Introduction
A series of complex and severe neurohormonal
abnormalities, including increased levels of cardiac and
circulating endothelin-1 (ET-1) and angiotensin II (AngII), have
been linked to the development of cardiac dysfunction and heart
failure. It has been demonstrated that ET-1 and AngII comprise a
mutually reciprocal signalling network and exhibit certain
reciprocal effects on the myocardium. In previous studies using
cultured neonatal rat ventricular myocytes (NRVMs), endogenous ET-1
mediates AngII-induced hypertrophy (1). The exogenous administration of ET-1 also
induced hypertrophic responses in cardiomyocytes (2). As the predominant receptors on myocytes,
AngII receptor type-1 (AT1R) and ET type A receptors
(ETAR), as well as AngII receptor type-2
(AT2R) and ET type B receptors (ETBR), share
several common subcellular signalling pathways (3). As a result of these local positive
feedback loops and signal redundancy, the roles of these receptors
in chronic processes, including in vivo hypertrophy and
cardiac failure, were synergistic or additive.
Adiponectin (APN), a circulating cytokine, has been
shown to be produced by adipocytes, skeletal myocytes, endothelial
cells, as well as cardiomyocytes, and serves an important role in
glucose and lipid metabolism (4,5). Numerous
previous reports have demonstrated increased APN levels in the
peripheral circulation in patients with chronic heart failure
(CHF); high plasma APN levels are significant prognostic indicators
in these patients (6–8). Several previous reports have postulated
roles for ET-1 in the regulation of APN production in adipocytes
and cardiomyocytes. In cultured human cardiomyocytes, ET-1 has been
reported to cause a significant increase in myocyte size and the
protein expression of APN (9).
However, the underlying mechanisms remain unknown. It is known that
ET-1 can bind to ETBR to stimulate nitric oxide (NO)
production, resulting in an increased formation of the second
messenger, cyclic GMP(cGMP)-dependent protein kinase. Our previous
study demonstrated a concentration-dependent increase in the mRNA
expression of APN when NRVMs were exposed to sodium nitroprusside
(SNP), a NO donor, as well as a cGMP agonist, 8-Br-cGMP. AngII
increased the production of APN in NRVMs through NO/cGMP
activation. Cardiomyocyte-produced APN may be the major source of
the observed upregulation in circulating APN levels in CHF
(10). Whether ET-1 and AngII serve
alternative, additive, or synergistic roles in APN activation via
the identical NO/cGMP signalling pathway in NRVMs remains to be
elucidated.
The present study sought to address the following:
i) Whether ET-1 upregulates APN gene expression and secretion in
cardiomyocytes; ii) whether ET-1 and AngII serve additive roles in
APN gene expression and secretion; iii) to determine the molecular
mechanism underlying the activation of APN in response to ET-1 and
AngII. The present results demonstrated that ET-1-induced
upregulation of the gene expression and secretion of APN
potentially occurs via the ETA and ETB receptors. ET-1 and AngII
were additive in the activation of APN. The use of an NO synthase
inhibitor (Nx-nitro-L-arginine methyl ester hydrochloride) and an
analogue of the cGMP antagonist (Rp-8-Br-CGMP-S) resulted in the
diminished additive roles of ET-1 and AngII on APN induction in
cardiomyocytes.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM),
penicillin, and streptomycin were obtained from Life Technologies
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Telmisartan,
PD123319, BQ-610 and BQ-788 were purchased from Phoenix
Pharmaceutical, Inc. (Belmont, CA, USA). ET-1, AngII, actinomycin
D, bovine serum albumin and all other chemicals were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Primary culture of NRVMs
NRVMs were prepared from ventricles of 1-3-day-old
Sprague-Dawley rats, as previously described (10). Briefly, following digestion in
phosphate-buffered saline containing 0.1% trypsin and 0.04% type II
collagenase, the cells were centrifuged at 320 × g at 37°C for 5
min and suspended in DMEM containing 15% fetal calf serum (FCS).
The cells were pre-seeded and cultured for 2 h to eliminate
non-myocardial cells. Non-attached cells were seeded at
1×106 cells/cm2 in the same medium as above.
Following incubation, the cells were washed and the medium was
replaced with DMEM containing 0.5% FCS for 24 h prior to each
experiment.
ELISA
Following various treatments, the medium was
collected and stored at −80°C for future use. APN contents in the
samples from the same set of experiments were determined using an
ELISA (R&D Systems, Inc., Minneapolis, MN, USA), according to
the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain recation (RT-qPCR)
The total RNA was extracted from the cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. cDNA was synthesised
using a TaqMan RT kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The qPCR was performed using the following
reaction: 1X gene master mix and 1X APN primer and probe mix (cat.
no. Hs00152932 m1; Applied Biosystems; Thermo Fisher Scientific,
Inc.) in 25 µl reactions in a 96-well plate. The reactions were
performed in duplicate for each sample. The plate was then placed
in an ABI Prism 7500 Sequence detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). PCR cycles were performed 40 times
using a three-step cycle procedure (denaturation at 94°C for 30
sec, annealing at 58°C for 30 sec and extension at 72°C for 30
sec), following the initial stage at 96°C for 4 min. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) RNA (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used as the endogenous control. For
the comparison of APN with GAPDH RNA, the cycle threshold value was
analysed (Cq) using the 2−ΔΔCq method (11). The data were subsequently reported as
the fold-change compared with the control. The primer sequence for
APN was as follows: Sense, 5′-GCCGTTCTCTTCACCTACGA-3′ and
antisense, 5′-TGGTCTCCCACCTCCAGAT-3′.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Group mean values were compared using a one-way
analysis of variance, followed by Tukey's multiple comparison test,
where appropriate. Statistics were performed using GraphPad/Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
ET-1 upregulates APN secretion and
exerts additive effects with AngII on APN secretion in NRVMs
To determine effect of ET-1 and AngII on APN
secretion, NRVMs were treated with ET-1 for 24 h and the released
APN in the medium was measured. As shown in Fig. 1A, ET-1 upregulated the secretion of
APN, reaching a maximum of 10 nM from 1.0-6.52±0.52-fold induction
(P<0.01; n=4). When compared with ET-1 alone, the combination of
ET-1 and AngII simultaneously induced higher levels of APN (between
2.31±0.23 and 9.98±0.77-fold induction; P<0.01; n=4). Similarly,
an additive effect of between 10 nM AngII and varying
concentrations of ET-1 on the release of APN was also observed
(Fig. 1B). Since the maximal effect
was observed at 10 nM ET-1 and 10 nM AngII, these concentrations
were used throughout the present study. Additionally, the
time-dependent effects of ET-1, and a combination of ET-1 and AngII
also demonstrated the additive effect on APN secretion (Fig. 1C).
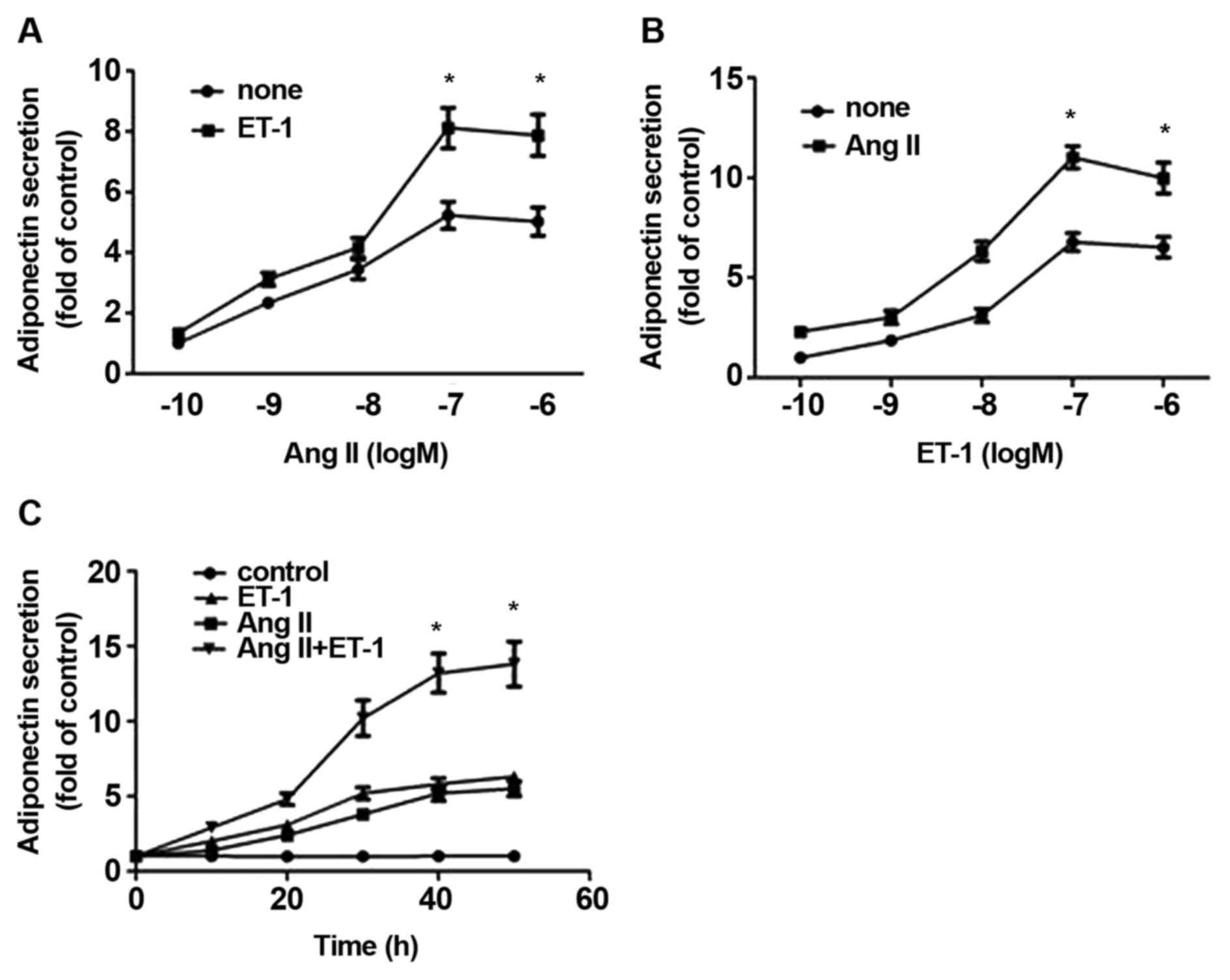 | Figure 1.Combined effects of ET-1 and AngII on
APN release from NRVMs. NRVMs were treated with (A) varying
concentrations of AngII alone or in combination with 10 nM ET-1, or
with (B) varying concentrations of ET-1 alone or in combination
with 10 nM AngII for 24 h. The medium was assessed to determine APN
content using ELISA. (C) Time-dependent effect of ET-1, AngII or a
combination of each on the release of APN. NRVMs were treated
without (control) or with 10 nM ET-1, AngII or both for 8, 16, 24
and 48 h and APN release was assessed. The data are presented as
the mean ± standard error of the mean of four separate experiments,
*P<0.05 compared with the corresponding control. ET-1,
endothelin-1; AngII, angiotensin II; APN, adiponectin; NRVMs,
neonatal rat ventricular myocytes. |
ET-1 upregulates APN gene expression
and exerts additive effects with AngII on the gene expression and
secretion of APN in NRVMs
Since the secretory process of APN in adipocytes is
likely to be subject to its gene expression, the enhancing effect
of ET-1 on APN secretion in NRVMs is most likely also due to
augmented gene expression. To assess this, a transcription
inhibitor (actinomycin D) was used. As shown in Fig. 2A, in the presence of actinomycin D, the
enhancing effect of ET-1, AngII and ET-1 plus AngII was inhibited
by ~95, 92 and 82%, respectively, suggesting that the primary
target site of regulation by ET-1 and AngII is the APN gene.
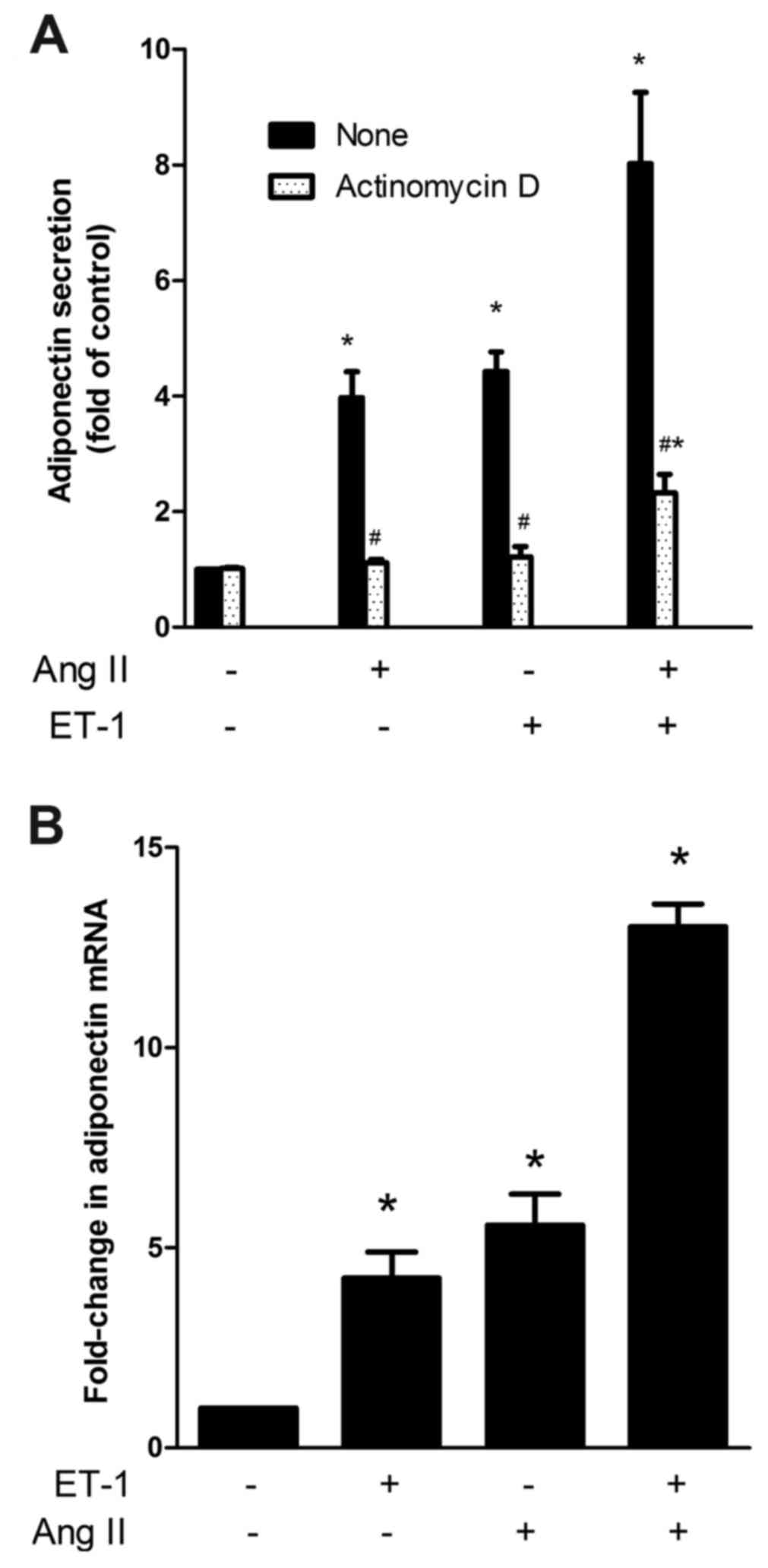 | Figure 2.(A) Effect of actinomycin D on APN
release induced by ET-1, AngII and a combination of both. NRVMs
were treated without or with 5 mg/ml actinomycin D for 30 min prior
to the addition of the vehicle (control), 10 nM ET-1, 10 nM AngII
or both for a further 24 h. The medium was measured to determine
APN content using ELISA. The data are presented as the mean ±
standard error of the mean (*P<0.05 compared with the
corresponding control, #P<0.05 compared with the
corresponding untreated group). (B) The effect of ET-1, AngII and a
combination of both on the mRNA expression of APN in NRVMs.
Following treatment with vehicle (control), ET-1, AngII or both for
24 h, the total RNA was extracted and estimated for APN mRNA
expression by reverse transcription-quantitative polymerase chain
reaction. The data are presented as the mean ± standard error of
the mean (*P<0.05 compared with the control). ET-1,
endothelin-1; AngII, angiotensin II; APN, adiponectin; NRVMs,
neonatal rat ventricular myocytes. |
To further determine whether the gene expression of
APN is similarly enhanced by ET-1 and AngII, the cells were treated
with ET-1, AngII and a combination of both, and were subsequently
evaluated using RT-qPCR. As shown in Fig.
2B, the mRNA expression of APN was augmented by ET-1 and AngII
in an additive manner.
ETAR and ETBR as
well as AT2R were all involved in the effect of ET-1 and
AngII on APN gene expression
Although it was previously demonstrated that AngII
upregulated the expression of APN in NRVMs via the AT2R
and that ET-1 downregulated APN gene expression via the
ETAR activation in 3T3-L1 adipocytes, the additive
effects of ET-1 and AngII on APN secretion may not be mediated by
the same receptor. Since the additive effect of ET-1 and AngII is
predominantly attributable to their effects on APN gene expression,
the present study employed the gene assay to assess which ET and
AngII receptor was involved; this was performed using BQ610 and
BQ788, which are selective antagonists for ETA and ETB receptors,
as well as telmisartan and PD123319, which are selective
antagonists for AT1 and AT2Rs, respectively.
As shown in Fig. 3, AngII-stimulated
APN gene expression was inhibited by PD123319 (P<0.01, n=4) and
telmisartan had a non-significant effect. ET-1-stimulated gene
expression of APN was inhibited by BQ610 (P<0.01, n=4) and BQ788
(P<0.01, n=4). Regarding the gene expression in response to a
combination of ET-1 and AngII, BQ610, BQ788 and PD123319, a
significantly reduced response was observed. The AT1
receptor was not involved. Using the difference between the
increase in gene expression in response to ET-1 plus AngII [(ET-1 +
AngII)-(control)] and the sum of the increases caused by ET-1
[(ET-1)-(control)] and AngII [(AngII)-(control)] alone as an index
to estimate the additive effect, it was revealed that BQ610 and
BQ788, as well as PD123319, inhibited the additive effect by 67
(P<0.01, n=4), 91 (P<0.01, n=4) and 80% (P<0.01, n=4),
respectively. Therefore, it appears that the additive effect of
ET-1 and AngII on APN gene expression was mediated by ETA and ETB,
as well as AT2Rs. The ETB receptor may serve a major
role.
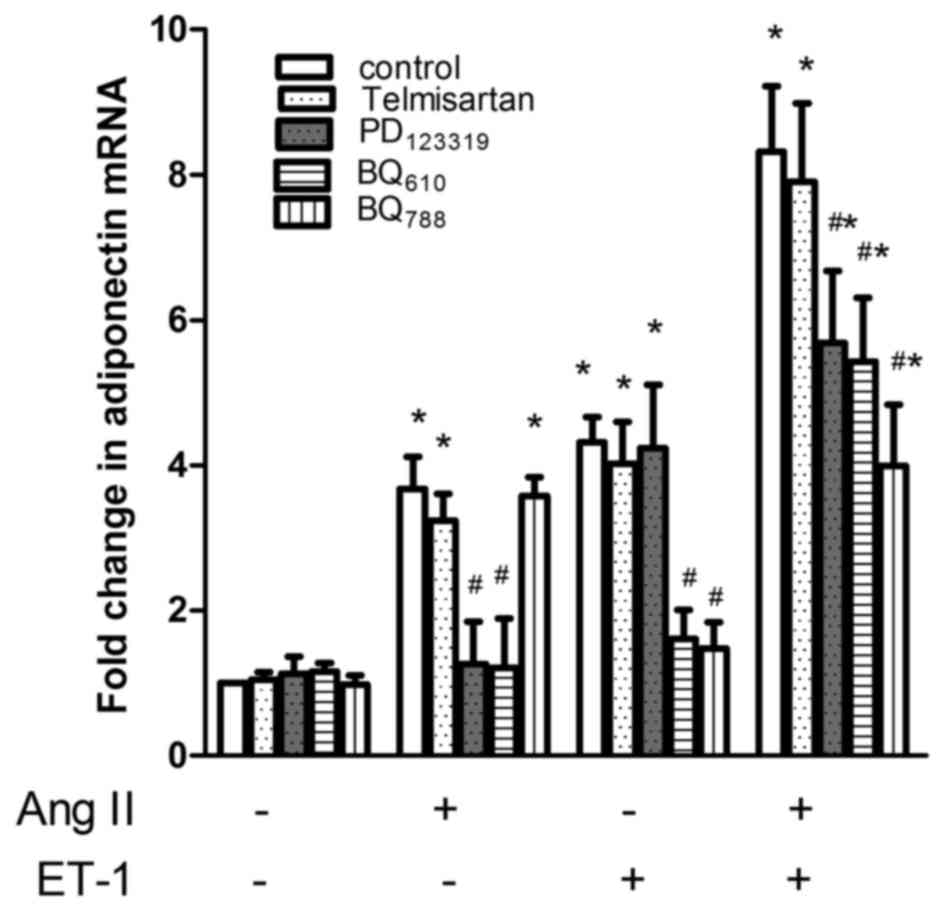 | Figure 3.Effect of ET, AngII receptor
antagonists on adiponectin mRNA in response to ET-1, AngII and a
combination of both drugs. NRVMs were pre-treated with 0, 10 mM
BQ610, 10 mM BQ788, 10 mM telmisatan or 10 mM PD123319 for 1 h and
the effect of vehicle (control), ET-1, AngII and a combination of
both on APN mRNA expression was assessed. The data are presented as
the mean ± standard error of the mean (*P<0.05 compared with the
corresponding control, #P<0.05 compared with the
corresponding untreated group). ET-1, endothelin-1; AngII,
angiotensin II; APN, adiponectin; NRVMs, neonatal rat ventricular
myocytes. |
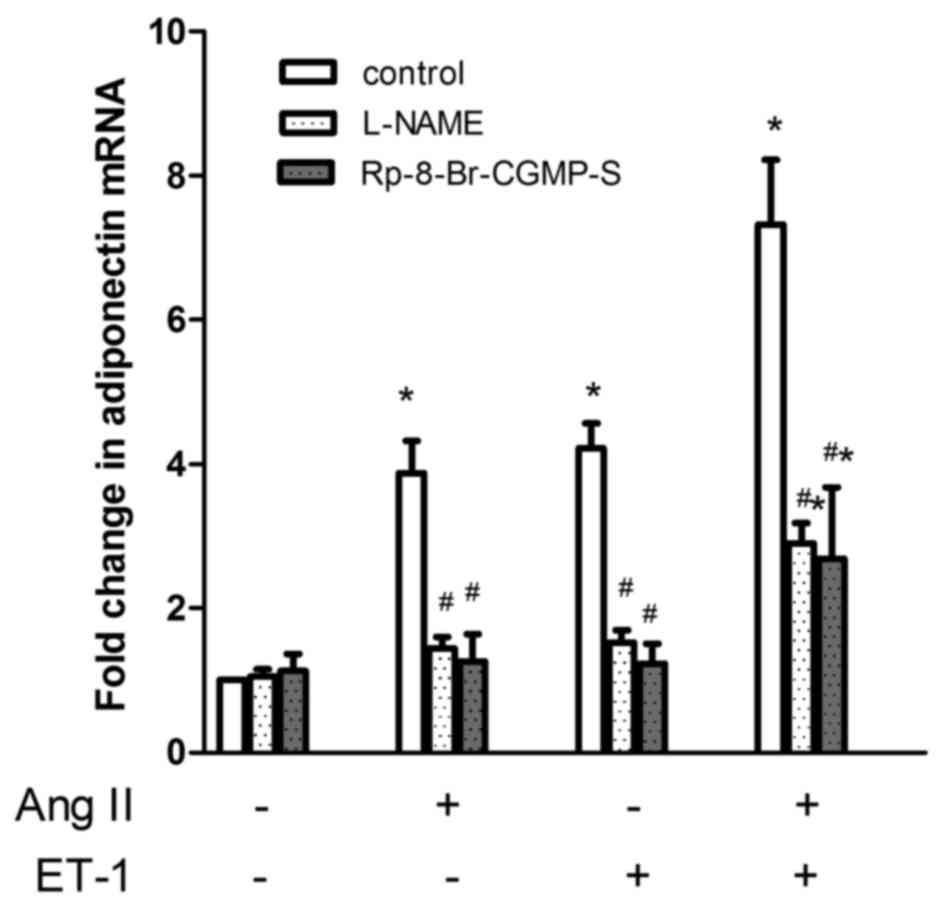
ET-1 and AngII upregulate the gene
expression of APN via the activation of NO/cGMP
To test the hypothesis that the NO/cGMP pathway
serves a role in the additive effects of ET-1 and AngII on APN gene
expression, NRVMs were pre-incubated for 1 h with either L-NAME
(10−3 M) or Rp-8-Br-cGMP-S (10−5 M), and were
then co-incubated with the same inhibitor, as well as
10−7 M AngII and/or ET-1 for a further 24 h. As shown in
Fig. 4, L-NAME or Rp-8-Br-cGMP-S alone
had no effect on the basal mRNA expression of APN; both AngII and
ET-1-stimulated APN gene expression was inhibited by L-NAME and
Rp-8-Br-cGMP-S, respectively (P<0.01, n=4). Regarding the gene
expression in response to a combination of ET-1 and AngII, both
L-NAME and Rp-8-Br-cGMP-S significantly reduced the response. Using
the difference between the increase in gene expression in response
to ET-1 plus AngII [(ET-1 + AngII)-(control)] and the sum of the
increases caused by ET-1 [(ET-1)-(control)] and AngII
[(AngII)-(control)] alone as an index to estimate the additive
effect, it was calculated that L-NAME and Rp-8-Br-cGMP-S inhibited
the additive effect by 87 (P<0.01, n=4) and 91% (P<0.01,
n=4), respectively.
Discussion
The major finding of the present study was that ET-1
upregulated the gene expression and secretion of APN in NRVMs, and
the NO/cGMP/PKG signalling pathways mediated this upregulation.
ET-1 and AngII caused and additive effect on APN activation.
The APN secretion data in the present study are
compatible with those in a previous report in 3T3-L1 adipocytes
(12). The authors reported that
treatment with ET-1 for 4 h induced the significant stimulation of
APN secretion; however, this effect was lost after 8 h of
treatment. In another previous study in 3T3-L1 adipocytes (13), ET-1 exposure for 24 h led to lower
levels of APN secretion compared with the relative control. This
different secretion response between NRVMs and 3T3-L1 adipocytes
may be due to the different cell type.
Recently, Yin et al (14) suggested that plasma ET-1 and APN
increased with the severity of heart failure. APN protein is
upregulated in cardiac tissue and is correlated with increased
serum concentrations of ET-1, the incubation of HCM with ET-1
significantly increased the protein expression of APN in a time-
and dose-dependent manner. However, the possible mechanism
responsible for the action of ET-1 was not discussed. The present
study revealed that ET-1, acting via ETAR and
ETBR, upregulated APN gene expression. Selective
antagonists of ETAR, ETBR, BQ610 and BQ788
decreased the mRNA expression of APN via inactivation of the
NO/cGMP/PKG pathway, since its effect was prevented through the
incubation of the cells with the NOS inhibitor, L-NAME, and cGMP
antagonist analogue, Rp-8-Br-cGMP-S.
Skurk et al (15) reported that APN is expressed in the
hearts of patients with dilated cardiomyopathy (DCM), independent
of their plasma levels; there is a local paracrine cardiac APN
system involved in the pathogenesis of DCM. Cardiomyocyte-derived
APN may have a paracrine effect on the cardiovascular system; APN
serves a protective role in suppressing the development of cardiac
hypertrophy and inhibits cardiovascular disease progression
(16). It is very likely that
upregulated ET and AngII in CHF induced cardiac APN expression,
exerting its protective effects on the cardiovascular system and
increasing the levels of APN in the peripheral circulation.
ET-1 and AngII serve important roles in the
structural and electrical remodelling of the myocardium. They
compose a mutually reciprocal signalling network in the myocardium
and have certain reciprocal associations at the receptor level.
AngII has been reported to upregulate the expression of
ETBR via the AT1R (17). Although our previous study (10) demonstrated that AngII upregulated the
expression of APN in NRVMs via the AT2R, the present
study demonstrated that selective antagonists for ETAR
and ETBR, as well as AT2R diminished the
additive roles of ET-1 and AngII on APN activation, indicating that
at the receptor level, ET-1 and AngII have a mutually reciprocal
association that indirectly activates the NO/cGMP/PKG signalling
pathway.
In conclusion, the present study demonstrated that
ET-1 upregulated the gene expression and secretion of APN in NRVMs,
and that ET-1 and AngII serve additive roles in APN activation in
NRVMs through the common NO/cGMP/PKG signalling pathway. Future
studies will focus on the evaluation of how APN affects the heart
and how APN can be used as a therapeutic treatment for heart
disease.
Acknowledgements
This present study was supported by grants from the
National Natural Science Foundation of China (nos. 81400217 and
81570345), the nature science foundation of Hebei Province (no.
H2014206389) and Hebei Provincial Health Bureau of medical key
subject (no. 20130156).
References
|
1
|
Correa MV, Nolly MB, Caldiz CI, de
Cingolani GE, Cingolani HE and Ennis IL: Endogenous endothelin 1
mediates angiotensin II-induced hypertrophy in electrically paced
cardiac myocytes through EGFR transactivation, reactive oxygen
species and NHE-1. Pflugers Arch. 466:1819–1830. 2014.PubMed/NCBI
|
|
2
|
de Jonge HW, Dekkers DH, Houtsmuller AB,
Sharma HS and Lamers JM: Differential signaling and hypertrophic
responses in cyclically stretched vs endothelin-1 stimulated
neonatal rat cardiomyocytes. Cell Biochem Biophys. 47:21–32. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin YJ, Kwok CF, Juan CC, Hsu YP, Shih KC,
Chen CC and Ho LT: Angiotensin II enhances endothelin-1-induced
vasoconstriction through upregulating endothelin type A receptor.
Biochem Biophys Res Commun. 451:263–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolf AM, Wolf D, Avila MA, Moschen AR,
Berasain C, Enrich B, Rumpold H and Tilg H: Up-regulation of the
anti-inflammatory adipokine adiponectin in acute liver failure in
mice. J Hepatol. 44:537–543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piñeiro R, Iglesias MJ, Gallego R, Raghay
K, Eiras S, Rubio J, Diéguez C, Gualillo O, González-Juanatey JR
and Lago F: Adiponectin is synthesized and secreted by human and
murine cardiomyocytes. FEBS Lett. 579:5163–5169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kistorp C, Faber J, Galatius S, Gustafsson
F, Frystyk J, Flyvbjerg A and Hildebrandt P: Plasma adiponectin,
body mass index, and mortality in patients with chronic heart
failure. Circulation. 112:1756–1762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
George J, Patal S, Wexler D, Sharabi Y,
Peleg E, Kamari Y, Grossman E, Sheps D, Keren G and Roth A:
Circulating adiponectin levels predict outcome in patients with
severe congestive heart failure. Heart. 92:1420–1424. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wannamethee SG, Whincup PH, Lennon L and
Sattar N: Circulating adiponectin levels and mortality in elderly
men with and without cardiovascular disease and heart failure. Arch
Intern Med. 167:1510–1517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takano H, Obata JE, Kodama Y, Kitta Y,
Nakamura T, Mende A, Kawabata K, Saito Y, Fujioka D, Kobayashi T,
et al: Adiponectin is released from the heart in patients with
heart failure. Int J Cardiol. 132:221–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo B, Li Y, Han R, Zhou H and Wang M:
Angiotensin II upregulation of cardiomyocyte adiponectin production
is nitric oxide/cyclic GMP dependent. Am J Med Sci. 341:350–355.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Juan CC, Chuang TY, Chang CL, Huang SW and
Ho LT: Endothelin-1 regulates adiponectin gene expression and
secretion in 3T3-L1 adipocytes via distinct signaling pathways.
Endocrinology. 148:1835–1842. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bedi D, Clarke KJ, Dennis JC, Zhong Q,
Brunson BL, Morrison EE and Judd RL: Endothelin-1 inhibits
adiponectin secretion through a phosphatidylinositol
4,5-bisphosphate/actin-dependent mechanism. Biochem Biophys Res
Commun. 345:332–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin WH, Chen YH, Wei J, Jen HL, Huang WP,
Young MS, Chen DC and Liu PL: Associations between endothelin-1 and
adiponectin in chronic heart failure. Cardiology. 118:207–216.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Skurk C, Wittchen F, Suckau L, Witt H,
Noutsias M, Fechner H, Schultheiss HP and Poller W: Description of
a local cardiac adiponectin system and its deregulation in dilated
cardiomyopathy. Eur Heart J. 29:1168–1180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li
R, Wang X, Koch WJ and Ma XL: Cardiomyocyte-derived adiponectin is
biologically active in protecting against myocardial
ischemia-reperfusion injury. Am J Physiol Endocrinol Metab.
298:E663–E670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanno K, Hirata Y, Tsujino M, Imai T,
Shichiri M, Ito H and Marumo F: Up-regulation of ETB receptor
subtype mRNA by angiotensin II in rat cardiomyocytes. Biochem
Biophys Res Commun. 194:1282–1287. 1993. View Article : Google Scholar : PubMed/NCBI
|