Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
chronic liver disease characterized by hepatic fat accumulation in
patients with little to no history of alcohol consumption (1). Up to 30% of the worldwide population is
affected by NAFLD, among which populations in developed countries
are particularly affected, including those in the United States
(2,3).
The occurrence and development of NAFLD are associated with
metabolic syndrome, which comprises a group of disorders including
obesity, increased plasma triglycerides (TGs) and reduced insulin
sensitivity (4). More specifically,
Li et al (5) reported that
obesity may be an independent risk factor for NAFLD. NAFLD is
considered a public health concern due to its potential to progress
to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis and
even hepatocellular carcinoma (6,7). The
primary therapy for NAFLD is lifestyle management to prevent
obesity; however, long-term changes in eating habits and weight
loss are difficult to achieve and maintain (8). Due to the lack of effective therapy
options and consequences of disease progression, the development of
novel pharmacological treatments for NAFLD should be actively
pursued.
Orlistat is among the few types of over-the-counter
diet pill available worldwide, and is a potent and long-lasting
gastrointestinal lipase inhibitor that directly blocks intestinal
absorption of dietary fat, and thus promotes weight loss (9,10). In
clinical trials, patients treated with orlistat exhibited lower
fasting glucose, fasting insulin, TGs, insulin resistance as
assessed via the homeostasis model assessment of insulin resistance
(HOMA-IR) and body mass index (BMI), along with greater weight loss
compared with those treated with placebo (11–13).
Furthermore, orlistat was demonstrated to reduce transaminase
levels (14), commonly used as
biomarkers to assess liver damage (14,15). As an
inhibitor of gastroenteric lipase, the long-term application of
orlistat is considered to have no toxic side effects (16); additionally, any adverse effects in
the contexts of cardiovascular outcome and carcinogenesis are as
yet unreported.
For these reasons, orlistat is expected to be an
effective drug for the management of NAFLD; however, its efficacy
remains controversial. Zelber-Sagi et al (17) identified that, compared with placebo,
orlistat did not cause a significant reduction in body weight in
patients with NAFLD. Thus, the purpose of the present systematic
review and meta-analysis was to evaluate the efficacy and safety of
orlistat in patients with NAFLD or NASH.
Materials and methods
Protocol
The meta-analysis was conducted by two investigators
independently. Any differences in opinion were resolved by
consensual agreement. The methods used adhered to the criteria of
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses guidelines (18).
Literature search strategy
In August 2017, a computer-assisted search was
performed of PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Embase
(https://www.embase.com/#search),
Cochrane Library (https://onlinelibrary-wiley-com.webproxy.potsdam.edu/cochranelibrary/search),
Web of Science (http://isiknowledge.com) and Wan Fang data (http://www.wanfangdata.com.cn/index.html) using the
following combinations of terms: ‘THLP’ or ‘tetrahydrolipastatin’
or ‘Xenical’ or ‘Roche brand of orlistat’ or ‘Hoffmann-La Roche
brand of orlistat’ or ‘Alli’ or ‘GlaxoSmithKline brand of orlistat’
and ‘non-alcoholic fatty liver disease’ or ‘NAFLD’ or ‘nonalcoholic
fatty liver disease’ or ‘fatty liver, nonalcoholic’ or ‘fatty
livers, nonalcoholic’ or ‘liver, nonalcoholic fatty’ or ‘livers,
nonalcoholic fatty’ or ‘nonalcoholic fatty liver’ or ‘nonalcoholic
fatty livers’ or ‘nonalcoholic steatohepatitis’ or ‘nonalcoholic
steatohepatitides’ or ‘steatohepatitides, nonalcoholic’ or
‘steatohepatitis, nonalcoholic’.
Inclusion and exclusion criteria
Database search results were screened using the
following inclusion criteria: i) NAFLD or NASH diagnosed using
results of liver biopsy or imaging; ii) orlistat prescribed to
patients with NAFLD or NASH; iii) if present, controls were
placebo, energy-controlled diet and/or vitamin E; and iv) the study
classified as a randomized controlled trial (RCT) or an
observational study. Exclusion criteria were: i) The use of animal
or cell models; ii) orlistat prescribed in combination with other
therapeutic drugs; iii) the study classified as a review or case
report; and iv) treatment of sub-cohort groups with orlistat. No
language restrictions were set.
Data extraction and quality
assessment
The following data were extracted from the included
studies: The first author, year of publication, sample size, length
of follow-up, patient characteristics (country, sex ratio, mean age
and obesity status), intervention (dose, duration and diet),
control (placebo or other), liver histological variables and
biomarkers for liver function [alanine aminotransferase (ALT),
aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT),
TGs, glucose and HOMA-IR]. Extracted data were verified by an
independent investigator and any disagreements were resolved
through discussion with a second investigator until a consensus was
reached. The Cochrane Risk of Bias tool (19) and the Newcastle-Ottawa Quality
Assessment Scale (20) were used to
evaluate RCTs and observational studies, respectively. Safety was
assessed based on reported treatment-related adverse events.
Statistical analysis
RevMan software (version 5.3; Cochrane, London, UK)
was used for statistical analysis. Post-treatment and baseline
values were extracted as means ± standard deviations. For combined
analyses, the mean difference (MD) or standardized mean difference
(SMD) with 95% confidence interval (CI) to pool the extracted
variables. The heterogeneity between studies was determined using
the I2 statistic; I2 values of 25, 50 and 75%
represented low, moderate and high heterogeneity, respectively
(21). In cases with I2
<50%, a fixed-effects model was used, whereas a random-effects
model was used if I2 >50%. P<0.05 was considered
to indicate statistical significance.
Results
Literature search
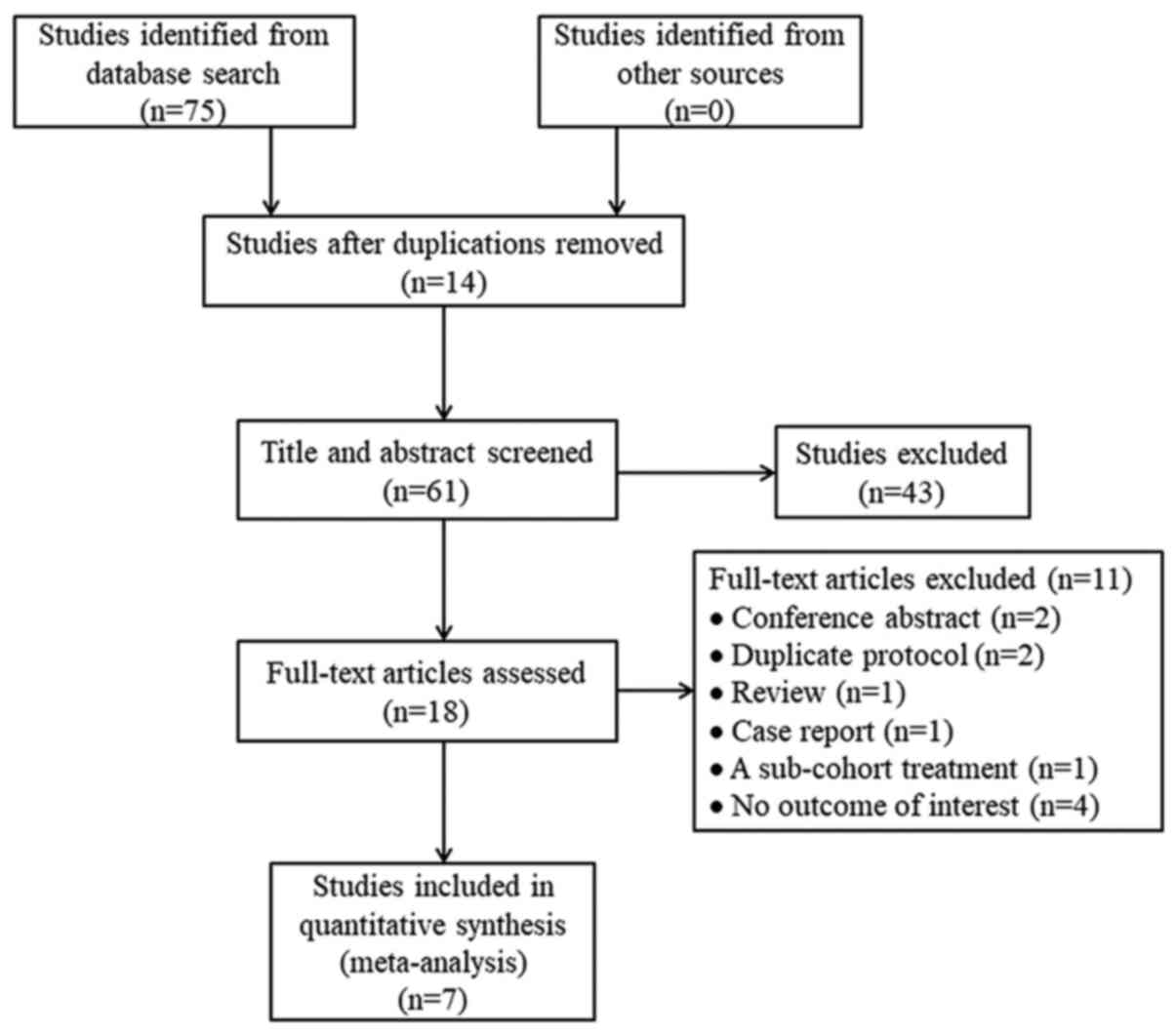
The database searches yielded 75 studies, of which
14 were excluded due to duplication. Following screening of titles
and abstracts for eligibility, 43 additional studies were excluded.
Of the remaining 18 studies, 11 were excluded following assessment
of the full-text: Two conference abstracts, two duplicate
protocols, one review, one case report, one study with sub-cohort
treatment with orlistat and four studies with no outcomes of
interest. Ultimately, seven studies were analyzed (11–13,17,22–24)
(Fig. 1).
Trial characteristics
Trial characteristics of the seven included studies
are summarized in Table I. Briefly,
the trials were published between 2004 and 2017 and included 330
participants. The mean age of participants ranged from 36.5 to 58.5
years, and in all but one trial, the obesity rate was 100%. The
duration of orlistat intervention ranged from 16 to 36 weeks, and
in all studies, the dose administered was 120 mg three times a day.
The study by Fan et al (22)
analyzed two treatment durations (12 and 24 weeks); due to more
comprehensive reporting on the 24-week data, the 24-week group was
selected for inclusion in the meta-analysis.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| Author (refs.) | Year of
publication | Country | n | Number of women, n
(%) | Dropout, n (%) | Mean age,
years | Intervention dose,
mg tid | Comparators | Duration | Obesity, % | Diagnosis | Study type |
|---|
| Chen et al
(12) | 2006 | China | 30 | 5 (16.7) | 0 (0) | 37.8 | 120 | No control | 24 weeks | 100.0 | NAFLD | Single-arm
trial |
| Fan et al
(22) | 2004 | China | 60 | 23 (38.3) | 2 (3.3) | 36.5 | 120 | No control | 24 weeks | 100.0 | NAFLD | Single-arm
trial |
| Harrison et
al (13) | 2004 | USA | 10 | 6 (60.0) | 0 (0) | 54.4 | 120 | No control | 6 months | 100.0 | NASH | Single-arm
trial |
| Harrison et
al (23) | 2009 | USA | 50 | 0 (0) | 9 (18.0) | 47 | 120 | Vitamin E (800 IU)
+ 1,400-calorie diet | 36 weeks | 100.0 | NASH | RCT |
| Hussein et
al (24) | 2007 | Israel | 16 | 10 (62.5) | 2 (12.5) | 42 | 120 | No control | 6 months | 100.0 | NASH | Single-arm
trial |
| Ali Khan et
al (11) | 2017 | India | 112 | 35 (31.3) | 14 (12.5) | 58.5 | 120 | Diet | 16 weeks | 100.0 | NAFLD | RCT |
| Zelber-Sagi et
al (17) | 2006 | Israel | 52 | 25 (48.1) | 8 (15.4) | 47.7 | 120 | Placebo | 6 months | 71.2 | NAFLD | RCT |
Quality assessment
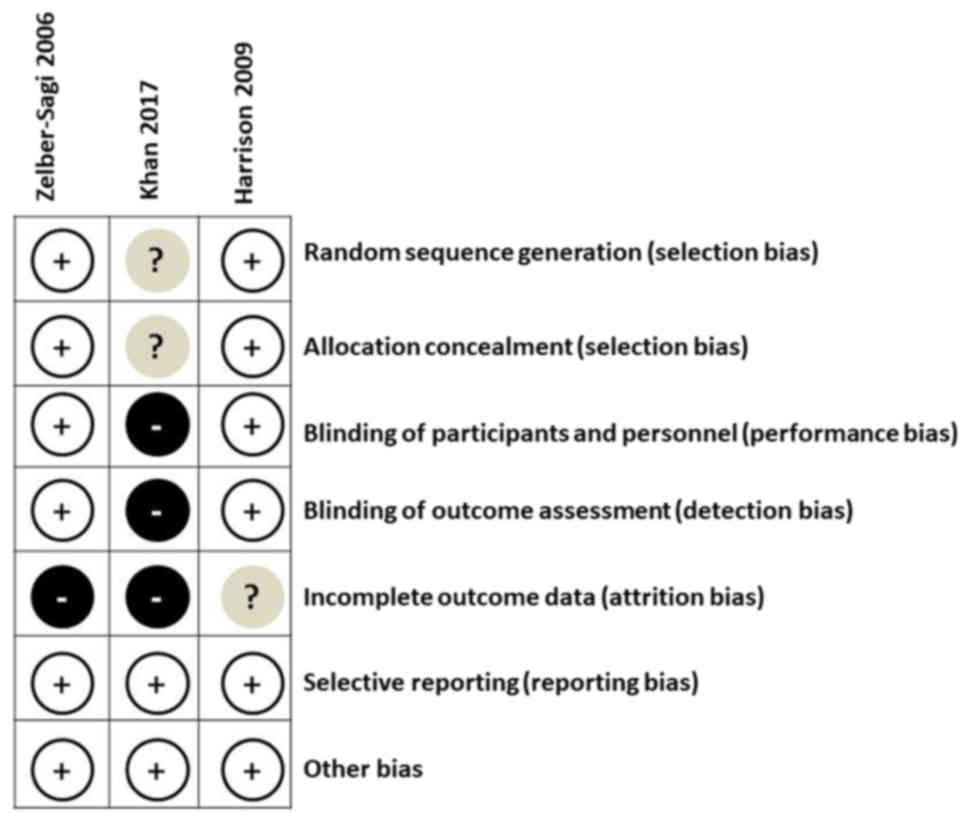
Quality assessment of the three included RCTs
(11,17,23) was
performed using the Cochrane Risk of Bias tool (Fig. 2). Overall, all three studies had a low
risk of bias in selective reporting and other areas. Additionally,
two of the studies (17,23) had low risk of bias in random sequence
generation, allocation concealment, blinding of participants and
personnel, and blinding of outcome assessment. The number and cause
of dropouts were identified for each study.
Quantitative data synthesis
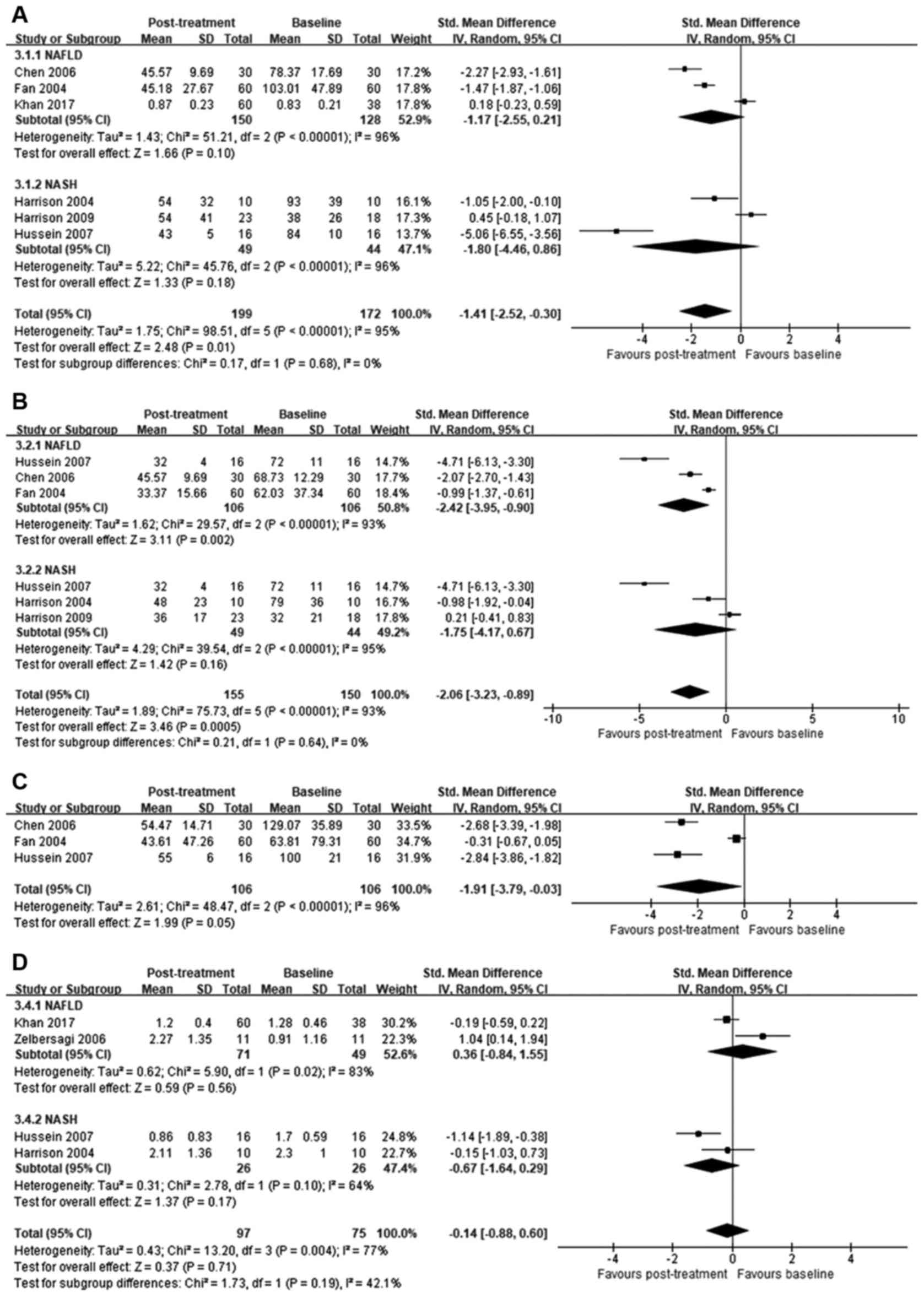
ALT. ALT levels were reported in all included
studies. Although between-study heterogeneity was high
(I2=95%), orlistat post-treatment significantly reduced
ALT levels compared to baseline [SMD=−1.41; 95% confidence interval
(95% CI)=−2.52 to −0.30; P=0.01]. Specific subgroup analysis
indicated no significant improvement in ALT levels in patients with
NAFLD (SMD=−1.17; 95% CI=−2.55 to 0.21; I2=96%; P=0.1)
or NASH (SMD=−1.80; 95% CI=−4.46 to 0.86; I2=96%;
P=0.18; Fig. 3A).
AST
AST levels were reported in six of the seven studies
analyzed. Compared to baseline, orlistat treatment significantly
reduced AST levels (SMD=−2.06; 95% CI=−3.23 to −0.89; P=0.0005);
however, between-study heterogeneity was high (I2=93%).
In the subgroup analysis, significant difference in AST was
identified in patients with NAFLD (SMD=−2.42; 95% CI=−3.95 to
−0.90; I2=93%; P=0.002), but not in those with NASH
(SMD=−1.75; 95% CI=−4.17 to 0.67; I2=95%; P=0.16;
Fig. 3B).
GGT
The effect of orlistat on GGT levels reported in
three studies was analyzed. Orlistat treatment significantly
reduced GGT levels compared to baseline (SMD=−1.91; 95% CI=−3.79 to
−0.03; P=0.05); however, between-study heterogeneity was high
(I2=96%; Fig. 3C).
Liver histology
In four studies included in the meta-analysis, liver
biopsies were administrated on admission and iterated following the
treatment with orlistat. Fibrosis was scored on a scale from 0 to 4
(25). No significant differences
between post-treatment and baseline scores were observed
(SMD=−0.14; 95% CI=−0.88 to 0.60; P=0.71) in patients treated with
orlistat. Between-study heterogeneity was high (I2=77%;
Fig. 3D).
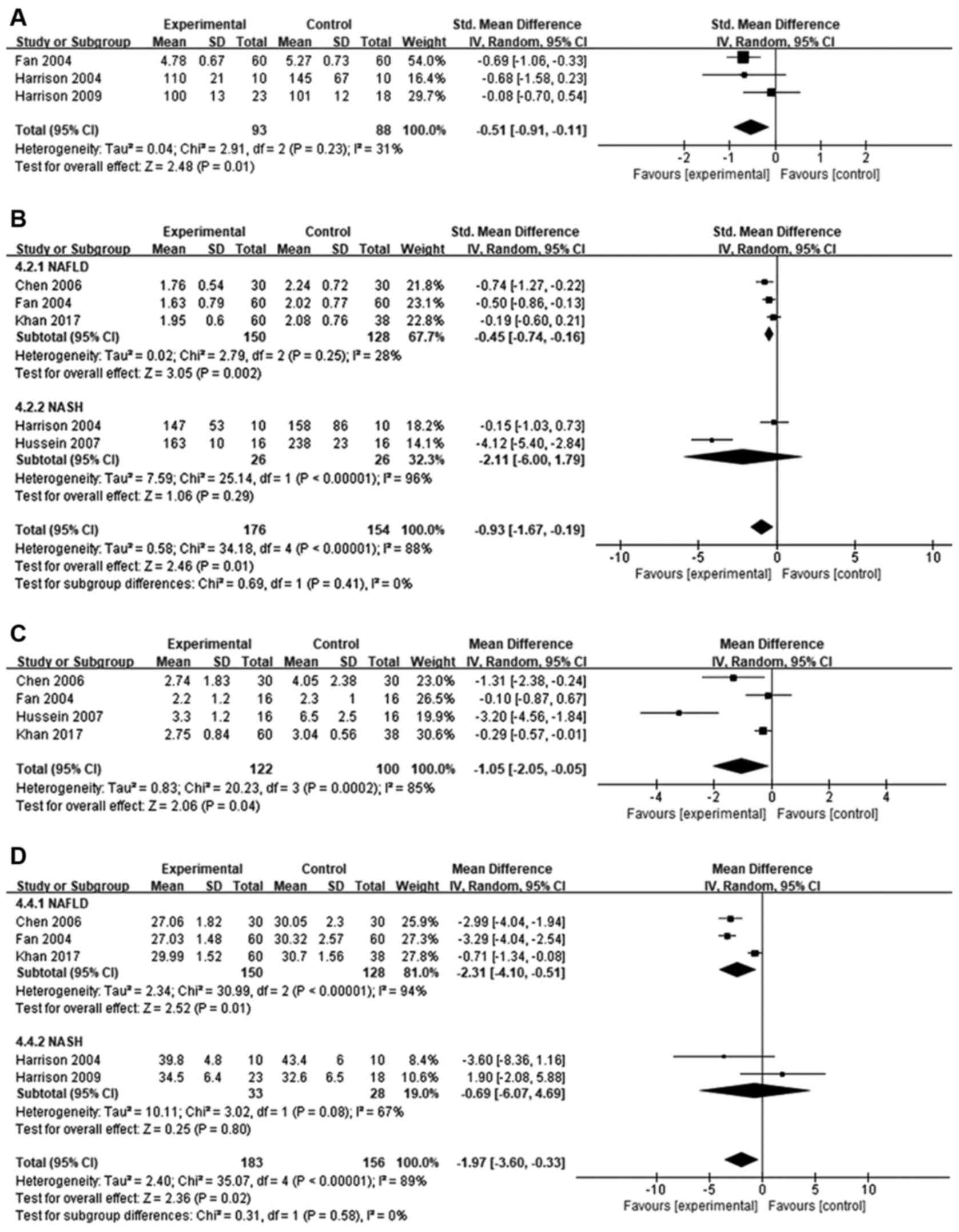
Glucose
Orlistat treatment caused significant reduction in
fasting plasma glucose levels compared to baseline in patients with
NAFLD and NASH (SMD=−0.51; 95% CI=−0.91 to −0.11; P=0.01).
Between-study heterogeneity was moderate (I2=31%;
Fig. 4A).
Plasma triglycerides
The effect of orlistat on TG levels in five studies
was analyzed. Overall, despite high heterogeneity
(I2=88%), TG levels were significantly reduced following
orlistat treatment compared to baseline (MD=−0.93; 95% CI=−1.67 to
−0.19; P=0.01). Subgroup analysis determined a significant
difference in patients with NAFLD (SMD=−0.45; 95% CI=−0.74 to
−0.16; I2=28%; P=0.002), but not in patients with NASH
(SMD=−2.11; 95% CI=−6.00 to 1.79; I2=96%; P=0.29;
Fig. 4B).
HOMA-IR
HOMA-IR levels were reported in four studies.
Compared to baseline, orlistat treatment caused a modest, but
significant, reduction in insulin resistance (MD=−1.05; 95%
CI=−2.05 to −0.05; I2=85%, P=0.04; Fig. 4C).
BMI
Data from five studies were used to evaluate BMI
(Fig. 4D). Compared to baseline,
orlistat treatment significantly reduced BMI (MD=−1.97; 95%
CI=−3.60 to −0.33; P=0.02), although between-study heterogeneity
was high (I2=89%). Subgroup analysis identified a
significant difference in patients with NAFLD (MD=−2.31; 95%
CI=−4.10 to −0.51; I2=94%; P=0.01) but not in patients
with NASH (MD=−0.69; 95% CI=−6.07 to 4.69; I2=67%;
P=0.80).
Adverse events
Of the seven studies included in the meta-analysis,
four reported adverse events (11,17,22,24).
Zelber-Sagi et al (17)
reported that 2 patients withdrew from the study due to
gastrointestinal side effects. Fan et al (22) reported that 2 patients withdrew from
the study due to fecal urgency and fecal incontinence following 8
weeks of treatment. Mild gastrointestinal discomfort symptoms,
including nausea, abdominal cramps, fecal urgency, fecal
incontinence and increased defecation were also reported (11,12,17,22,24).
No serious treatment-related adverse effects on cardiovascular
outcome or carcinogenesis were reported in any of the included
studies.
Discussion
The present study to the best of our knowledge is
the first systematic review and meta-analysis investigating the
efficacy and safety of orlistat intervention in patients with NAFLD
or NASH. Following a review of the literature, seven trials were
identified that satisfied the inclusion criteria. Although the
results of the current analysis are limited by the relatively small
number of studies included, the data suggest improvements in
biochemical, metabolic and anthropometric indicators of liver
disease and in overall safety by orlistat treatment in patients
with NAFLD or NASH.
In the studies analyzed, orlistat improved
biochemical markers of liver disease. Orlistat treatment reduced
the levels of ALT, AST and GGT compared to baseline in patients
with NAFLD; however, the subgroup analysis did not reveal
significant differences in patients with NASH. Aminotransferases
are sensitive indicators of liver damage and cellular integrity and
are mainly located in hepatocytes (14,15,26). When
hepatocytes are damaged, for example during liver disease, blood
aminotransferase levels increase (15). It is considered that orlistat may
reduce damage to hepatocytes by decreasing fat accumulation in the
liver, and thereby decrease aminotransferase levels (23).
However, the effect of orlistat on liver
histological variables remains unclear. Overall assessment of the
four studies noting liver fibrosis score revealed no significant
changes in the scores following treatment with orlistat compared to
baseline. These results are consistent with those of Tilg and
Moschen (27) who demonstrated that
orlistat failed to improve the histopathology of NAFLD. Therefore,
orlistat should not be considered as a medication for alleviating
fibrosis in patients with NAFLD or NASH.
As an anti-obesity drug, orlistat has been
demonstrated to reduce postprandial lipid levels following high-fat
meals. A study by Gabriel et al (28) identified that administration of
orlistat suppressed the postprandial rise of TG levels in healthy
adult volunteers following consumption of meals with 50% fat. Nakou
et al (29) observed a greater
decrease in TG levels with ezetimibe plus orlistat therapy compared
with monotherapy with ezetimibe alone. In the current
meta-analysis, TG levels were markedly improved following orlistat
treatment compared to baseline, suggesting that orlistat may
alleviate lipid accumulation, although statistical significance was
not achieved in patients with NASH.
The present results also indicated that orlistat
treatment reduced plasma glucose levels compared to baseline in
patients with NAFLD and NASH. Kujawska-Łuczak et al
(30) observed similar results while
examining the effect of orlistat on glucose/insulin homeostasis in
obese premenopausal women. Orlistat has been further indicated to
improve glycemic control and insulin resistance, as well as reduce
body weight gain and BMI, in patients with obesity and type 2
diabetes (31,32). Based on these data, the effect of
orlistat on insulin resistance and BMI in patients with NAFLD was
analyzed. HOMA-IR was used to evaluate insulin resistance and BMI
substituted for incomplete data on body weight. As expected,
improvement in BMI was associated with improved insulin resistance,
and HOMA-IR scores and BMI were significantly lower following
orlistat treatment compared to baseline.
Previous results have indicated that hepatic
steatosis and inflammation may be improved by weight loss or
reduction of BMI (33). However,
Harrison et al (23) reported
that no significant differences in hepatic steatosis and
inflammation were found between orlistat/diet/vitamin E and
diet/vitamin E groups. The limited information collected on this
aspect in our included studies hampered analysis of hepatic
steatosis, necroinflammation and ballooning. Of the included
studies, only two reported on steatosis and inflammation (23,24), and
one on ballooning (23). Thus,
further studies are required to investigate whether orlistat may
improve liver histology.
Several limitations of the current analysis should
be noted. First, a relatively small number of studies and sample
sizes were included in the meta-analysis. Second, a high
heterogeneity was observed, probably in part due to the small
sample sizes, inconsistent duration of treatment, and lack of
control groups, which prevented the evaluation of clinically
significant conclusions. Third, only three studies investigated
liver histological variables in patients with NAFLD and NASH.
In conclusion, the present systematic review and
meta-analysis assessed the effect of the anti-obesity drug orlistat
on various disease biomarkers in patients with NAFLD and NASH. The
results suggest that, although orlistat does not consistently
reverse liver fibrosis, it appears to improve other biochemical,
metabolic and anthropometric indicators and to a certain extent,
may contribute to prognosis. However, due to the limited sample
size and high heterogeneity, these findings did not prove
statistically significant in all subgroup analyses. Nonetheless,
the data support the use of orlistat as a therapeutic strategy to
improve biochemical indicators of liver damage, but not as a drug
of choice for the management of NAFLD or NASH. Larger sample sizes
and additional liver histological parameters (including hepatic
steatosis, ballooning, necroinflammation and fibrosis) should be
assessed over a longer follow-up period in future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81470303), the
Top-notch Academic Programs Project of Jiangsu Higher Education
Institutions (grant no. PPZY2015B161) and the Jiangsu Province
Students Innovation and Entrepreneurship Training Program (grant
no. 201710313022Z).
Authors' contributions
HW and YC conducted the meta-analysis including
design and drafting of the manuscript. LW and ZX verified the data
extracted following the literature search. HW, YL and JC were
responsible for proofreading and revising the review critically for
intellectual content. All authors read and approved the final
manuscript.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NAFLD
|
non-alcoholic fatty liver
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
GGT
|
γ-glutamyl transpeptidase
|
|
TG
|
triglyceride
|
|
HOMA-IR
|
homeostasis model assessment of
insulin resistance
|
|
BMI
|
body mass index
|
|
RCT
|
randomized controlled trial
|
|
MD
|
mean difference
|
|
SMD
|
standard mean difference
|
|
95% CI
|
95% confidence interval
|
References
|
1
|
Bellentani S, Scaglioni F, Marino M and
Bedogni G: Epidemiology of non-alcoholic fatty liver disease. Dig
Dis. 28:155–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lazo M and Clark JM: The epidemiology of
nonalcoholic fatty liver disease: A global perspective. Semin Liver
Dis. 28:339–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu JZ, Dai YN, Wang YM, Zhou QY, Yu CH
and Li YM: Prevalence of nonalcoholic fatty liver disease and
economy. Dig Dis Sci. 60:3194–3202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vanni E, Bugianesi E, Kotronen A, de
Minicis S, Yki-Järvinen H and Svegliati-Baroni G: From the
metabolic syndrome to NAFLD or vice versa? Dig Liver Dis.
42:320–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Liu DW, Yan HY, Wang ZY, Zhao SH and
Wang B: Obesity is an independent risk factor for non-alcoholic
fatty liver disease: Evidence from a meta-analysis of 21 cohort
studies. Obes Rev. 17:510–519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao ZH, Liu XL and Fan JG: Research on
the natural history of non-alcoholic fatty liver disease should be
taken seriously. Zhonghua Gan Zang Bing Za Zhi. 25:81–84. 2017.(In
Chinese). PubMed/NCBI
|
|
7
|
Bhala N, Angulo P, van der Poorten D, Lee
E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping
JH, Bugianesi E, et al: The natural history of nonalcoholic fatty
liver disease with advanced fibrosis or cirrhosis: An international
collaborative study. Hepatology. 54:1208–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duvnjak M, Tomasic V, Gomercic M, Smircic
Duvnjak L, Barsic N and Lerotic I: Therapy of nonalcoholic fatty
liver disease: Current status. J Physiol Pharmacol. 60(Suppl 7):
57–66. 2009.PubMed/NCBI
|
|
9
|
Narayanaswami V and Dwoskin LP: Obesity:
Current and potential pharmacotherapeutics and targets. Pharmacol
Ther. 170:116–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kushner RF: Medical management of obesity.
Semin Gastrointest Dis. 13:123–132. 2002.PubMed/NCBI
|
|
11
|
Ali Khan R, Kapur P, Jain A, Farah F and
Bhandari U: Effect of orlistat on periostin, adiponectin,
inflammatory markers and ultrasound grades of fatty liver in obese
NAFLD patients. Ther Clin Risk Manag. 13:139–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Z, Liang B, Wang Y and Fan X: The
analysis of the efficacy and risk factors in obesity with
non-alcoholic fatty liver disease subjects treated with orlistat.
Chin J Clin Hepatol. 22:123–124. 2006.(In Chinese).
|
|
13
|
Harrison SA, Fincke C, Helinski D,
Torgerson S and Hayashi P: A pilot study of orlistat treatment in
obese, non-alcoholic steatohepatitis patients. Aliment Pharmacol
Ther. 20:623–628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fernández S and Córdoba M: Progesterone
causes metabolic changes involving aminotransferases and creatine
kinase in cryopreserved bovine spermatozoa. Anim Reprod Sci.
164:90–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruinsma BG, Wu W, Ozer S, Farmer A,
Markmann JF, Yeh H and Uygun K: Warm ischemic injury is reflected
in the release of injury markers during cold preservation of the
human liver. PLoS One. 10:e01234212015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Filippatos TD, Derdemezis CS, Gazi IF,
Nakou ES, Mikhailidis DP and Elisaf MS: Orlistat-associated adverse
effects and drug interactions: A critical review. Drug Saf.
31:53–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zelber-Sagi S, Kessler A, Brazowsky E,
Webb M, Lurie Y, Santo M, Leshno M, Blendis L, Halpern Z and Oren
R: A double-blind randomized placebo-controlled trial of orlistat
for the treatment of nonalcoholic fatty liver disease. Clin
Gastroenterol Hepatol. 4:639–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. Int J Surg. 8:336–341.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Furlan AD, Pennick V, Bombardier C and van
Tulder M; Editorial Board, Cochrane Back Review Group: 2009 updated
method guidelines for systematic reviews in the Cochrane Back
Review Group. Spine. 34:1929–1941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Xue J, Chen P, Chen L, Yan S and Liu
L: Prevalence of nonalcoholic fatty liver disease in mainland of
China: A meta-analysis of published studies. J Gastroenterol
Hepatol. 29:42–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan Z, Qiu D, Xie W, Hu H, Chen Y, Sun Z,
Liu S and Zeng M: Clinical study of obesity associated
non-alcoholic fatty liver disease. Chin Hepatol. 9:155–158.
2004.(In Chinese).
|
|
23
|
Harrison SA, Fecht W, Brunt EM and
Neuschwander-Tetri BA: Orlistat for overweight subjects with
nonalcoholic steatohepatitis: A randomized, prospective trial.
Hepatology. 49:80–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hussein O, Grosovski M, Schlesinger S,
Szvalb S and Assy N: Orlistat reverse fatty infiltration and
improves hepatic fibrosis in obese patients with nonalcoholic
steatohepatitis (NASH). Dig Dis Sci. 52:2512–2519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Angulo P, Hui JM, Marchesini G, Bugianesi
E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, et
al: The NAFLD fibrosis score: A noninvasive system that identifies
liver fibrosis in patients with NAFLD. Hepatology. 45:846–854.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahboub P, Ottens P, Seelen M, 't Hart N,
van Goor H, Ploeg R, Martins PN and Leuvenink H: Gradual rewarming
with gradual increase in pressure during machine perfusion after
cold static preservation reduces kidney ischemia reperfusion
injury. PLoS One. 11:e01520062016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tilg H and Moschen A: Weight loss:
Cornerstone in the treatment of non-alcoholic fatty liver disease.
Minerva Gastroenterol Dietol. 56:159–167. 2010.PubMed/NCBI
|
|
28
|
Gabriel FS, Samson CE, Abejuela ZR,
Sicat-Gabriel PR, Sumpio JP, Zacarias MB and Mercado-Asis LB:
Postprandial effect of orlistat on the peaking of lipid level after
sequential high fat meals. Int J Endocrinol Metab. 10:458–463.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakou ES, Filippatos TD, Agouridis AP,
Kostara C, Bairaktari ET and Elisaf MS: The effects of ezetimibe
and/or orlistat on triglyceride-rich lipoprotein metabolism in
obese hypercholesterolemic patients. Lipids. 45:445–450. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kujawska-Łuczak M, Musialik K, Szulińska
M, Swora-Cwynar E, Kargulewicz A, Grzymisławska M, Pupek-Musialik D
and Bogdański P: The effect of orlistat versus metformin on body
composition and insulin resistance in obese premenopausal women:
3-month randomized prospective open-label study. Arch Med Sci.
13:725–731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scheen AJ and van Gaal LF: Combating the
dual burden: Therapeutic targeting of common pathways in obesity
and type 2 diabetes. Lancet Diabetes Endocrinol. 2:911–922. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smith-Marsh D: Pharmacological strategies
for preventing type 2 diabetes in patients with impaired glucose
tolerance. Drugs Today (Barc). 49:499–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ; American
Gastroenterological Association; American Association for the Study
of Liver Diseases; American College of Gastroenterologyh: The
diagnosis and management of non-alcoholic fatty liver disease:
Practice guideline by the American Gastroenterological Association,
American Association for the Study of Liver Diseases, and American
College of Gastroenterology. Gastroenterology. 142:1592–1609. 2012.
View Article : Google Scholar : PubMed/NCBI
|