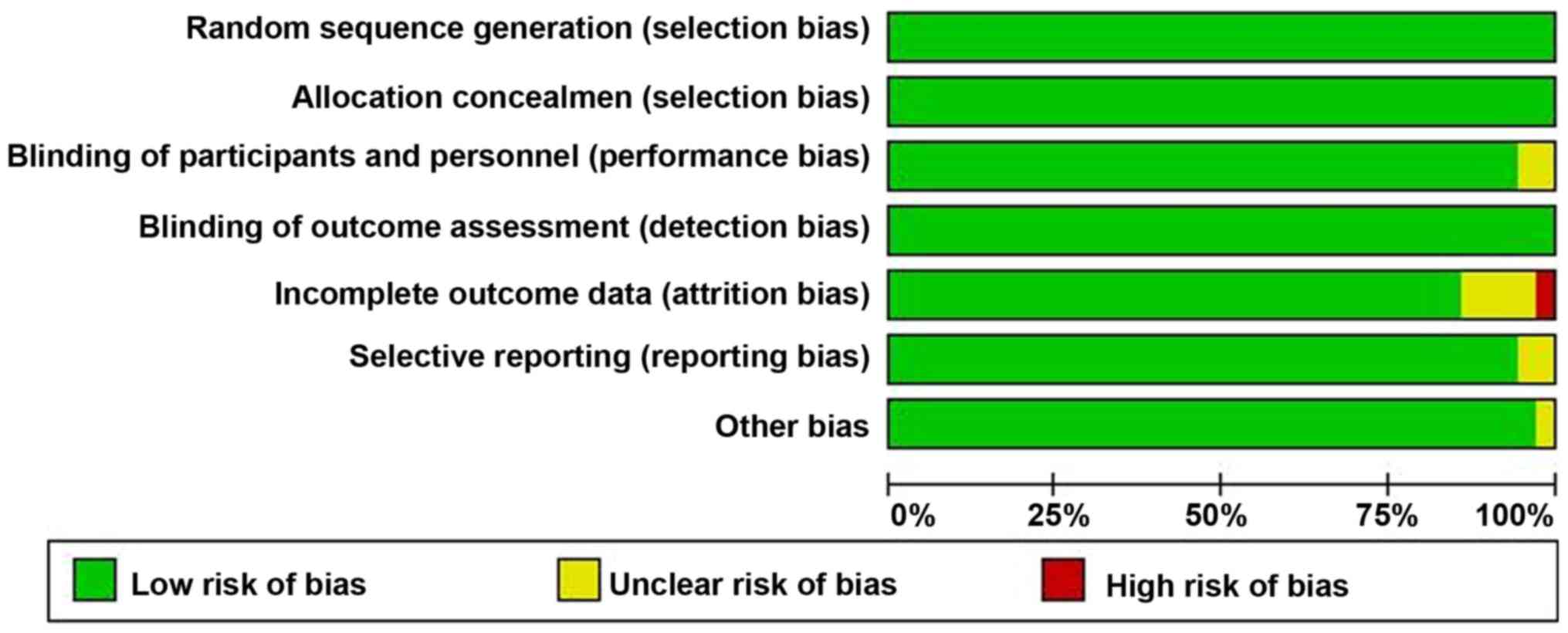
(Fig. 1). Each risk of bias item for
each included study was shown in (Figs.
2 and 3).
A total of 35 studies with 8,147 cases and 12,182
controls were included in this analysis (Fig. 4). The most popular investigated
genotypes were TNF-α 308, TNF-α 238, which were
presented in 33 and 16 studies, respectively (11–44). Other
genotypes, such as TNF-α 857, TNF-α 863, and TNF-α
1031 were also included in this meta-analysis. Genotype and
allele distributions of TNF-α 308 are presented in Table I. Median frequencies of TNF-α
308A allele were 13.46% in western populations and 7.20% in
eastern populations. Corresponding frequencies for the TNF-α
238A allele were 5.52 and 3.92%, respectively. While the
frequencies for TNF-α 857, TNF-α 1031, TNF-α
863 were 19.64, 20.28, 14.98%, respectively in western
populations and 15.90, 20.30, 15.87%, respectively in eastern
populations.
 | Table I.Study characteristics. |
Table I.
Study characteristics.
| First author | Year | Loc. | Control source | Case | Control | 308-A (%) |
PHWE | 238-A (%) |
PHWE | 857-T (%) |
PHWE | 1031-C (%) |
PHWE | 863-A (%) |
PHWE | Refs. |
|---|
| Jang | 2001 | E | HB | 52 | 92 |
3.80 | 0.70 | 7.07 | 0.39 | – | – | – | – | – | – | (23) |
| Yea | 2001 | E | HB | 83 |
113 |
3.10 | 0.73 | 6.19 | 0.36 | – | – | – | – | – | – | (42) |
| Wu | 2002 | E | HB |
150 |
220 | 12.05 | 0.00 | 1.82 | 0.01 | – | – | – | – | – | – | (39) |
| Persson | 2011 | W | PB |
314 |
210 | 15.24 | 0.55 | – | – | – | – | – | – | – | – | (47) |
| Machado | 2003 | W | PB |
287 |
304 | 12.66 | 0.65 | – | – | – | – | – | – | – | – | (30) |
| Wu | 2003 | E | PB |
220 |
230 | 13.26 | 0.00 | 2.17 | 0.01 | – | – | – | – | – | – | (40) |
| Fei | 2004 | E | HB | 56 |
164 |
6.7 | 0.69 | – | – | – | – | – | – | – | – | (16) |
| Glas | 2004 | W | PB | 88 |
145 | 15.17 | 0.67 | 3.79 | 0.63 | – | – | – | – | – | – | (19) |
| Lee | 2004 | E | PB |
341 |
261 |
8.43 | 0.49 | 4.79 | 0.42 | – | – | – | – | – | – | (27) |
| Ohyama | 2004 | E | PB |
300 |
472 | – | – | – | – | 18.50 | 0.90 | – | – | – | – | (33) |
| Wu | 2004 | E | PB |
204 |
210 | 12.38 | 0.00 | 1.67 | 0.01 | 14.29 | 0.11 | 23.21 | 0.23 | 15.95 | 0.80 | (38) |
| Garza-Gonzalez | 2005 | W | HB | 63 |
215 |
8.60 | 0.61 | – | – | – | – | – | – | – | – | (18) |
| Guo | 2005 | E | PB |
264 |
437 |
5.95 | 0.00 | – | – | – | – | – | – | – | – | (20) |
| Lee | 2005 | E | PB |
122 |
120 |
7.08 | 0.40 | – | – | 15.90 | 0.20 | 20.69 | 0.32 | 16.86 | 0.70 | (26) |
| Li | 2005 | E | PB | 59 |
264 |
7.20 | 0.56 | – | – | – | – | – | – | – | – | (28) |
| Lu | 2005 | E | PB |
250 |
300 |
4.67 | 0.08 | 3.83 | 0.49 | – | – | – | – | – | – | (29) |
| Perri | 2005 | W | PB |
184 |
362 | 10.91 | 0.15 | – | – | – | – | – | – | – | – | (35) |
| Zambon | 2005 | W | HB |
129 |
644 | 12.27 | 0.91 | 5.90 | 0.38 | 19.64 | 0.34 | 23.21 | 0.87 | – | – | (43) |
| Kamangar | 2006 | W | PB |
112 |
208 | 13.46 | 0.29 | 1.20 | 0.86 | – | – | – | – | – | – | (24) |
| Kim | 2006 | E | PB |
237 |
461 |
6.83 | 0.91 | – | – | – | – | – | – | – | – | (26) |
| Morgan | 2006 | W | PB |
168 |
161 |
3.73 | 0.62 | – | – | – | – | – | – | – | – | (32) |
|
Garcia-Gonzalez | 2007 | W | PB |
404 |
404 | 11.26 | 0.35 | 10.27 | 0.01 | – | – | – | – | – | – | (17) |
| Hou | 2007 | W | PB |
305 |
428 | 16.24 | 0.19 | – | – | 13.10 | 0.36 | 17.35 | 0.32 | 14.98 | 0.49 | (22) |
| Sugimoto | 2007 | E | HB |
105 |
172 |
0.87 | 0.91 | – | – | 15.70 | 0.01 | 17.35 | 0.63 | 14.98 | 0.39 | (37) |
| Canedo | 2008 | W | PB |
508 |
713 | 18.86 | 0.00 | – | – | – | – | – | – | – | – | (12) |
| Crusius | 2008 | W | HB |
236 | 1,125 | 14.93 | 0.17 | 5.52 | 0.23 | – | – | – | – | – | – | (13) |
| Zeng | 2007 | E | PB |
130 |
142 | 15.49 | 0.37 | 9.15 | 0.37 | – | – | – | – | – | – | (44) |
| Jia | 2008 | E | HB |
106 |
108 | 14.35 | 0.00 | – | – | – | – | – | – | – | – | (11) |
| Melo | 2009 | W | HB | 30 |
100 |
7.50 | 0.53 | – | – | – | – | – | – | – | – | (31) |
| Yang | 2009 | E | PB | 83 |
322 |
5.28 | 0.32 | 3.93 | 0.46 | 16.00 | 0.90 | 19.91 | 0.77 | 15.79 | 0.01 | (41) |
| Zang | 2009 | E | PB |
296 |
319 | – | – | 3.92 | 0.47 | – | – | – | – | – | – | (51) |
| Yin | 2011 | E | HB |
311 |
485 |
9.79 | 0.09 | 3.92 | 0.37 | – | – | – | – | – | – | (52) |
| Santos | 2012 | W | PB | 64 |
137 | 14.60 | 0.96 | – | – | – | – | – | – | – | – | (36) |
| Oliveira | 2012 | W | PB |
200 |
240 | 15.21 | 0.01 | – | – | 21.04 | 0.01 | – | – | – | – | (34) |
| Hong | 2013 | E | HB | 1,686 | 1,894 |
8.53 | 0.95 | – | – | – | – | – | – | – | – | (21) |
TNF-α 308
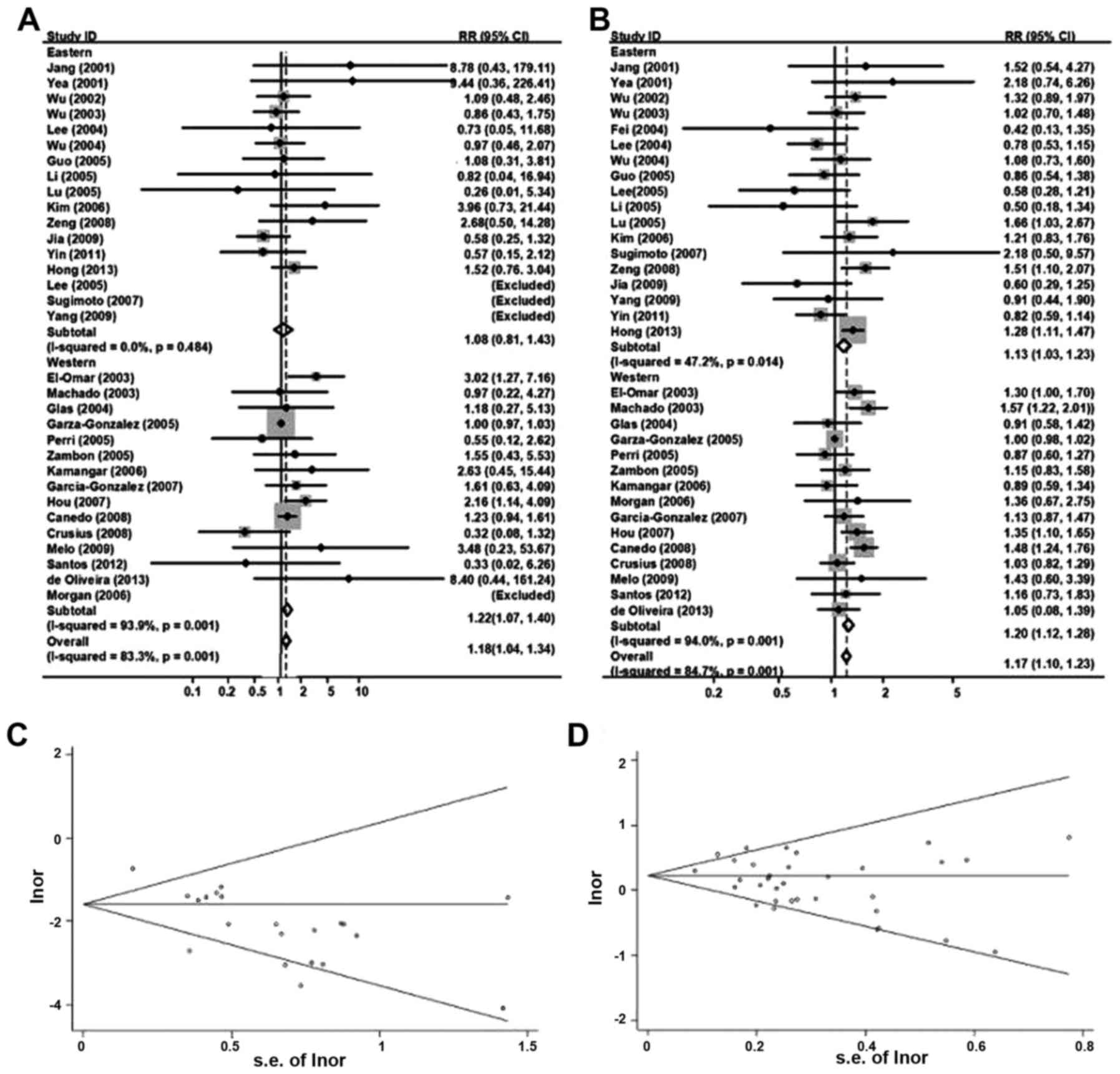
Fig. 4A displayed the
random-effect overall OR (95% CIs) of TNF-α 308
polymorphisms under homozygous genotype comparison [AA vs. GG: 1.18
(1.04–1.34)]. Since the frequencies of AA were too low, AA and AG
groups were summed up as ‘A carriers’ groups for subsequent
comparison with GG groups, which did not change the previous
conclusion much [GA/AA vs. GG: 1.17 (1.10–1.23); Fig. 4B].
Classified by ethnicity, it reported an obvious
relevance between TNF-α 308 and gastric cancer inclination
in eastern populations [AA vs. GG: 1.08 (0.80–1.42); GA/AA vs. GG:
1.13 (1.03–1.23), random-effects model]. A similar association was
also identified in western populations (AA vs. GG: 1.22
(1.07–1.40); GA/AA vs. GG: 1.20 (1.12–1.28), random-effects
model).
Analysis stratified by control population source
(hospital-based, HB or population-based, PB) was also conducted
(Table II). There was an obvious
association between TNF-α 308 and gastric cancer inclination
in both HB subgroup [GA/AA vs. GG: 1.13 (1.04–1.23)] and PB
subgroup [GA/AA vs. GG: 1.19 (1.10–1.28), AA vs. GG: 1.35
(1.11–1.64)]. For non-cardia cancers only, the summary ORs (95%
CIs) for GA/AA vs. GG and AA vs. GG were 0.98 (0.86–1.12) and
1.01(0.67–1.54), respectively, which were not statistically
significant (Table II). When the
analysis was limited to H. pylori-positive cases, these ORs
(95% CIs) for AA vs. GG and GA/AA vs. GG were 2.07 (0.74–5.79) and
1.27 (1.04–1.55), respectively.
 | Table II.Overall and group-specific summary
statistics for TNF-α 308, TNF-α 238, TNF-α
857, TNF-α 1031 and TNF-α 863 in gastric
cancer. |
Table II.
Overall and group-specific summary
statistics for TNF-α 308, TNF-α 238, TNF-α
857, TNF-α 1031 and TNF-α 863 in gastric
cancer.
|
|
|
| Test of
association | Test of
heterogeneity |
|---|
|
|
|
|
|
|
|---|
| Variables | No. of studies | Comparison | OR (95% CI) | P-value (Z
test) | I2
(%) | P-value |
|---|
| TNF-α
308 |
|
|
|
|
|
|
|
Hospital based | 12 | AA vs. GG | 1.00
(0.86–1.15) | 0.96 | 22 | 0.24 |
|
| 12 | GA + AA vs. GG | 1.13
(1.04–1.23) | 0.01 | 90 | <0.001 |
|
Population based | 21 | AA vs. GG | 1.35
(1.11–1.64) | 0.02 | 0 | 0.50 |
|
| 21 | GA + AA vs. GG | 1.19
(1.10–1.28) | <0.001 | 46 | 0.10 |
|
Non-cardia cancers | 9 | AA vs. GG | 1.14
(0.75–1.72) | 0.53 | 0 | 0.45 |
|
| 12 | GA + AA vs. GG | 0.98
(0.86–1.12) | 0.76 | 8 | 0.36 |
| H.
pylori-positive | 4 | AA vs. GG | 2.07
(0.74–5.79) | 0.17 | 15 | 0.31 |
|
| 6 | GA + AA vs. GG | 1.27
(1.04–1.55) | 0.02 | 28 | 0.23 |
| TNF-α
238 |
|
|
|
|
|
|
|
Hospital based | 6 | AA vs. GG | 3.35
(1.46–7.67) | 0.01 | 46 | 0.10 |
|
| 16 | GA + AA vs. GG | 0.84
(0.65–1.11) | 0.12 | 3 | 0.40 |
|
Population based | 4 | AA vs. GG | 0.51
(0.22–1.20) | 0.12 | 32 | 0.22 |
|
| 11 | GA + AA vs. GG | 1.22
(1.04–1.43) | 0.02 | 17 | 0.28 |
|
Non-cardia cancers | 2 | AA vs. GG | 3.04
(0.90–10.20) | 0.07 | 67 | 0.08 |
|
| 5 | GA + AA vs. GG | 1.08
(0.80–1.47) | 0.61 | 0 | 0.68 |
| TNF-α
857 |
|
|
|
|
|
|
|
Hospital based | 2 | TT vs. CC | 1.92
(0.93–3.97) | 0.08 | 58 | 0.12 |
|
| 2 | TT + TC vs. CC | 1.08
(0.88–1.33) | 0.46 | 61 | 0.11 |
|
Population based | 6 | TT vs. CC | 1.71
(1.20–2.43) | <0.001 | 0 | 0.45 |
|
| 6 | TT + TC vs. CC | 1.15
(1.04–1.27) | 0.01 | 40 | 0.14 |
|
Non-cardia cancers | 5 | TT vs. CC | 2.13
(1.46–3.09) | <0.001 | 0 | 0.57 |
|
| 5 | TT + TC vs. CC | 1.16
(1.03–1.31) | 0.01 | 51 | 0.09 |
| TNF-α
1031 |
|
|
|
|
|
|
|
Hospital based | 4 | CC vs. TT | 1.45
(0.92–2.28) | 0.11 | 0 | 0.73 |
|
| 4 | CC + CT vs. TT | 0.85
(0.75–0.97) | 0.01 | 0 | 0.85 |
|
Population based | 2 | CC vs. TT | 1.59
(0.85–2.97) | 0.15 | 73 | 0.05 |
|
| 2 | CC + CT vs. TT | 1.19
(0.99–1.43) | 0.06 | 83 | 0.85 |
|
Non-cardia cancers | 4 | CC vs. TT | 1.39
(0.88–2.19) | 0.15 | 26.50 | 0.64 |
|
| 4 | CC + CT vs. TT | 1.00
(0.88–1.14) | 0.99 | 75 | 0.01 |
| TNF-α
863 |
|
|
|
|
|
|
|
Hospital based | 1 | AA vs. CC | 2.62
(0.60–11.38) | 0.17 | 0 | 0.96 |
|
| 1 | AC + AA vs. CC | 1.54
(1.10–2.13) | 0.14 | 76 | 0.04 |
|
Population based | 4 | AA vs. CC | 1.35
(0.86–2.13) | 0.19 | 0 | 0.96 |
|
| 4 | AC + AA vs. CC | 0.81
(0.70–0.95) | 0.01 | 0 | 0.86 |
|
Non-cardia cancers | 3 | AA vs. CC | 1.03
(0.55–1.95) | 0.93 | 0 | 0.79 |
|
| 3 | AC + AA vs. CC | 0.97
(0.82–1.15) | 0.76 | 79 | 0.01 |
For publication bias investigation, Fig. 4C and D used Begg's funnel plot for the
association between TNF-α 308 and the cancer risk under
homozygous and dominant genetic model comparison, and no evidence
for bias was identified using Egger's weighted regression method
(AA vs. GG, P for bias=0.43; GA/AA vs. GG, P for bias=0.20). To
further confirm these reports, the authors carried out the
sensitivity analysis. It indicated that there was little
modification of the assessment following rejection of any single
studies.
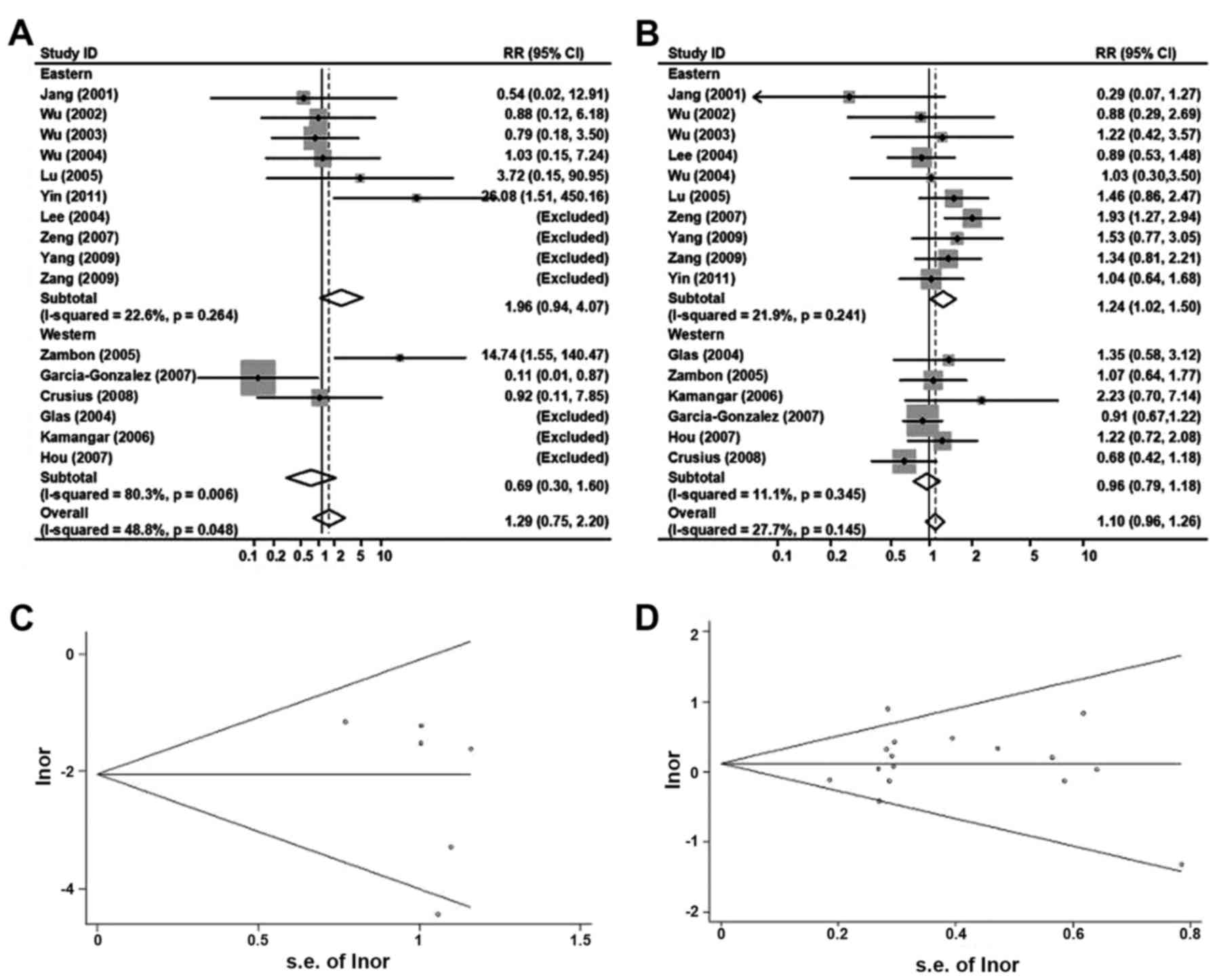
TNF-α 238
Analyzed by the same procedure as TNF-α 308
above, Fig. 5A and B summarized the
ORs and 95% CIs for the associations between TNF-α 238
polymorphisms and overall risk of the gastric cancer [AA vs. GG:
1.29 (0.75–2.20); GA/AA vs. GG: 1.10(0.98–1.26), random-effects
model].
In the analyses stratified by ethnicity, the ORs
(95% CIs) of TNF-α 238 and gastric cancer risk in eastern
populations were 1.96 (0.94–4.07) for AA vs. GG and 1.24
(1.02–1.50) for GA/AA vs. GG. Corresponding ORs (95% CIs) were 0.69
(0.30–1.60) and 0.98 (0.79–1.18) in western populations.
For GA/AA vs. GG, the overall ORs (95% CIs) of
TNF-α 238 and gastric cancer risk was 0.84 (0.65–1.11) in
the HB subgroup and 1.22 (1.04–1.43) in the PB subgroup. For AA vs.
GG, the HB subgroup demonstrated a more significant association,
with a OR (95% CI) of 3.35 (1.46–7.67). For non-cardia cancers, the
OR (95% CI) for GA/AA vs. GG genotypes was 1.08 (0.80–1.47).
However, AA vs. GG genotypes were rejected for the null values to
AA genotype frequency.
To rule out any possible publication bias, Begg's
funnel plot were indicated in Fig. 5C and
D, and no evidence for bias was detected (AA vs. GG, P for
bias=0.93; AA vs. GG, p for bias=0.31). In the subgroup analyses of
populations, the results did not alter obviously when the authors
rejected the relatively small studies.
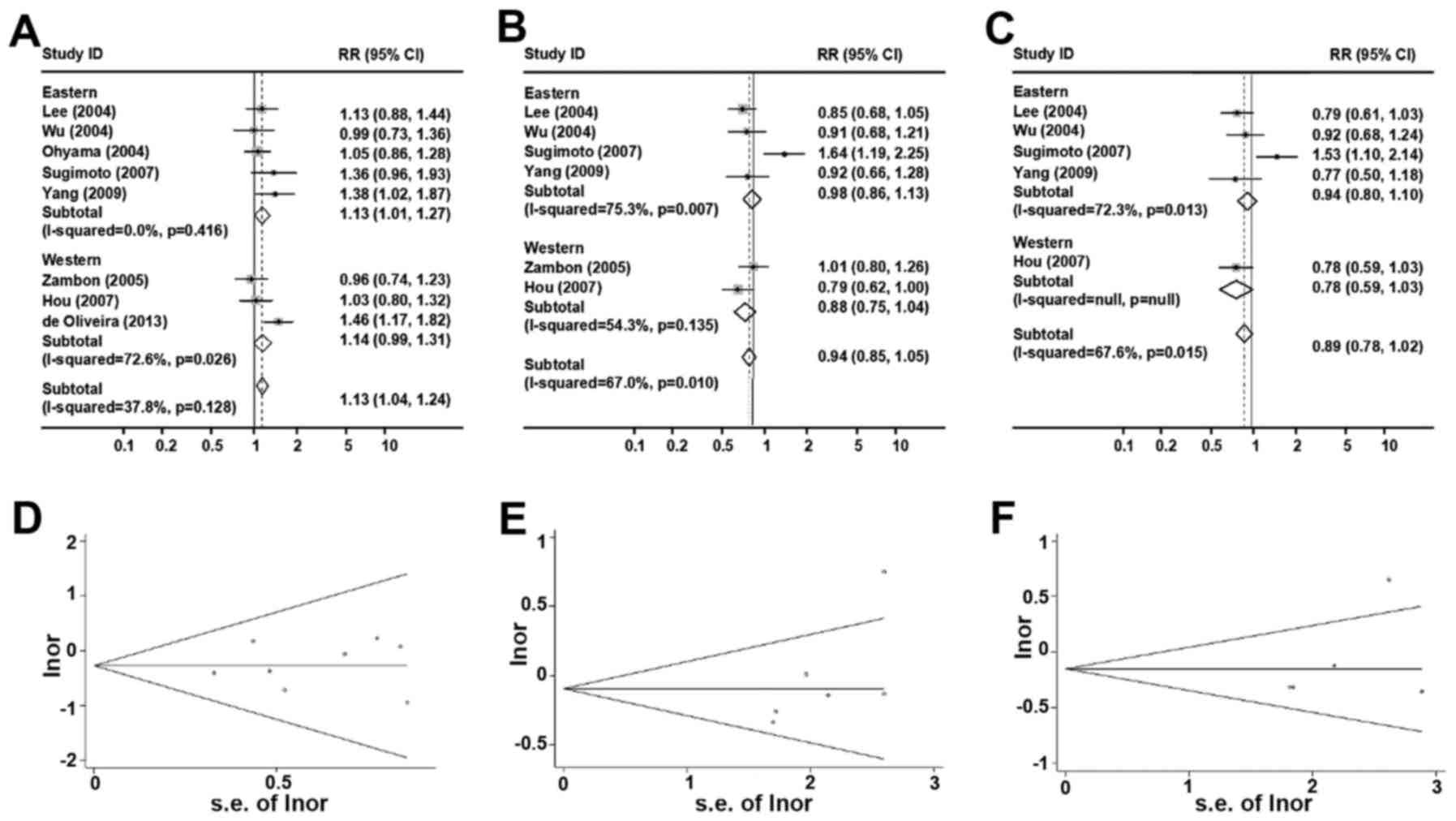
TNF-α 857, TNF-α 1031 and TNF-α
863
Fig. 6 summarized the
ORs and 95% CIs for the relationships between the genotypes of
TNF-α 857 (TC/TT vs. CC; Fig.
3A), TNF-α 1031 (CT/CC vs. TT; Fig. 6B), TNF-α 863 (AC/AA vs. CC;
Fig. 6C) and gastric cancer risk.
Since the case populations of the included studies were small,
especially the homozygous subgroups, only dominant genetic model of
these three TNF-α polymorphisms was investigated. For overall
analysis, the random-effect OR (95% CI) for TNF-α 857 was
1.13 (1.04–1.24), indicating TNF-α 857 is a potential risk
factor of gastric cancer. Similar ORs (95% CIs) were obtained in
the analyses stratified by ethnicity and control population source,
and also subgroup of non-cardia cancers (Table II).
TNF-α 1031 [CT/CC vs. TT: 0.94 (0.85–1.05)]
and TNF-α 863 [AC/AA vs. CC: 0.89 (0.78–1.02)] both seemed
to be associated with a reduced risk of gastric cancer, but neither
of them was statistically significant probably due to a small
population size. Analyses stratified by ethnicity and control
population source were also performed; no inconsistence between
different populations was identified in Fig. 6. However, in a population-based
subgroup, the OR (95% CI) was 0.81 (0.70–0.95) for the association
between TNF-α 863 (AC/AA vs. CC) and gastric cancer risk.
(Table II; P=0.007).
Begg's funnel plot for the relationship among these
three TNF-α polymorphisms and gastric cancer was presented
in Figs. 6D-F. For TNF-α 857,
TNF-α 1031, TNF-α 863, there were no evidence for
bias using the method of Begg (P for bias=0.80, 0.11, 0.42,
respectively).
Discussion
Inflammation is usually considered as a significant
factor involving in the pathogenesis of cancer, and the
polymorphisms of inflammation related genes have been extensively
studied for many years (2).
TNF-α is the most well-studied inflammatory factor gene in
gastric cancers, and it was proved that TNF-α related cell
functions were greatly affected by the polymorphisms in the
promoter region of TNF-α gene (3). So far, polymorphisms at 238 (rs361525),
308 (rs1800629), 857 (rs1799724), and 1031 (rs1799964) positions
were all reported to be related with risk of cancer (3,45), but all
the conclusions are still in controversial and the results of
previous relevant studies were ambiguous. Up to now, at least five
meta-analyses about the relationships between TNF-α
polymorphisms and gastric cancer risks have been published, and
even those meta-analyses held inconsistent opinions (3,46–48). Furthermore, to the best of the authors'
knowledge, no systematic review about all the above five SNPs has
been published yet. Thus, this review and a bran-new meta-analysis
can display a further proof about the relationship between those
polymorphisms and the risk of gastric cancer comprehensively and
systematically.
For TNF-α 308, a total of 33 studies, more
than those included in previous meta-analysis (3,49), were
included in the present study. Analysis on TNF-α 308
reported a significant relationship between certain genotypes and
gastric cancer risk in worldwide populations. A previous
meta-analysis summarizing data on TNF-α-308 variants have
suggested some race-specific associations, with increased gastric
cancer risk in different ethnic populations (3). The presented results indicated that the
median prevalence of TNF-α 308A allele in western
populations was almost twice as high as in eastern populations
(13.46 vs. 7.20%) and the OR was also slightly higher in western
populations. The PB subgroup demonstrated a much more significant
association probably perhaps HB controls may not be representative
of the universal population and such studies usually had biases.
Together, the results proved that TNF-α 308 ‘A carrier’
genotypes were potential risk factors of statistical significance
in gastric cancers.
For TNF-α 238, a total of 18 studies was
identified to be associated with gastric cancer risks, nearly
doubled those of the previous meta-analyses (23,41,45). Consistent with the previous results,
TNF-α 238 polymorphisms were not significantly associated
with the risk of gastric cancer when pulled all the ORs together.
As a relatively large population size was used in current data,
stratified analyses could be carried out. Interestingly, TNF-α
238 presented a totally variable effect in different
populations, therefore displaying an obvious relationship with
gastric cancer risks in eastern populations, but not western
populations. This difference could be explained by the high
incidence of H. pylori infection among the Asian
populations, so that the inflammation related genes such as TNF-α
may serve a much more important role there. Furthermore, a
significant association between TNF-α 238 polymorphisms and
gastric cancer risks was just observed in the PB subgroup but not
in the HB subgroup, probably because HB controls may not be
representative of the general. In addition, the incidence of H.
pylori infection of the PB and HB subgroups may also be
distinctive, which may potentially contribute to the conclusion
above. In conclusion, a more detailed investigation with larger
numbers of universal participants is required to confirm the
relevance between the TNF-α 238 polymorphisms and gastric
cancer risks, and confirm the difference between subgroups.
The present study also stated clearly that the T
allele of the TNF-α 857 polymorphisms be associated with a
higher risk of gastric cancer. The random-effect OR (95% CI) for
TNF-α 857 was 1.13 (1.04–1.24) for overall analysis,
implying TNF-α 857 T allele is a potential risk factor of
gastric cancers. The relevance was also endorsed by a report about
increased transcriptional activity of the TNF-α gene with
the 857 T allele and the pathological role of excessive expressed
TNF-α (9). Additionally, it is
the first time that the relationship between TNF-α 857
polymorphisms and gastric cancer risks was reviewed by a
meta-analysis.
In the present meta-analysis, not enough evidence
was promulgated to authenticate the existing association between
TNF-α 1031, TNF-α 863 polymorphisms and gastric
cancer risks. However, both of the SNPs were suggested to be
related with a reduced risk of gastric cancer, according to the ORs
and 95% CIs. The association between TNF-α 1031, TNF-α
863 and gastric cancers was of approximate significance, so
more studies are required. Additionally, it is to be remarked that
in population-based subgroup, the OR (95% CI) for TNF-α 863
becomes statistically significant, which supports the above
suggestions from another side.
H. pylori infection is a key risk factor of
gastric cancer. H. pylori strains and host genotypes
possibly affected the host inflammatory response and
epithelial-cell physiology, thus aggravated the risk of gastric
cancer (50). Zambon et al
(43) reported that H. pylori
infection was associated with the TNF-α 308 genotype. The
current study demonstrated that the TNF-α 308 polymorphisms
had much more effect on the risk of catching gastric cancer in the
populations with H. pylori infection, which indicated the
existence of interaction between H. pylori infection and
TNF-α pathway in gastric carcinogenesis. However, for
TNF-α 238 and other SNPs, studies with detailed H.
pylori infection status information were so limited that data
could not be stratified according to H. pylori infection
status.
Some limitations of this meta-analysis should also
be taken into account. Firstly, the sample size was too small to
conduct stratified analyses, which weakened the conclusions,
especially in the analyses of TNF-α 857, TNF-α 1031
and TNF-α 863. More studies need to be picked up to achieve
a much more credible conclusion. Secondly, detailed information was
lacking in this meta-analysis, for which many analyses, for example
analyses stratified by histology and sex, could not be carried out.
Also, gene-gene, gene-phenotype and gene-environment interactions
should also be checked in further studies provided that individual
raw data were available.
Based on these analyses collectively, this
systematic review had collected all the available data related with
the TNF-α polymorphisms and gastric cancer, and this meta-analysis
indicated that TNF-α 308, TNF-α 238 and TNF-α
857 were moderately associated with an increased risk of
gastric cancer. However, the association between TNF-α 1031,
TNF-α 863 polymorphisms and gastric cancer risk was of
similar significance. To understand the molecular carcinogenesis
panorama of gastric cancer, further prospective studies in
combination with analysis of other cytokines and environmental
factors are required.
Acknowledgements
The present meta-analysis was supported by the
Natural Science Foundation of Shandong Province (grant no.
ZR2015HL078) which was been in charged by Yuan Tian. The authors
would like to thank all members of the Oncology Lab of Qianfoshan
Hospital. We would like to thank Peking University and Shandong
University for assistance with some statistical analyses.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camargo MC, Mera R, Correa P, Peek RM Jr,
Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J and
Schneider BG: Interleukin-1beta and interleukin-1 receptor
antagonist gene polymorphisms and gastric cancer: A meta-analysis.
Cancer Epidemiol Biomarkers Prev. 15:1674–1687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gorouhi F, Islami F, Bahrami H and
Kamangar F: Tumour-necrosis factor-A polymorphisms and gastric
cancer risk: a meta-analysis. Br J Cancer. 98:1443–1451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strieter RM and Kunkel SL: The
immunopathology of chemotactic cytokines. Adv Exp Med Biol.
351:19–28. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campbell DA, Field M, McArdle CS, Cooke TG
and Gallagher G: Polymorphism at the tumour necrosis factor locus:
A marker of genetic predisposition to colorectal cancer? Lancet.
347:17061996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huizinga TW, Westendorp RG, Bollen EL,
Keijsers V, Brinkman BM, Langermans JA, Breedveld FC, Verweij CL,
van de Gaer L, Dams L, et al: TNF-alpha promoter polymorphisms,
production and susceptibility to multiple sclerosis in different
groups of patients. J Neuroimmunol. 72:149–153. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hellmig S, Fischbach W, Goebeler-Kolve ME,
Fölsch UR, Hampe J and Schreiber S: A functional promotor
polymorphism of TNF-α is associated with primary gastric B-Cell
lymphoma. Am J Gastroenterol. 100:2644–2649. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lindholm E, Bakhtadze E, Cilio C, Agardh
E, Groop L and Agardh CD: Association between LTA TNF and AGER
polymorphisms and late diabetic complications. PLoS One.
3:e25462008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Higuchi T, Seki N, Kamizono S, Yamada A,
Kimura A, Kato H and Itoh K: Polymorphism of the 5′-flanking region
of the human tumor necrosis factor (TNF)-alpha gene in Japanese.
Tissue Antigens. 51:605–612. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sterne JA, Egger M and Smith GD:
Systematic reviews in health care: Investigating and dealing with
publication and other biases in meta-analysis. BMJ. 323:101–105.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia A, Gong J, Li YC and Hao ZM:
Correlation between polymorphisms of IL-4, tumor necrosis factor
alpha gene and non-cardiac gastric cancer in Han people of Shaanxi
province. Chin J Gastroenterol Hepatol. 17:642–644. 2008.
|
|
12
|
Canedo P, Durães C, Pereira F, Regalo G,
Lunet N, Barros H, Carneiro F, Seruca R, Rocha J and Machado JC:
Tumor necrosis factor alpha extended haplotypes and risk of gastric
carcinoma. Cancer Epidemiol Biomarkers Prev. 17:2416–2420. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crusius JB, Canzian F, Capellá G, Peña AS,
Pera G, Sala N, Agudo A, Rico F, Del Giudice G, Palli D, et al:
Cytokine gene polymorphisms and the risk of adenocarcinoma of the
stomach in the European prospective investigation into cancer and
nutrition (EPIC-EURGAST). Ann Oncol. 19:1894–1902. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Y, Qisan W, Fei W and Tao M:
Relationship between tagSNPs and haplotype of TNF-A gene and
gastric cancer in Uygur and Han ethnic groups in Xinjiang.
Carcinogenesis. Teratogenesis Mutagen. 24:261–265. 2012.
|
|
15
|
El-Omar EM, Rabkin CS, Gammon MD, Vaughan
TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot
WJ, et al: Increased risk of noncardia gastric cancer associated
with proinflammatory cytokine gene polymorphisms. Gastroenterology.
124:1193–1201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fei BY, Xia B, Deng CS, Xia XQ, Xie M,
Crusius JB and Pena AS: Association of tumor necrosis factor
genetic polymorphism with chronic atrophic gastritis and gastric
adenocarcinoma in Chinese Han population. World J Gastroenterol.
10:1256–1261. 2004.PubMed/NCBI
|
|
17
|
García-González MA, Lanas A, Quintero E,
Nicolás D, Parra-Blanco A, Strunk M, Benito R, Simón M Angel,
Santolaria S, Sopeña F, et al: Spanish Gastroenterological
Association AEG: Gastric cancer susceptibility is not linked to
pro-and anti-inflammatory cytokine gene polymorphisms in whites: A
Nationwide Multicenter Study in Spain. Am J Gastroenterol.
102:1878–1892. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garza-González E, Bosques-Padilla FJ,
El-Omar E, Hold G, Tijerina-Menchaca R, Maldonado-Garza HJ and
Pérez-Pérez GI: Role of the polymorphic IL-1B, IL-1RN and TNF-A
genes in distal gastric cancer in Mexico. Int J Cancer.
114:237–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Glas J, Török HP, Schneider A, Brünnler G,
Kopp R, Albert ED, Stolte M and Folwaczny C: Allele 2 of the
interleukin-1 receptor antagonist gene is associated with early
gastric cancer. J Clin Oncol. 22:4746–4752. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo W, Wang N, Li Y and Zhang JH:
Polymorphisms in tumor necrosis factor genes and susceptibility to
esophageal squamous cell carcinoma and gastric cardiac
adenocarcinoma in a population of high incidence region of North
China. Chin Med J (Engl). 118:1870–1878. 2005.PubMed/NCBI
|
|
21
|
Hong Y, Ge Z, Jing C, Shi J, Dong X, Zhou
F, Wang M, Zhang Z and Gong W: Functional promoter −308G>A
variant in tumor necrosis factor α gene is associated with risk and
progression of gastric cancer in a Chinese population. PLoS One.
8:e508562013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou L, El-Omar EM, Chen J, Grillo P,
Rabkin CS, Baccarelli A, Yeager M, Chanock SJ, Zatonski W, Sobin
LH, et al: Polymorphisms in Th1-type cell-mediated response genes
and risk of gastric cancer. Carcinogenesis. 28:118–123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jang WH, Yang YI, Yea SS, Lee YJ, Chun JH,
Kim HI, Kim MS and Paik KH: The −238 tumor necrosis factor-alpha
promoter polymorphism is associated with decreased susceptibility
to cancers. Cancer Lett. 166:41–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamangar F, Abnet CC, Hutchinson AA,
Newschaffer CJ, Helzlsouer K, Shugart YY, Pietinen P, Dawsey SM,
Albanes D, Virtamo J, et al: Polymorphisms in inflammation-related
genes and risk of gastric cancer (Finland). Cancer Causes Control.
17:117–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim N, Cho SI, Yim JY, Kim JM, Lee DH,
Park JH, Kim JS, Jung HC and Song IS: The effects of genetic
polymorphisms of IL-1 and TNF-A on Helicobacter pylori-induced
gastroduodenal diseases in Korea. Helicobacter. 11:105–112. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JY, Kim HY, Kim KH, Kim SM, Jang MK,
Park JY, Lee JH, Kim JH and Yoo JY: Association of polymorphism of
IL-10 and TNF-A genes with gastric cancer in Korea. Cancer Lett.
225:207–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SG, Kim B, Yook JH, Oh ST, Lee I and
Song K: TNF/LTA polymorphisms and risk for gastric cancer/duodenal
ulcer in the Korean population. Cytokine. 28:75–82. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Xia B, Yang Y, Li J and Xia HH: TNF
gene polymorphisms and Helicobacter Pylori infection in gastric
carcinogenesis in Chinese population. Am J Gastroenterol.
100:290–294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu W, Pan K, Zhang L, Lin D, Miao X and
You W: Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8,
IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer
in a Chinese population. Carcinogenesis. 26:631–636. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Machado JC, Figueiredo C, Canedo P,
Pharoah P, Carvalho R, Nabais S, Alves C Castro, Campos ML, van
Doorn LJ, Caldas C, et al: A proinflammatory genetic profile
increases the risk for chronic atrophic gastritis and gastric
carcinoma. Gastroenterology. 125:364–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barbosa HP Melo, Martins LC, Dos Santos
SE, Demachki S, Assumpção MB, Aragão CD and de Oliveira Corvelo TC:
Interleukin-1 and TNF-α polymorphisms and Helicobacter pylori in a
Brazilian Amazon population. World J Gastroenterol. 15:1465–1471.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morgan DR, Dominguez RL, Keku TO, Heidt
PE, Martin CF, Galanko JA, Omofoye OA and Sandler RS: Gastric
cancer and the high combination prevalence of host cytokine
genotypes and Helicobacter pylori in Honduras. Clin Gastroenterol
Hepatol. 4:1103–1111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohyama I, Ohmiya N, Niwa Y, Shirai K,
Taguchi A, Itoh A, Hirooka Y, Wakai K, Hamajima N, Mori N, et al:
The association between tumour necrosis factor-alpha gene
polymorphism and the susceptibility to rugal hyperplastic gastritis
and gastric carcinoma. Eur J Gastroenterol Hepatol. 16:693–700.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oliveira JG, Duarte MC and Silva AE:
IL-1ra anti-inflammatory cytokine polymorphism is associated with
risk of gastric cancer and chronic gastritis in a Brazilian
population, but the TNF-β pro-inflammatory cytokine is not. Mol
Biol Rep. 39:7617–7625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Perri F, Piepoli A, Bonvicini C, Gentile
A, Quitadamo M, Di Candia M, Cotugno R, Cattaneo F, Zagari MR,
Ricciardiello L, et al: Cytokine gene polymorphisms in gastric
cancer patients from two Italian areas at high and low cancer
prevalence. Cytokine. 30:293–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Santos JC, Ladeira MS, Pedrazzoli J Jr and
Ribeiro ML: Relationship of IL-1 and TNF-α polymorphisms with
Helicobacter pylori in gastric diseases in a Brazilian population.
Braz J Med Biol Res. 45:811–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sugimoto M, Furuta T, Shirai N, Nakamura
A, Xiao F, Kajimura M, Sugimura H and Hishida A: Different effects
of polymorphisms of tumor necrosis factor-alpha and interleukin-1
beta on development of peptic ulcer and gastric cancer. J
Gastroenterol Hepatol. 22:51–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu MS, Chen LT, Shun CT, Huang SP, Chiu
HM, Wang HP, Lin MT, Cheng AL and Lin JT: Promoter polymorphisms of
tumor necrosis factor-alpha are associated with risk of gastric
mucosa-associated lymphoid tissue lymphoma. Int J Cancer.
110:695–700. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu MS, Huang SP, Chang YT, Shun CT, Chang
MC, Lin MT, Wang HP and Lin JT: Tumor necrosis factor-alpha and
interleukin-10 promoter polymorphisms in Epstein-Barr
virus-associated gastric carcinoma. J Infect Dis. 185:106–109.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu MS, Wu CY, Chen CJ, Lin MT, Shun CT and
Lin JT: Interleukin-10 genotypes associate with the risk of gastric
carcinoma in Taiwanese Chinese. Int J Cancer. 104:617–623. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang JJ, Ko KP, Cho LY, Shin A, Gwack J,
Chang SH, Shin HR, Yoo KY, Kang D and Park SK: The role of TNF
genetic variants and the interaction with cigarette smoking for
gastric cancer risk: A nested case-control study. BMC Cancer.
9:2382009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yea SS, Yang YI, Jang WH, Lee YJ, Bae HS
and Paik KH: Association between TNF-α promoter polymorphism and
Helicobacter pylori cagA subtype infection. J Clin Pathol.
54:703–706. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zambon CF, Basso D, Navaglia F, Belluco C,
Falda A, Fogar P, Greco E, Gallo N, Rugge M, Di Mario F, et al:
Pro- and anti-inflammatory cytokines gene polymorphisms and
Helicobacter pylori infection: Interactions influence outcome.
Cytokine. 29:141–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zeng QD, Lü LH, Xing PX, Lü B and Wang YS:
Relationship between cytokine gene polymorphism and development of
gastric adenocarcinoma. Zhonghua Yi Xue Za Zhi. 87:1037–1039.
2007.(In Chinese). PubMed/NCBI
|
|
45
|
Zhou P, Lv GQ, Wang JZ, Li CW, Du LF,
Zhang C and Li JP: The TNF-α-238 polymorphism and cancer risk: A
meta-analysis. PLoS One. 6:e220922011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peleteiro B, Lunet N, Carrilho C, Durães
C, Machado JC, La Vecchia C and Barros H: Association between
cytokine gene polymorphisms and gastric precancerous lesions:
Systematic review and meta-analysis. Cancer Epidemiol Biomarkers
Prev. 19:762–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Persson C, Canedo P, Machado JC, El-Omar
EM and Forman D: Polymorphisms in inflammatory response genes and
their association with gastric cancer: A HuGE systematic review and
meta-analyses. Am J Epidemiol. 173:259–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang J, Cao C, Luo H, Xiong S, Xu Y and
Xiong W: Tumour necrosis factor alpha −308G/A polymorphism and risk
of the four most frequent cancers: A meta-analysis. Int J
Immunogenet. 38:311–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu PH, Tang Y, Li C, Shen W, Ji L, Guo YJ
and Tao GQ: Meta-analysis of association of tumor necrosis factor
alpha-308 gene promoter polymorphism with gastric cancer. Zhonghua
Yu Fang Yi Xue Za Zhi. 44:209–214. 2010.(In Chinese). PubMed/NCBI
|
|
50
|
Peek RM Jr and Blaser MJ: Helicobacter
pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer.
2:28–37. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zang G, Miao L, Mu Y, Qiao C, Liu J, Ke X,
Zheng C and Sun H: Adenoviral mediated transduction of adenoid
cystic carcinoma by human TRAIL gene driven with hTERT tumor
specific promoter induces apoptosis. Cancer Biol Ther. 8:966–972.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yin Y, Chen W, Tang C, Ding H, Jang J,
Weng M, Cai Y and Zou G: NF-κB, JNK and p53 pathways are involved
in tubeimoside-1-induced apoptosis in HepG2 cells with oxidative
stress and G2/M cell cycle arrest. Food Chem Toxicol. 49:3046–3054.
2011. View Article : Google Scholar : PubMed/NCBI
|