Introduction
It is well known that blood contains circulating
cell-free DNA (cfDNA) (1–9). There are several hypotheses concerning
the origin of cfDNA, including the cell death hypothesis and the
hypothesis that living cells actively secrete DNA as signaling
molecules. CfDNA is a potential marker for pathological processes
and is of importance for diagnostic purposes (1–9).
cfDNA in a fetus was first reported to be present in
maternal blood in 1997, when Lo et al (10) demonstrated that maternal plasma
contains Y-chromosomal sequence of the male fetus. This discovery
led to development of new non-invasive prenatal diagnosis
techniques. cfDNA analysis detects genetic abnormalities of the
fetus (11). In 2014, whole fetal
genome was sequenced from cfDNA in maternal plasma (12).
Apart from genetic abnormalities of the fetus, cfDNA
analysis allows monitoring pregnancy-related complications such as
preeclampsia and preterm birth (13–15). In this
case instead of a search for gene mutations, concentration of
general cfDNA or fetal cfDNA in maternal plasma is analyzed. Fetal
cfDNA comprises up to 10% of all cfDNA in maternal plasma and is
believed to be released by necrotic and apoptotic cells of placenta
(16).
Intrauterine growth restriction (IUGR) is a complex
disorder of pregnancy with varying etiology. It is characterized by
the failure of the fetus to achieve its normal growth potential and
is associated with perinatal morbidity and mortality. Long-term
consequences include a higher risk of cardiovascular diseases
(13,17). One of the primary causes of IUGR is
placental dysfunction that often leads to increased death of
placental cells. Death of cells of placenta results in increased
concentration of cell-free fetal DNA in maternal plasma,
contributing to the overall cfDNA concentration.
Data concerning changes in concentrations of fetal
and maternal cfDNA in cases of IUGR are scarce and controversial.
In the first research (16), authors
did not find any discrepancy between cfDNA concentrations in cases
of normal pregnancies and the ones with IUGR. Later studies
indicated that cfDNA concentrations are increased in cases of
pregnancies with IUGR (18–21). It is of importance that in these
studies the authors did not take into account the processes of
elimination of cfDNA from plasma. Increase in cfDNA concentration
as a consequence of cell death leads to activation of cfDNA
eliminating system, thus, effectively decreasing the concentration
of cfDNA. The authors have observed the effect of significant
decrease of cfDNA concentration in cases of chronic processes (that
are accompanied by increased cell death) such as cardiovascular
diseases (22). The same effect occurs
in people chronically working with radiation. This can be due to
the activity of DNase I - one of the components of cfDNA
elimination system (23).
In the current study, the authors assessed cfDNA
concentration changes simultaneously with DNase I activity in
plasma of non-pregnant women, women with normal pregnancies and
pregnancies complicated by IUGR. The results have demonstrated that
cfDNA concentration in maternal blood plasma is not a reliable
marker of IUGR in third trimester of pregnancy. Simultaneous
analysis of cfDNA concentration and DNase I activity can, however,
provide valuable information about IUGR development.
Materials and methods
Sample of participants
The investigation was carried out in accordance with
the latest version of the Declaration of Helsinki and approved by
the Regional Ethics Committee of Research Centre For Medical
Genetics (Moscow, Russia; approval no. 5). All participants signed
an informed written consent to participate after the nature of the
procedures had been fully explained.
Analyzed sample consisted of three groups of women
of relatively the same age (22–40 years old) living in Moscow in
the same social environment. The groups are as follows: Group I,
non-pregnant, healthy women (n=40), consisting of students and
employees of the medical facility (volunteers); group II, women
with normal pregnancy >37 weeks (n=40), that later bore healthy
children without signs of hypoxia or hypotrophy; group III, women
with pregnancy complicated with IUGR >30 weeks, that later on
bore children with IUGR signs (n=40). A total 5 ml of blood was
collected under strict aseptic conditions from a peripheral vein of
the subject using a syringe flushed with heparin (0.1 ml/5 ml
blood).
Prenatal screening
Fetal development during pregnancy was screened with
help of ultrasound anthropometry. Biparietal diameter of the head,
chest circumference, abdominal circumference and femur length were
measured. When these measurements were behind the population mean,
by 2 weeks IUGR of stage 1 was diagnosed; 2 to 4 weeks stage 2
IUGR; more than 4 weeks stage 3 of IUGR. The final IUGR status was
diagnosed after birth based on body mass. Normative parameters were
between 75 and 25 percentile curves, first stage of IUGR 25 curve
10; second stage of IUGR 10 curve 3; third stage below curve 3. In
addition to this, the weight-for-height index was used: Healthy,
>60; IUGR stage 1, 55–60; stage 2, 50–55; stage 3, <50.
The functional state of the fetuses during pregnancy
and labor was assessed with help of functional diagnostic methods
such as cardiotocography and dopplerometry of uterine, umbilical
and fetal blood vessels.
Plasma cfDNA concentration (cfDNA
index)
Cells were removed from the blood by centrifugation
at 460 × g, followed by mixing of 3 ml plasma with 0.3 ml of the
solution containing 1% sodium lauroyl sarcosinate, 0.02 M EDTA, and
75 µg/ml RNase A (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
incubation for 45 min, then the treatment with proteinase K (200
µg/ml; Promega Corporation, Madison, WI, USA) for 24 h at 37°C.
Following two cycles of the purification with saturated phenolic
solution, DNA fragments were precipitated by adding two volumes of
ethanol in the presence of 2 M ammonium acetate. The precipitate
was then washed with 75% ethanol twice, then dried and dissolved in
water. The concentration of DNA (cfDNA index) was determined by
measuring fluorescence intensity on ‘LS 55’ (PerkinElmer, Inc.,
Waltham, MA, USA) spectrometer after DNA staining with the
PicoGreen (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The relative standard error of the index cfDNA was 10±4%.
DNase I activity in plasma
Levels of DNase I activity in plasma samples were
measured by the single radial enzyme diffusion method, as described
previously (24). The assay method can
determine pg to fg quantities of DNase I in 1 ml serum samples
within 30 min. One unit of enzyme assayed corresponds to 1 ng
purified human DNase I. Relative standard error of the index DNase
I was 15±5%.
Statistical analysis and modeling
All reported results were reproduced at least three
times as independent biological replicates. The significance of the
observed differences was analyzed using the non-parametric
Mann-Whitney U-tests. P<0.05 was considered to indicate a
statistically significant difference. Data were analyzed with
StatPlus2007 Professional software (http://www.analystsoft.com/).
ApoModel2 software was written by Roman Veiko
(Research Centre For Medical Genetics, Moscow, Russia) in Object
Pascal language with the help of an integrated media Turbo Delphi
2006 development. Software designed to simulate changes for the
cfDNA under the influence of external and internal factors (e.g.,
exposure to radiation or pathology associated with the induction of
apoptosis in cells in the body). The input data of the model were:
The level of damages in the cell; the level of damages in the cell
after reaching which it enters apoptosis; an increase in the number
of lesions after exposure; the amount of DNA in the cell (in pg);
the number of cells; the initial percentage of cells with damage;
the number of damaging actions; DNA percentage remaining in the
cell after degradation.
Functional input parameters were: The time function
of reducing the amount of extracellular DNA as a result of
elimination; function for apoptotic cells release of DNA into the
extracellular environment. Functions can be set using a special
formula designer and generator values. The output parameters of the
model were the magazine states of the system, which were created
during the simulation. The magazine reflected the number of dead
cells, the number of surviving cells and the amount of
extracellular and intracellular DNA.
Results
cfDNA concentration (cfDNA index)
CfDNA was isolated from the samples of blood plasma
of 120 women. DNA concentrations were determined using the DNA
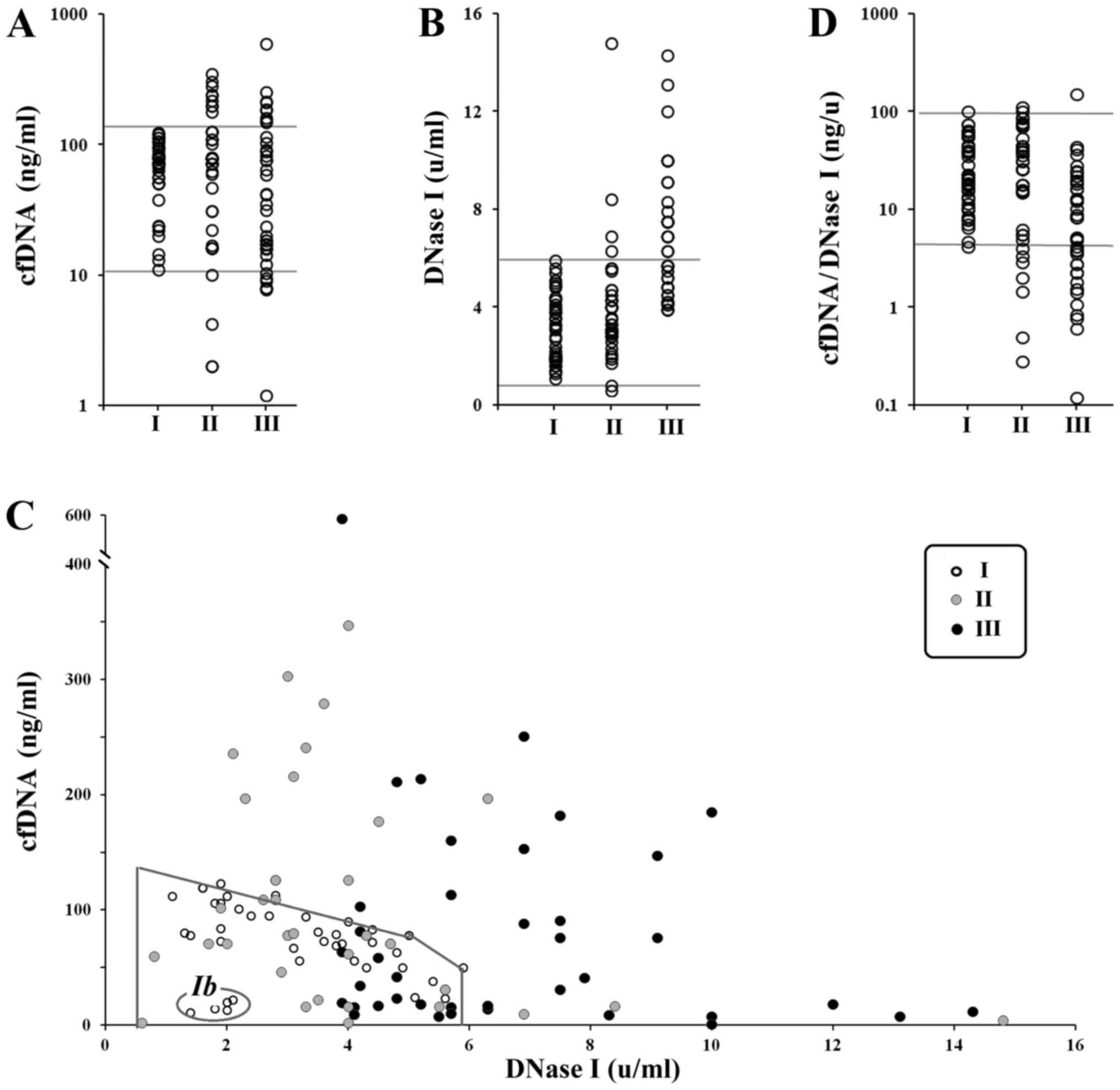
binding dye PicoGreen. Fig. 1A
presents cfDNA concentration in the blood plasma (Table I for descriptive statistics). Group I
(n=40), non-pregnant women; group II (n=40), women with normal
pregnancy (gestational age 30–41 weeks); and group III (n=40),
women with pregnancies complicated by IUGR (gestational age 30–41
weeks).
 | Table I.Statistics for the parameters defined
in the study. |
Table I.
Statistics for the parameters defined
in the study.
| Parameter | Cohort I (n=40) | Cohort II (n=40) | Cohort III
(n=40) |
|---|
| cfDNA, ng/ml |
| Mean | 72±32 | 101±88 |
83±109 |
| Var | 0.45 | 0.87 | 1.32 |
|
Median | 76 | 78 | 41 |
|
Interval | 11–123 | 2–347 | 1–596 |
| DNase I, U/ml |
| Mean | 3.1±1.4 |
3.9±2.3 | 6.7±2.6 |
| Var | 0.45 | 0.60 | 0.39 |
|
Median | 2.9 | 3.4 | 5.7 |
|
Interval | 1.1–5.9 | 0.6–14.8 | 3.9–14.3 |
| cfDNA/DNase I,
ng/µl |
| Mean | 30±24 |
34±30 | 15±25 |
| Var | 0.79 | 0.91 | 1.7 |
|
Median | 21 | 26 | 8.3 |
|
Interval | 4–102 | 0.3–112 | 0.1–153 |
There was no statistical difference between the
three groups regarding cfDNA index (P>0.05). However, in nine
women of groups II and III (22%), the authors observed elevated
concentration of сfDNA as compared to the controls (Fig. 1A). In the third group, cfDNA
concentrations were somewhat lower than in group II. If the single
highest outlier is removed from group III (596 ng/ml), there is a
persistent decrease in cfDNA concentration in group III compared to
group II (P<0.05; NII=40, NIII=39).
Thus, the expected increase of cfDNA concentration
in the blood of pregnant women compared to non-pregnant was not
observed. Moreover, in troubled pregnancies, where a significant
increase in cfDNA concentration was expected, the authors
discovered a decrease in the concentration of cfDNA compared to
healthy pregnancy. One of the primary reasons of cfDNA
concentration decrease can be activation of the cfDNA elimination
system of blood. One of the factors here is the activity of the
blood plasma enzyme responsible for the cfDNA fragmentation, DNase
I.
DNase I activity (DNase I index)
DNase I activity in blood plasma of 120 women was
determined by the standard radial diffusion method (24). The DNase I indices for the groups are
provided in Fig. 1A and in Table I. No significant difference was
detected between the groups I and II by this index (P>0.05).
The third group, however, differs from group I
(P<0.0000001) and group II (P<0.00001). Thus, plasma of
pregnant women with IUGR contains highly active DNase I compared to
blood plasma of healthy pregnant or non-pregnant women. A total of
19 out of 40 (47.5%) women from the group III had very high
activity of DNase I in their blood, that were not present in the
non-pregnant women sample. In group II, only 4 (10%) patients had
increased DNase I activity compared to non-pregnant women.
Dependence of cfDNA concentration on
DNase I activity
Dependence of cfDNA concentration on DNase I
activity is shown of Fig. 1C. Marked
area is the field of data for non-pregnant women (group I).
Non-pregnant women can be divided into two groups: Ia and Ib. Group
Ia consists of 35 women (87.5%) and is characterized by linear
dependence of cfDNA concentration on DNase I activity. Ib consists
of 5 women who had low cfDNA concentration (11–22 ng/ml) and low
DNase I activity (1.4–2.1 U/ml).
Marked area on the Fig.
1C corresponding to non-pregnant women included 25 (subgroup
IIa) and 16 (IIIa) women from groups II and III. It is of
importance, that the activity of DNase I for the subgroup IIIa in
this area is higher than the activity of DNase I for groups I and
IIa (P>0.05).
All the other patients from groups II (subgroup IIb)
and III (subgroup IIIb) are beyond the marked area that contains
all the non-pregnant women. For these women, the authors identified
a linear dependence between cfDNA concentration and DNase I
activity. Linear regression equations (cfDNA=A-b*DNase I)
reflecting the dependence in all three groups are shown below.
Ia) cfDNA=123-13*DNase I (n=35), k=−0.8,
P<<0,0001; IIb) cfDNA=275-21*DNase I (n=15), k=−0.7,
P<0.01; IIIb) cfDNA=354-30*DNase I (n=24), k=−0.62,
P<0.002.
Coefficient A in the equation is the hypothetic
average cfDNA concentration in absence of DNase I (DNase I=0). This
level reflects the amount of dying cells under the same conditions.
Thus, the levels of cell death are: Group I (non-pregnant) <
group II (healthy pregnancy) < group III (IUGR).
Coefficient b reflects the rate of cfDNA elimination
when the DNase I is activated. This coefficient is increasing in a
row: group I (non-pregnant) < group II (healthy pregnancy) <
group III (IUGR).
Increase in DNase I activity affects cfDNA
concentration in blood plasma of group III patients much stronger
than patients of other groups. The authors introduced an index that
reflects the amount of cfDNA for one unit of DNase I activity:
cfDNA/DNase I (Fig. 1D and Table I for descriptive statistics). The
difference between cfDNA/DNase I indices for samples I and II is
not statistically significant (P>0.05). Sample III differs from
sample I (P<0.001) and sample II (P<0.001).
Dependence of IUGR features on cfDNA,
DNase I and cfDNA/DNase I
IUGR can be characterized by objective parameters
such as mass and height of the fetus and mass-to-height index. The
authors analyzed the dependence of these characteristics and
duration of gestation on cfDNA concentration, DNase I activity and
the cfDNA/DNase I index with help of linear regression method
(Table II). cfDNA concentration and
cfDNA/DNase I index correlate negatively with all characteristics
of the fetus. The higher the cfDNA concentration and the
cfDNA/DNase I index are, the lower are the mass and height of the
fetus and the shorter is duration of gestation. DNase I activity
does not correlate with these factors (Table II).
 | Table II.Linear regression analysis of fetus
features and pregnancy duration dependence on cfDNA concentration,
DNase I activity and cfDNA/DNase I index for group III (IUGR). |
Table II.
Linear regression analysis of fetus
features and pregnancy duration dependence on cfDNA concentration,
DNase I activity and cfDNA/DNase I index for group III (IUGR).
|
| Fetus mass
(600–3,270 g) | Fetus height (29–52
cm) | Mass-to-height
(21–64 g/cm) | Pregnancy duration
(30–41 weeks) |
|---|
|
|
|
|
|
|
|---|
| Parameter | k | P-value | k | P-value | k | P-value | k | P-value |
|---|
| cfDNA | −0.63 | <0.0001 |
−0.74 | <0.0001 |
−0.63 | <0.0001 | −0.6 | <0.0001 |
| DNase I |
0.01 | 0.92 |
0.02 | 0.8 |
0.01 | 0.8 |
0.1 | 0.6 |
| cfDNA/DNase I | −0.55 |
0.0004 |
−0.62 | <0.0001 |
−0.54 |
0.0005 | −0.5 | 0.001 |
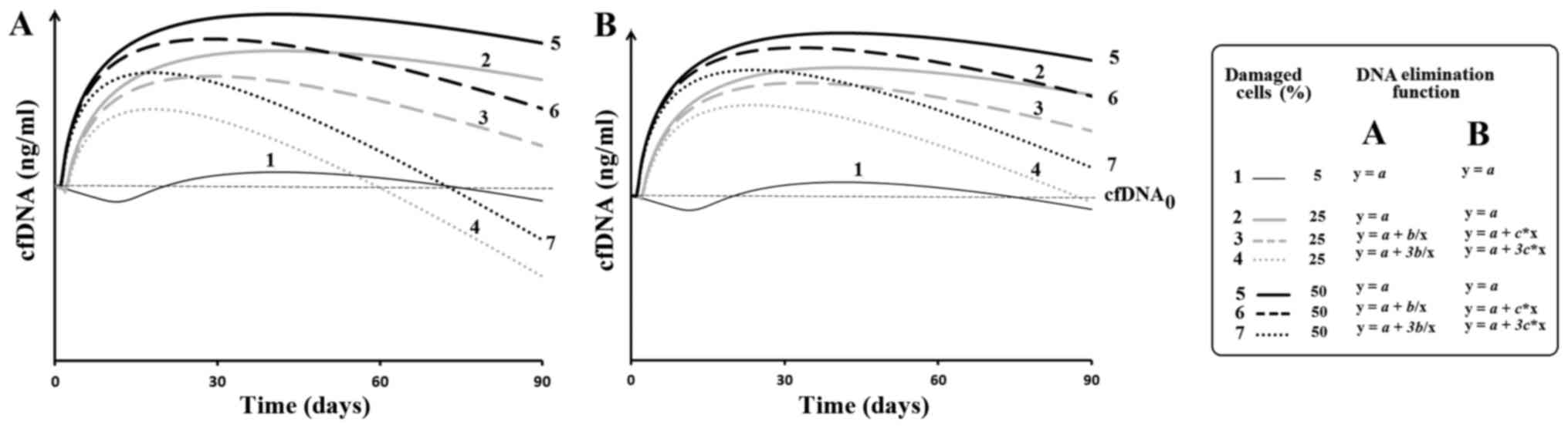
Modeling of changes of cfDNA
concentration
Apomodel 2 software allowed the authors to set the
level of injury and subsequent apoptosis, to analyze the amount of
DNA entering into the extracellular medium from the dead cells and
to take into account the processes that lead to the elimination of
cfDNA. It is assumed that a damaging agent is acting on the cells.
If the damage is above a given level, the cell is destroyed and DNA
enters into the extracellular medium. cfDNA is constantly removed
from the medium. Fig. 2 provides the
results of modeling. The dependence of the amount of cfDNA in the
medium from the time of observation. The time interval of
observation was 3 months. For the function of the DNA release from
the dead cells arbitrarily accepted expression: y=1/x, where x=time
after a regular exposure.
Discussion
It is a matter of common knowledge that various
endogenous and exogenous factors can affect the cell death rate in
the organism. Dying cells release DNA into the bloodstream
contributing to the pool of circulating cfDNA (1–9). cfDNA is an
active biological agent. For example, cfDNA can alter hemodynamic
properties of the blood (2,25). It can induce oxidative stress,
stimulate proinflammatory cytokine synthesis and induce sterile
inflammation (26). The organism
defense against cfDNA is the cfDNA elimination system of the blood.
cfDNA elimination system knockout in animals causes lupus-like
autoimmune disease (27,28). Healthy non-pregnant women have a
negative correlation between cfDNA concentration in blood and DNase
I activity (Fig. 1C). Apparently,
DNase I activity is the main factor regulating elimination of the
cfDNA from the bloodstream in this case. As been indicated
previously, ~10% of people have low cfDNA concentration and low
DNase I activity (23). These people
have low level of cell apoptosis in their body. People from this
group are more resistant to external negative factors such as
chronic ionizing radiation. Possible reasons for low cell death may
include increased activity of antioxidant and reparation systems.
The group Ib (Fig. 1C) consists of
women who have low level of cell death.
Modeling of the basic processes that lead to a
change in the amount of circulating DNA shows that, despite a
10-fold increase in the level of cell damage, the authors observe a
significant reduction in amount of cfDNA due to activation of the
cfDNA elimination (Fig. 2). The
modeling results correspond well with the previously obtained
experimental data. It has been reported that chronic human exposure
to ionizing radiation leads to a significant reduction in the cfDNA
concentration while the level of cell damage remains above the
control level (23). In addition, it
was demonstrated that chronic cardiovascular diseases also lead to
a decrease in total cfDNA concentration, while acute myocardial
infarction accompanied by a sharp increase of the DNA in the first
day of the disease (22).
In case of pathological problems with placenta that
is associated with IUGR, cells of the placenta have increased cell
death rate due to both apoptosis and necrosis (28,29). cfDNA
of the dead cells of the placenta enter maternal bloodstream,
increasing cfDNA concentration. This placental cfDNA is believed to
increase apoptosis in maternal cells, thus, causing the cfDNA
concentration to increase even more. Thus, in cases of IUGR it is
logical to expect increase of total cfDNA concentration in maternal
blood plasma. Apparently, this happens during early stages of the
pathological processes, before the defense mechanism of cfDNA
elimination is activated (Fig. 2).
Increased DNase I activity is a sign of pathological
process, that is accompanied by cell death. The authors have
previously found it to be true in cases of heart attack, ischemic
heart disease (22) and in patients
that are chronically subjected to ionizing radiation (23). In cases of pregnancy with IUGR, the
results indicated a persistent increase in DNase I activity in
blood plasma that is an evidence of increased cell death rate in
the patient's organism. Interestingly, subgroup IIa and IIIa
exhibited abnormally abrupt decrease in cfDNA level under
relatively low activity of DNase I compared to group I. In cases of
these women cfDNA concentration does not depend on DNase I
activity. In the patients from subgroups IIa and IIIa the decrease
in cfDNA concentration as the DNase I activity increases is more
abrupt than in group I.
All these facts are suggesting that DNase I is not
the single component of the cfDNA eliminating system during
pregnancy. Other important factors may be cfDNA-antibody binding
and increased kidney function that eliminates short cfDNA
fragments. In addition, expression of other cfDNA hydrolyzing
enzymes in blood plasma of pregnant women can be increased. This
assumption requires further experimental investigation.
High activity of cfDNA elimination system during
pregnancy distorts the results and makes it difficult to analyze
cfDNA concentration, especially in cases of IUGR. This may be a
possible explanation why the data concerning cfDNA concentration in
blood of pregnant women with pathologies are so controversial
(1). However, if three factors (cfDNA
concentration, DNase I activity, cfDNA/DNase I index) are taken
into account, it is possible to develop a screening method that
will allow monitoring cell death rate in pregnant patients. These
data informs us about cell death rate and cfDNA elimination system
activity.
References
|
1
|
Gahan PB and Stroun M: The Biology of
Circulating Nucleic Acids in Plasma and Serum (CNAPS)Extracellular
Nucleic Acids. Kikuchi Yo and Rykova EY: Springer; Berlin: pp.
167–189. 2010, View Article : Google Scholar
|
|
2
|
Mittra I, Nair NK and Mishra PK: Nucleic
acids in circulation: Are they harmful to the host? J Biosci.
37:301–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rykova EY, Morozkin ES, Ponomaryova AA,
Loseva EM, Zaporozhchenko IA, Cherdyntseva NV, Vlassov VV and
Laktionov PP: Cell-free and cell-bound circulating nucleic acid
complexes: Mechanisms of generation, concentration and content.
Expert Opin Biol Ther. 12 Suppl 1:S141–S153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Vaart M and Pretorius PJ:
Characterization of circulating DNA in healthy human plasma. Clin
Chim Acta. 395:1862008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Vaart M and Pretorius PJ:
Circulating DNA. Its origin and fluctuation. Ann N Y Acad Sci.
1137:18–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Vaart M and Pretorius PJ: The
origin of circulating free DNA. Clin Chem. 53:22152007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gahan PB: Biology of circulating nucleic
acids and possible roles in diagnosis and treatment in diabetes and
cancer. Infect Disord Drug Targets. 12:360–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gahan PB, Anker P and Stroun M: Metabolic
DNA as the origin of spontaneously released DNA? Ann N Y Acad Sci.
1137:7–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gahan PB and Stroun M: The virtosome-a
novel cytosolic informative entity and intercellular messenger.
Cell Biochem Funct. 28:529–538. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo YM, Corbetta N, Chamberlain PF, Rai V,
Sargent IL, Redman CW and Wainscoat JS: Presence of fetal DNA in
maternal plasma and serum. Lancet. 350:485–487. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gahan PB: Circulating nucleic acids in
plasma and serum: Applications in diagnostic techniques for
noninvasive prenatal diagnosis. Int J Womens Health. 5:177–186.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bianchi DW and Wilkins-Haug L: Integration
of noninvasive DNA testing for aneuploidy into prenatal care: What
has happened since the rubber met the road? Clin Chem. 60:78–87.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sifakis S, Koukou Z and Spandidos DA:
Cell-free fetal DNA and pregnancy-related complications (Review).
Mol Med Rep. 11:2367–2372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hahn S, Rusterholz C, Hösli I and Lapaire
O: Cell-free nucleic acids as potential markers for preeclampsia.
Placenta. 32 Suppl:S17–S20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dugoff L, Barberio A, Whittaker PG,
Schwartz N, Sehdev H and Bastek JA: Cell-free DNA fetal fraction
and preterm birth. Am J Obstet Gynecol. 215:231.e1–7. 2016.
View Article : Google Scholar
|
|
16
|
Sekizawa A, Jimbo M, Saito H, Iwasaki M,
Matsuoka R, Okai T and Farina A: Cell-free fetal DNA in the plasma
of pregnant women with severe fetal growth restriction. Am J Obstet
Gynecol. 188:480–484. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gourvas V, Dalpa E, Konstantinidou A,
Vrachnis N, Spandidos DA and Sifakis S: Angiogenic factors in
placentas from pregnancies complicated by fetal growth restriction
(Review). Mol Med Rep. 6:23–27. 2012.PubMed/NCBI
|
|
18
|
Smid M, Galbiati S, Lojacono A, Valsecchi
L, Platto C, Cavoretto P, Calza S, Ferrari A, Ferrari M and
Cremonesi L: Correlation of fetal DNA levels in maternal plasma
with Doppler status in pathological pregnancies. Prenat Diagn.
26:785–790. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alberry MS, Maddocks DG, Hadi MA, Metawi
H, Hunt LP, Abdel-Fattah SA, Avent ND and Soothill PW:
Quantification of cell free fetal DNA in maternal plasma in normal
pregnancies and in pregnancies with placental dysfunction. Am J
Obstet Gynecol. 200:98.e1–98.e6. 2009. View Article : Google Scholar
|
|
20
|
Al Nakib M, Desbrière R, Bonello N,
Bretelle F, Boubli L, Gabert J and Levy-Mozziconacci A: Total and
fetal cell-free DNA analysis in maternal blood as markers of
placental insufficiency in intrauterine growth restriction. Fetal
Diagn Ther. 26:24–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veĭko NN, Bulycheva NV, Roginko OA, Veĭko
RV, Ershova ES, Kozdoba OA, Kuz'min VA, Vinogradov AM, Iudin AA and
Speranskiĭ AI: Ribosomal repeat in the cell free DNA as a marker
for cell death. Biomed Khim. 54:78–93. 2008.(In Russian).
PubMed/NCBI
|
|
22
|
Korzeneva IB, Kostuyk SV, Ershova LS,
Osipov AN, Zhuravleva VF, Pankratova GV, Porokhovnik LN and Veiko
NN: Human circulating plasma DNA significantly decreases while
lymphocyte DNA damage increases under chronic occupational exposure
to low-dose gamma-neutron and tritium β-radiation. Mutat Res.
779:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Macanovic M and Lachmann PJ: Measurement
of deoxyribonuclease I (DNase) in the serum and urine of systemic
lupus erythematosus (SLE)-prone NZB/NZW mice by a new radial enzyme
diffusion assay. Clin Exp Immunol. 108:220–226. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gannushkina IV and Konorova IL: Cell-free
plasmic DNA as a blood factor determining hemodynamics in health
and in vascular pathology]. Patol Fiziol Eksp Ter. 2–10. 2008.(In
Russian). PubMed/NCBI
|
|
25
|
Speranskii AI, Kostyuk SV, Veiko NN and
Kalashnikova EA: Enrichment of extracellular DNA from the
cultivation medium of human peripheral blood mononuclears with
genomic CpG rich fragments results in increased cell production of
IL-6 and TNF-a via activation of the NF-kB signaling pathway.
Biomed Khim. 62:331–340. 2016.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seredkina N, Zykova SN and Rekvig OP:
Progression of murine lupus nephritis is linked to acquired renal
Dnase1 deficiency and not to up-regulated apoptosis. Am J Pathol.
175:97–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fenton K, Fismen S, Hedberg A, Seredkina
N, Fenton C, Mortensen ES and Rekvig OP: Anti-dsDNA antibodies
promote initiation, and acquired loss of renal Dnase1 promotes
progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice. PLoS
One. 4:e84742009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kolialexi A, Tsangaris GT, Antsaklis A,
Tzortzatou F, Amentas C, Koratzis A and Mavrou A: Apoptosis in
maternal peripheral blood during pregnancy. Fetal Diagn Ther.
16:32–37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kolialexi A, Tsangaris GT, Mavrou A,
Antsaklis A, Tzortzatou F, Touliatou V and Metaxotou C: Use of
annexin V antibody to identify apoptotic cells during pregnancy.
Ann N Y Acad Sci. 945:145–150. 2001. View Article : Google Scholar : PubMed/NCBI
|