Introduction
(Pro)renin receptor [(P)RR] is a single-spanning
membrane protein comprised of 350 amino acid residues (1–3). (P)RR is
coded by the ATP6AP2 gene, which is located on the X chromosome at
locus p11.4 (NCBI gene ID: 10159). Although (P)RR was originally
identified as a regulator of the renin-angiotensin system essential
for maintaining blood pressure and fluid balance,
angiotensin-independent functions of (P)RR have been reported,
including functions as an accessory protein of the vacuolar
H+-ATPase (4) and a
cofactor of the Wnt receptor complex (5). Other studies have indicated that (P)RR
is also implicated in pathophysiological conditions including
retinopathy (6), nephropathy
(7), mental retardation (8) and pancreatic ductal adenocarcinoma
(9).
(P)RR undergoes intracellular processing to produce
three different forms: A full-length form (1,2), a
truncated membrane-bound form (10)
and a truncated soluble form without the transmembrane domain
termed soluble (P)RR [s(P)RR] (11,12).
s(P)RR has been detected in plasma (11) and urine (13). In patients with essential
hypertension, serum s(P)RR levels have been associated with various
types of renal dysfunction (14).
Additionally, previous study identified significantly higher plasma
concentrations of s(P)RR in patients with pancreatic ductal
adenocarcinoma than in healthy controls (9). Lu et al (15) reported that s(P)RR exerted
antidiuretic action via frizzled-8 dependent β-catenin signaling,
which leads to enhanced renal aquaporin-2 expression and urine
concentrating capability. These authors first proposed the
physiological role of s(P)RR in regulating fluid homeostasis
(15), and in turn, s(P)RR in body
fluids has been proposed as a useful biomarker for diseases
(9,14)
as well as an indicator of physiological function (15).
Aliskiren is an orally-active direct renin inhibitor
(16). It specifically binds to the
active site of human renin, which catalyzes the rate-limiting step
of the renin-angiotensin system (16). This medicine is prescribed to patients
with mild to moderate hypertension (17). Aliskiren has been reported to reduce
(P)RR expression in the renal compartments of
streptozotocin-induced diabetic rats (18), and also in cultured human aortic
smooth muscle cells (19). However,
little is known about the effect of aliskiren on the concentration
of s(P)RR in body fluids.
Our group previously reported that s(P)RR was
released from cultured human umbilical vein endothelial cells
(HUVECs) (20), which can be used as
an in vitro model to investigate general properties of the
human endothelium (21). In the
present study, the effect of aliskiren on the protein levels of
s(P)RR released from cultured HUVECs was examined.
Materials and methods
Preparation of recombinant
proteins
Recombinant human prorenin was expressed in Chinese
hamster ovary (CHO) cells and purified as previously described
(22). In brief, cells harboring cDNA
coding for human prorenin were cultured in Dulbecco's modified
Eagle's medium (DMEM; Nissui Pharmaceutical Co., Ltd., Tokyo,
Japan) supplemented with 5% fetal bovine serum (FBS; PAA
Laboratories GmbH, Pasching, Austria), 0.1 mM non-essential amino
acids (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 2
mM glutamine, 100 U/ml streptomycin and 0.6 mM methotrexate. The
cells were maintained at 37°C under 5% CO2 in
25-cm2 cell culture flasks (CELLSTAR®;
Greiner Bio-One GmbH, Frickenhausen, Germany) until they were 100%
confluent. Human prorenin was purified from the CHO cell
conditioned medium by cation-exchange chromatography on a Resource
S column (GE Healthcare, Amersham, UK). The concentration of
purified prorenin was measured using a human prorenin enzyme-linked
immunosorbent assay (ELISA) kit (cat no. IHPRENKT-NP; Innovative
Research, Inc., Novi, MI, USA). Prior to experimentation, purified
prorenin was incubated at 37°C for 1 h (23) to avoid cryo-activation due to
preservation (24). Recombinant sheep
angiotensinogen (AOG) was also expressed in CHO cells and purified
as previously described (25,26).
Culture of HUVECs
HUVECs (cat no.: KJB-110; DS Pharma Biomedical Co.,
Ltd., Osaka, Japan) were cultured in human endothelial cell medium
(DS Pharma Biomedical Co., Ltd.) supplemented with 2% FBS,
essential endothelial growth factors (included in the endothelial
cell medium), 0.1% heparin, 1% ascorbic acid, 0.04% hydrocortisone
and 0.1% gentamicin/amphotericin-1000. The cells were maintained at
37°C under 5% CO2 in 25-cm2 cell culture
flasks (CELLSTAR®) until they were 80–90% confluent.
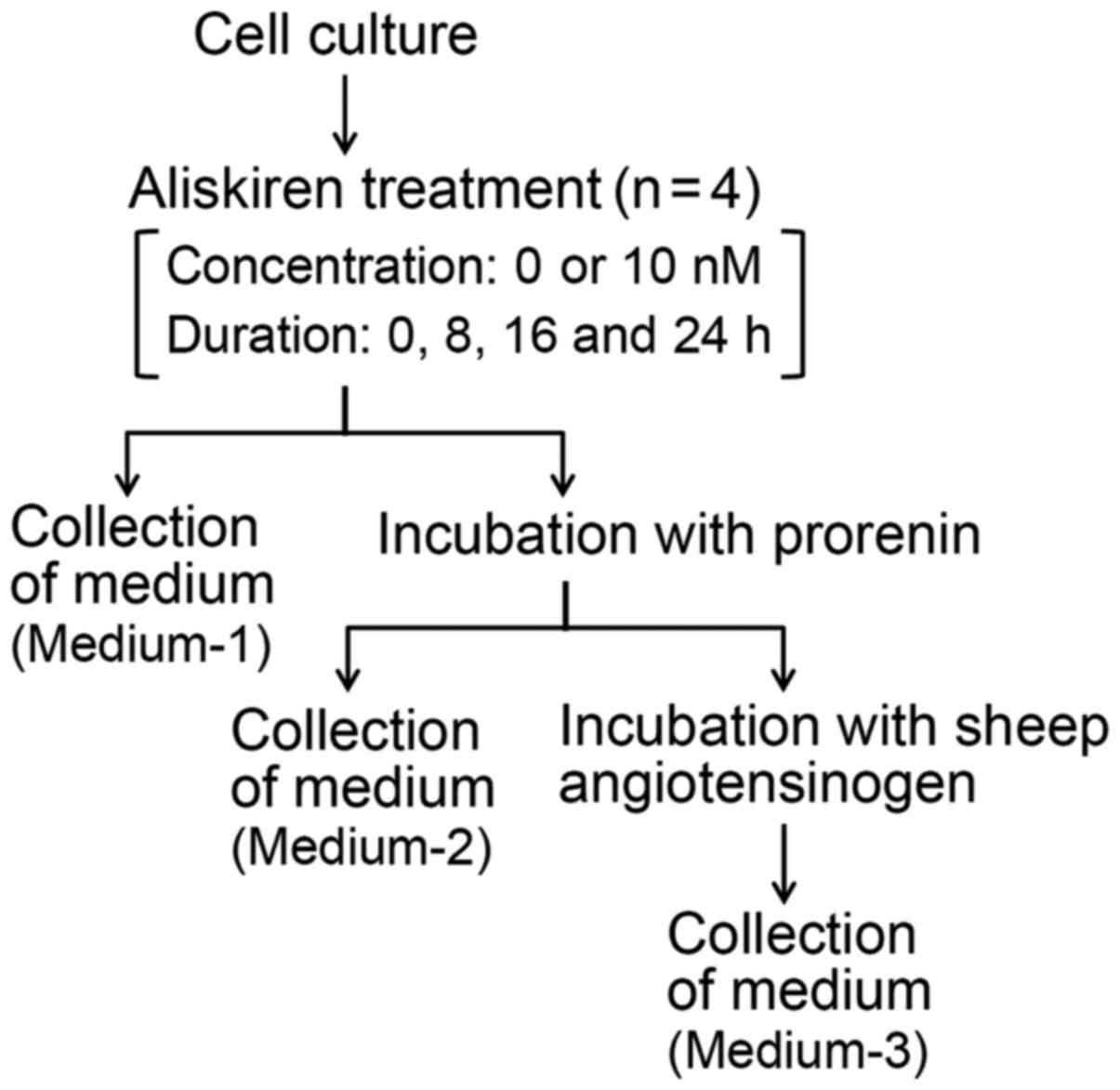
Treatment of HUVECs with
aliskiren
HUVECs were seeded at a density of 150,000
cells/well in 12-well sterile plates, and incubated at 37°C. After
24 h, the medium was removed, and the cells were washed with
2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid
(HEPES)-buffered saline [(HBS); 1.0 g/l dextrose, 5.0 g/l HEPES,
0.37 g/l KCl, 8.0 g/l NaCl and 0.14 g/l
Na2HPO4·2H2O, pH 7.4]. The cells
were then treated with or without aliskiren (10 nM; Novartis Pharma
AG, Basel, Switzerland) at 37°C for different durations (0, 8, 16,
and 24 h) in FBS-free DMEM (Fig. 1).
Following each completed duration of treatment: i) the cultured
medium (hereafter referred to as Medium-1) was collected to
determine the concentration of s(P)RR, and the cells were washed 3
times with HBS and incubated at 37°C with prorenin (1.0 nM) for 1
h; ii) the medium containing the unbound prorenin (hereafter
referred to as Medium-2) was collected, and the cells were further
washed 3 times with HBS and incubated at 37°C with sheep AOG (0.3
µM) for 30 min in 5 mM sodium phosphate buffer (pH 7.2) containing
5 mM diisopropyl fluorophosphates, 5 mM EDTA, 100 mM NaCl and 0.1%
(w/v) bovine serum albumin fraction V (Roche Diagnostics GmbH,
Mannheim, Germany); and finally iii) the medium containing the
possible generated angiotensin I (AngI; hereafter referred to as
Medium-3) was collected. These experimental procedures are depicted
in Fig. 1. The cells treated without
aliskiren served as controls.
For cell counting, HUVECs were seeded, cultured and
treated with or without aliskiren, as described above. After each
duration of treatment, the total number of cells per well was
counted using a hemocytometer. This cell culture was performed
independently of collecting the three media described above.
Determination of concentration of
membrane-bound prorenin in HUVECs
The concentration of prorenin contained in Medium-2
was determined using the human prorenin ELISA kit, and was
considered as the concentration of unbound prorenin
(CUB). The percentage of prorenin bound to the cell
membrane was calculated using the following equation:
(CIn - CUB)/CIn ×100, where
CIn was the initial concentration of prorenin that was
incubated with HUVECs (1.0 nM).
Measurement of renin activity derived
from membrane-bound prorenin in HUVECs
The concentration of AngI in Medium-3 was determined
by ELISA using an assay developed and prepared in our laboratory
(27). The AngI concentration was
divided by the reaction time (30 min) to calculate renin
activity.
Determination of s(P)RR concentration
in the culture medium of HUVECs
The concentration of s(P)RR contained in Medium-1
was determined by sandwich ELISA using an assay developed and
prepared in our laboratory (20) with
a primary anti-(P)RR antibody (anti-human renin R polyclonal
antibody; cat no. AF5716; R&D systems, Inc., Minneapolis, MN,
USA) and a secondary anti-(P)RR antibody [biotin-labeled
anti-237/250-antibody (20)]. The
resulting antigen-antibody complex was tagged with a
high-sensitivity streptavidin-horseradish peroxidase conjugate, and
detected by a chromogenic reaction using hydrogen peroxide and
3,3′,5,5′-tetramethylbenzedine. The s(P)RR standards were prepared
as previously described (28).
Statistical analysis
GraphPad Prism 7.0 software (GraphPad Software,
Inc., La Jolla, CA, USA) was used to analyze the data. Data were
expressed as the mean ± standard deviation of four replicates for
each treatment. The propagation of error formula (29) was used to calculate the standard
deviation of either of three ratio values: bound prorenin per cell,
renin activity per cell and s(P)RR per cell. A two-tailed unpaired
Student's t-test was performed to compare between two groups
(aliskiren-treated cells and controls). P<0.05 was considered to
indicate statistical significance.
Results
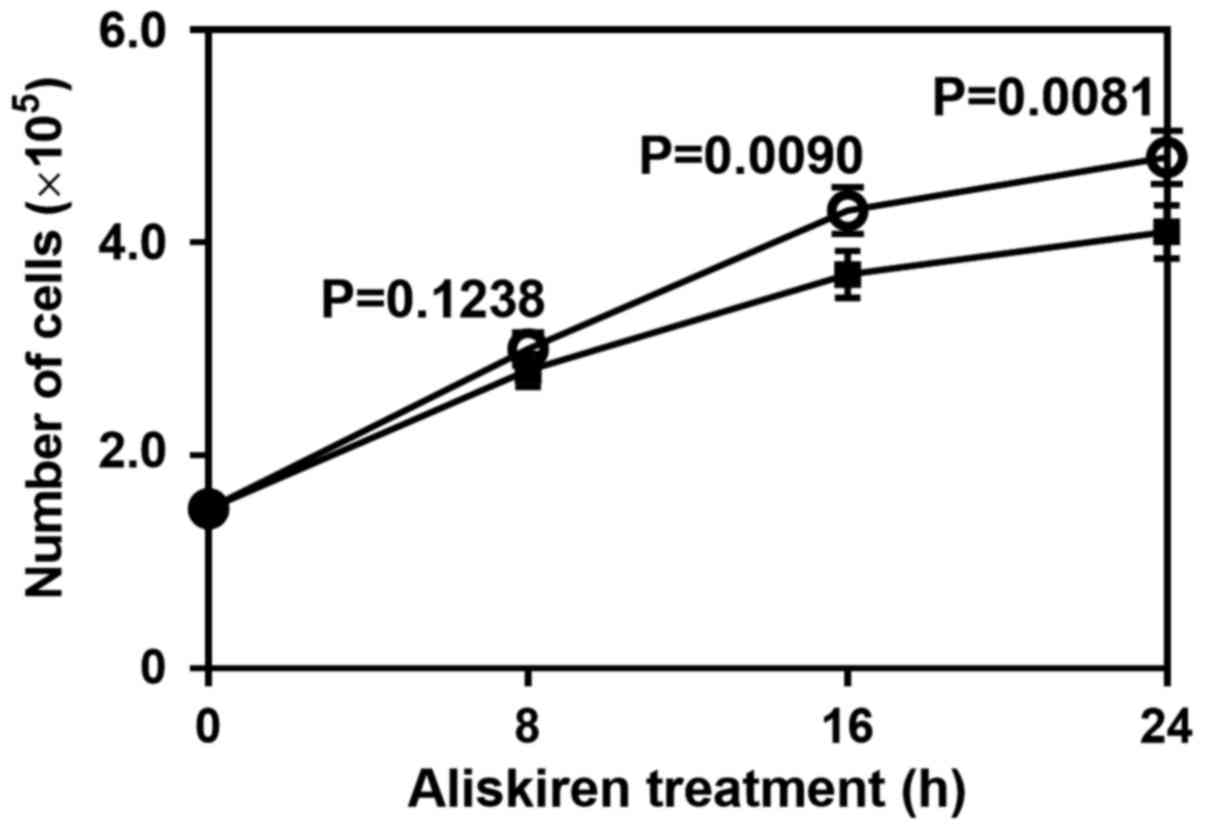
Effect of aliskiren on the growth of
HUVECs
HUVECs were cultured in the presence or absence of
aliskiren. The cell number of aliskiren-treated HUVECs was lower
than that of the controls, most notably at 16 h (P=0.0090) and 24 h
(P=0.0081; Fig. 2). Thus, aliskiren
appeared to reduce the growth of HUVECs.
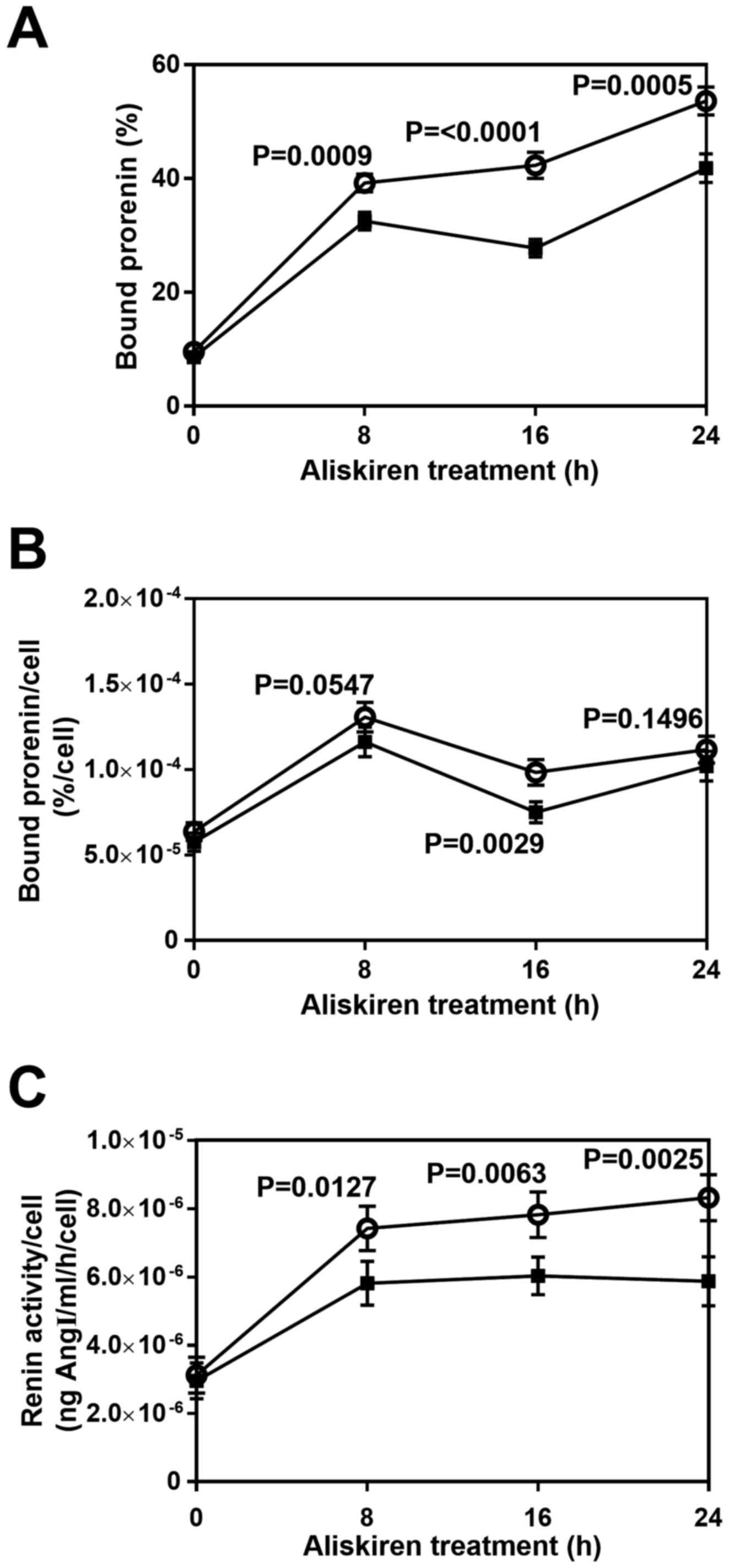
Effect of aliskiren on the
cell-surface expression of full-length (P)RR
(P)RR binds renin and its inactive precursor,
prorenin (1–3). To estimate the expression of full-length
(P)RR on the plasma membrane of HUVECs, recombinant prorenin
(exogenous prorenin) was added to the culture medium (Fig. 1), and the amount of prorenin bound to
the cell membrane was estimated. Following aliskiren treatment for
different durations (0, 8, 16 and 24 h), cells were incubated with
exogenous prorenin, and the quantity of bound prorenin was
evaluated (Fig. 3A). Approximately 10
and 50% of the exogenous prorenin bound to control membranes after
0 and 24 h of treatment, respectively (Fig. 3A, open circles). The quantity of bound
prorenin was significantly decreased with aliskiren treatment for
8–24 h (P<0.001; Fig. 3A, filled
squares). Additionally, it was observed that the concentration of
endogenous prorenin secreted by HUVECs was almost 1,000 times lower
than that of exogenous prorenin (data not shown), indicating that
endogenous prorenin would have a negligible effect on the
estimation of exogenous prorenin bound to HUVEC membrane. The
quantity of bound prorenin per cell with aliskiren treatment was
similar to that of untreated cells except at 16 h, where
significant difference was determined (P=0.0029; Fig. 3B).
To further estimate the amount of full-length (P)RR
on HUVEC membranes, the renin activity of membrane-bound exogenous
prorenin was measured. As reported previously (1,30,31), the receptor-bound prorenin may become
activated to exert renin activity, which can be estimated by
measuring the production of AngI following incubation with sheep
AOG (Fig. 1). In each condition
<3% total sheep AOG was utilized, which indicated that initial
velocities of the renin-AOG reaction were estimated appropriately.
The renin activity per cell of aliskiren-treated HUVECs was
significantly lower than that of the control group by 8 h
(P=0.0127), and to the greatest extent at 24 h (P=0.0025; Fig. 3C). After 8 h of treatment, both
aliskiren-treated HUVECs and controls exhibited a plateau in renin
activity per cell (Fig. 3C).
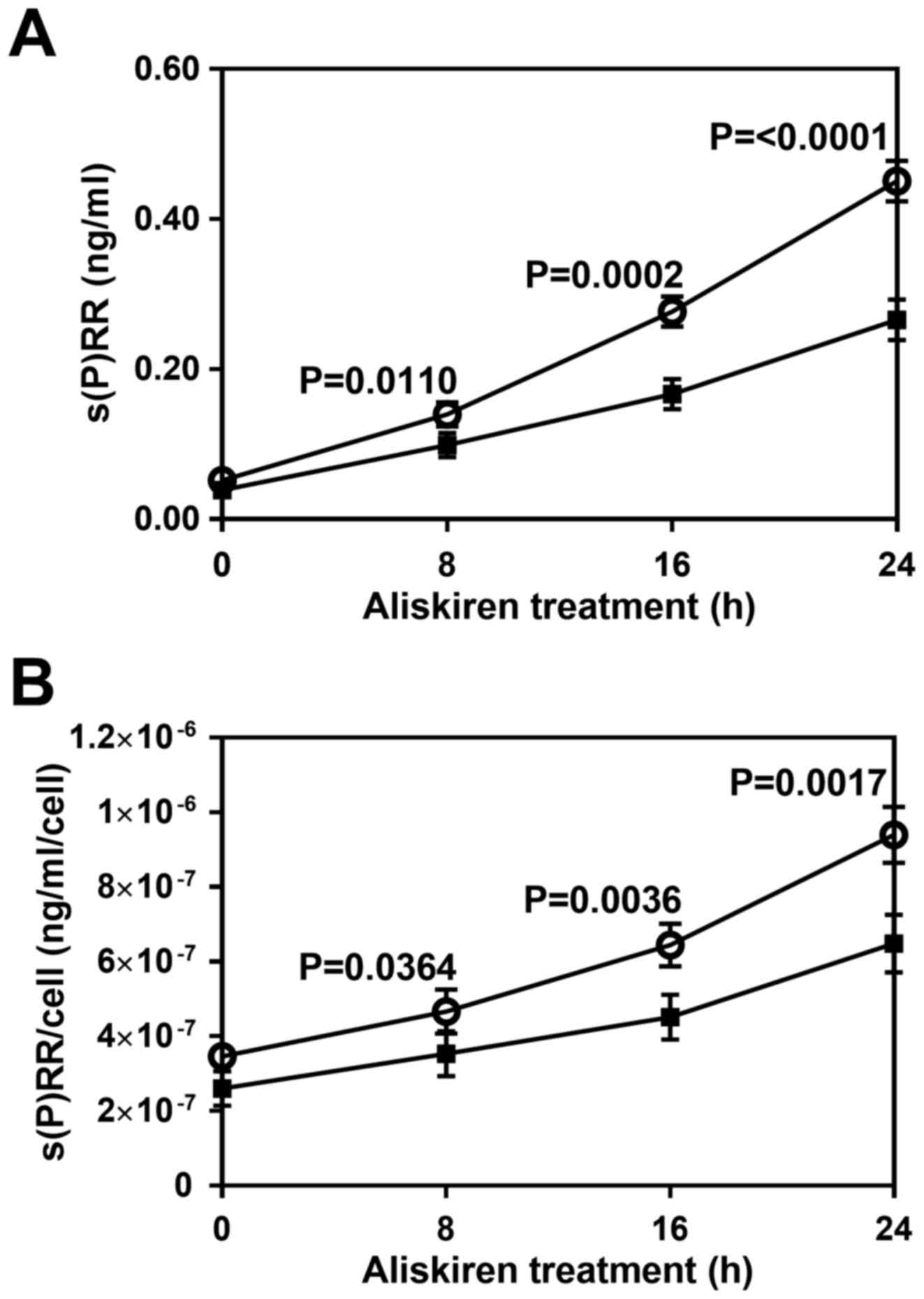
Effect of aliskiren on the level of
s(P)RR released from HUVECs
To examine the effect of aliskiren on the level of
s(P)RR released, HUVECs were treated with or without the drug for
different durations (0–24 h), and s(P)RR concentration was measured
in the culture medium collected following treatment. Not only total
s(P)RR concentration (Fig. 4A) but
also s(P)RR concentration per cell (Fig.
4B) was significantly reduced by the treatment with aliskiren
by 8 h (P<0.05) and most notably at 16–24 h (P<0.01).
Discussion
In the present study, HUVECs were used as an in
vitro model to investigate the effect of aliskiren on the
protein levels of full-length (P)RR as well as s(P)RR. To estimate
its effect on the cell-surface expression of full-length (P)RR,
exogenous prorenin as well as AOG were utilized. Previous studies
have reported that the mannose-6-phosphate receptor present on the
plasma membrane binds to renin and prorenin through oligosaccharide
chains, and that this receptor binding to prorenin does not result
in the generation of extracellular or intracellular angiotensin
(for a review see ref. 24). In
addition, the extracellular domain of (P)RR may take part in the
activation of prorenin, thereby leading to renin activity in
solution (1,30) and on the cell surface (1,31). In the
current study, it was observed that exogenous prorenin could bind
to the HUVEC membranes, and that renin activity was generated from
membrane-bound prorenin. These results indicated that full-length
(P)RR is expressed on the cell surface of HUVECs.
When HUVECs were treated with aliskiren, the renin
activity generated per cell was decreased. This result indicates
that cell-surface expression of full-length (P)RR is reduced by
aliskiren treatment, which is consistent with a previous report
(19). The observed plateau in the
level of renin activity per cell suggests that HUVECs possess a
given level of full-length (P)RR on the cell membrane.
Our group previously reported that s(P)RR was
released from HUVECs (20). From
current results, it was suggested that the quantity of s(P)RR
released from HUVECs was decreased by aliskiren treatment. To the
best of our knowledge, this is the first report indicated that
aliskiren, an anti-hypertensive drug, reduces the level of the
soluble form of (P)RR. The current findings suggest that the
protein levels of s(P)RR may be decreased during anti-hypertensive
treatments with aliskiren. Such a possibility should be considered
when monitoring s(P)RR levels for the diagnosis of diseases.
A recent study reported that s(P)RR could exert
antidiuretic functions and enhance urine concentrating capability
by increasing the expression of renal aquaporin-2, which is
involved in reabsorption of water (15). As indicated in the current study, the
administration of aliskiren may instigate a decrease in the levels
of s(P)RR, which would reduce reabsorption of water. Aliskiren may
act via an alternative route for lowering blood pressure via
aliskiren-induced reduction of s(P)RR. The present findings provide
novel insight into the efficacy of aliskiren. This hypothesis
should be clarified by examining the effect of aliskiren on s(P)RR
levels as well as the β-catenin signaling that increases renal
aquaporin-2 expression (15).
In conclusion, the quantity of s(P)RR appears
dependent on the extent that (P)RR is produced and processed in
HUVECs. Further biochemical and clinical investigations are
warranted to elucidate the underlying mechanisms in HUVEC, which
should provide researchers with an understanding of how aliskiren
changes the quantity of s(P)RR, as a potential antidiuretic
molecule and prospective disease biomarker.
Acknowledgements
Not applicable.
Funding
The current study was supported in part by a
Grant-in-Aid for Scientific Research from the Ministry of
Education, Science and Culture of Japan (grant no. 1907165) and by
a Heisei 28 (Fiscal Year 2016) Health Science Center Foundation
grant from the General Incorporated Foundation Health Science
Center (Kanagawa, Japan).
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY analyzed the data, performed the statistical
analysis and wrote the manuscript. KBB performed the experiments,
analyzed the data and wrote the manuscript. AHMNN participated in
the design of the study and analyzed the data. TN contributed to
the writing of the manuscript. FS conceived the study and
participated in its design and coordination. AE contributed to the
coordination of the study, and helped write the manuscript. All
authors edited the manuscript and approved the final version to be
published.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
The present address of KBB is the Department of
Research and Development, Ichimaru Pharcos Co., Ltd., 318-1 Asagi,
Motosu, Gifu 501–0475, Japan. This study was undertaken
independently of the current work of KBB.
Glossary
Abbreviations
Abbreviations:
|
AngI
|
angiotensin I
|
|
AOG
|
angiotensinogen
|
|
CHO
|
Chinese hamster ovary
|
|
CIn
|
initial concentration of prorenin
|
|
CUB
|
concentration of unbound prorenin
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
FBS
|
fetal bovine serum
|
|
HBS
|
2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid-buffered
saline
|
|
HEPES
|
2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
(P)RR
|
(pro)renin receptor
|
|
s(P)RR
|
soluble (pro)renin receptor
|
References
|
1
|
Nguyen G, Delarue F, Burcklé C, Bouzhir L,
Giller T and Sraer JD: Pivotal role of the renin/prorenin receptor
in angiotensin II production and cellular responses to renin. J
Clin Invest. 109:1417–1427. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krop M, Lu X, Danser AH and Meima ME: The
(pro)renin receptor. A decade of research: What have we learned?
Pflugers Arch. 465:87–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nabi AHMN, Biswas KB, Ebihara A, Nakagawa
T and Suzuki F: Renin angiotensin system in the context of renin,
prorenin, and the (pro)renin receptor. Rev Agric Sci. 1:43–60.
2013.
|
|
4
|
Kinouchi K, Ichihara A, Sano M, Sun-Wada
GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda
M, et al: The (pro)renin receptor/ATP6AP2 is essential for vacuolar
H+-ATPase assembly in murine cardiomyocytes. Circ Res. 107:30–34.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cruciat CM, Ohkawara B, Acebron SP,
Karaulanov E, Reinhard C, Ingelfinger D, Boutros M and Niehrs C:
Requirement of prorenin receptor and vacuolar H+-ATPase-mediated
acidification for Wnt signaling. Science. 327:459–463. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanda A, Noda K, Saito W and Ishida S:
(Pro)renin receptor is associated with angiogenic activity in
proliferative diabetic retinopathy. Diabetologia. 55:3104–3113.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matavelli LC, Huang J and Siragy HM:
(Pro)renin receptor contributes to diabetic nephropathy by
enhancing renal inflammation. Clin Exp Pharmacol Physiol.
37:277–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Contrepas A, Walker J, Koulakoff A, Franek
KJ, Qadri F, Giaume C, Corvol P, Schwartz CE and Nguyen G: A role
of the (pro)renin receptor in neuronal cell differentiation. Am J
Physiol Regul Integr Comp Physiol. 297:R250–R257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shibayama Y, Fujimori T, Nguyen G, Hirose
T, Totsune K, Ichihara A, Kitada K, Nakano D, Kobori H, Kohno M, et
al: (Pro)renin receptor is crucial for Wnt/β-catenin-dependent
genesis of pancreatic ductal adenocarcinoma. Sci Rep. 5:88542015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ludwig J, Kerscher S, Brandt U, Pfeiffer
K, Getlawi F, Apps DK and Schägger H: Identification and
characterization of a novel 9.2-kDa membrane sector-associated
protein of vacuolar proton-ATPase from chromaffin granules. J Biol
Chem. 273:10939–10947. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cousin C, Bracquart D, Contrepas A, Corvol
P, Muller L and Nguyen G: Soluble form of the (pro)renin receptor
generated by intracellular cleavage by furin is secreted in plasma.
Hypertension. 53:1077–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa T, Suzuki-Nakagawa C, Watanabe A,
Asami E, Matsumoto M, Nakano M, Ebihara A, Uddin MN and Suzuki F:
Site-1 protease is required for the generation of soluble
(pro)renin receptor. J Biochem. 161:369–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gonzalez AA, Lara LS, Luffman C, Seth DM
and Prieto MC: Soluble form of the (pro)renin receptor is augmented
in the collecting duct and urine of chronic angiotensin
II-dependent hypertensive rats. Hypertension. 57:859–864. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morimoto S, Ando T, Niiyama M, Seki Y,
Yoshida N, Watanabe D, Kawakami-Mori F, Kobori H, Nishiyama A and
Ichihara A: Serum soluble (pro)renin receptor levels in patients
with essential hypertension. Hypertens Res. 37:642–648. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu X, Wang F, Xu C, Soodvilai S, Peng K,
Su J, Zhao L, Yang KT, Feng Y, Zhou SF, et al: Soluble (pro)renin
receptor via β-catenin enhances urine concentration capability as a
target of liver X receptor. Proc Natl Acad Sci USA.
113:E1898–E1906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wood JM, Maibaum J, Rahuel J, Grütter MG,
Cohen NC, Rasetti V, Rüger H, Göschke R, Stutz S, Fuhrer W, et al:
Structure-based design of aliskiren, a novel orally effective renin
inhibitor. Biochem Biophys Res Commun. 308:698–705. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pantzaris ND, Karanikolas E, Tsiotsios K
and Velissaris D: Renin inhibition with aliskiren: A decade of
clinical experience. J Clin Med. 6:62017. View Article : Google Scholar
|
|
18
|
Feldman DL, Jin L, Xuan H, Contrepas A,
Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, et al:
Effects of aliskiren on blood pressure, albuminuria, and (pro)renin
receptor expression in diabetic TG(mRen-2)27 rats. Hypertension.
52:130–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferri N, Greco CM, Maiocchi G and Corsini
A: Aliskiren reduces prorenin receptor expression and activity in
cultured human aortic smooth muscle cells. J Renin Angiotensin
Aldosterone Syst. 12:469–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Biswas KB, Nabi AN, Arai Y, Nakagawa T,
Ebihara A, Ichihara A, Inagami T and Suzuki F: Qualitative and
quantitative analyses of (pro)renin receptor in the medium of
cultured human umbilical vein endothelial cells. Hypertens Res.
34:735–739. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nachman RL and Jaffe EA: Endothelial cell
culture: Beginnings of modern vascular biology. J Clin Invest.
114:1037–1040. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nasir UM, Takahashi K, Nagai T, Nakagawa
T, Suzuki F and Nakamura Y: Two peaks in pH dependence of
renin-angiotensinogen reaction. Biosci Biotechnol Biochem.
62:338–340. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki F, Hayakawa M, Nakagawa T, Nasir
UM, Ebihara A, Iwasawa A, Ishida Y, Nakamura Y and Murakami K:
Human prorenin has ‘gate and handle’ regions for its
non-proteolytic activation. J Biol Chem. 278:22217–22222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Danser AH and Deinum J: Renin, prorenin
and the putative (pro)renin receptor. Hypertension. 46:1069–1076.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagase M, Suzuki F, Sawai Y, Orihashi T,
Inui Y, Nakagawa T and Nakamura Y: Purification and some properties
of recombinant sheep angiotensinogen expressed in Chinese hamster
ovary cells. Biomed Research Tokyo. 18:439–443. 1997. View Article : Google Scholar
|
|
26
|
Ebihara A, Nasir UM, Yoshida S, Kondou T,
Nakagawa T, Fukamizu A, Suzuki F, Nakamura Y and Murakami K: Sialic
acid residue of ovine angiotensinogen does not affect the
reactivity to human renin. Biomed Research Tokyo. 21:105–109. 2000.
View Article : Google Scholar
|
|
27
|
Suzuki F, Yamashita S, Takahashi A, Ito M,
Miyazaki S, Nagata Y and Nakamura Y: Highly sensitive microplate
ELISA for angiotensin I using 3,3′5,5′tetramethylbenzidine. Clin
Exp Hypertens. A12:83–95. 1990.
|
|
28
|
Nabi AH, Biswas KB, Nakagawa T, Ichihara
A, Inagami T and Suzuki F: Prorenin has high affinity multiple
binding sites for (pro)renin receptor. Biochim Biophys Acta.
1794:1838–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ku HH: Notes Use Propag Error Formulas. J
Res Natl Bur Stand. 70C:263–273. 1966.
|
|
30
|
Nabi AH, Kageshima A, Uddin MN, Nakagawa
T, Park EY and Suzuki F: Binding properties of rat prorenin and
renin to the recombinant rat renin/prorenin receptor prepared by a
baculovirus expression system. Int J Mol Med. 18:483–488.
2006.PubMed/NCBI
|
|
31
|
Nurun NA, Uddin NM, Nakagawa T, Iwata H,
Ichihara A, Inagami T and Suzuki F: Role of ‘handle’ region of
prorenin prosegment in the non-proteolytic activation of prorenin
by binding to membrane anchored (pro)renin receptor. Front Biosci.
12:4810–4817. 2007. View
Article : Google Scholar : PubMed/NCBI
|