Introduction
Cancer is the second leading cause of mortality in
the world after cardiovascular diseases and was responsible for 8.7
million mortalities in 2015 (1).
Breast cancer is the most common type of malignancy in US women
with prevalence rate of 25% (2).
According to the International Agency for Research on Cancer
GLOBOCAN estimates, this type of malignancy accounted for 1.7
million cancers and 521,900 mortalities in 2012 worldwide (3).
In Iran, breast cancer is the most common type of
cancer in women with an incidence that shows a yearly increase
(4–6).
An estimated 266,120 new cases of invasive breast cancer are
expected to be diagnosed in 2018 in women in the US, along with
63,960 new cases of non-invasive (in situ) breast cancer
(7).
Approximately 80% of breast cancers are estrogen
receptor (ER) positive and are therefore eligible for adjuvant
endocrine therapy (8). Tamoxifen is
the most commonly-used anti-estrogenic drug in adjuvant therapy for
hormone-dependent breast cancer, and is able to minimize the risk
of recurrence and mortality rate, particularly in pre-menopausal
patients (9). Furthermore, the role
of this agent as a chemopreventive in high risk women has been made
evident (10). Tamoxifen acts through
competing with endogenous estrogen for binding to ER and blocks the
proliferative actions of estrogen on breast cells (11).
Tamoxifen is considered a ‘prodrug’, requiring
metabolic activation by the cytochrome P450 2D6 (CYP2D6) enzyme.
The main metabolites of tamoxifen are endoxifen and
4-hydroxitamoxifen, which have a 30–100 times stronger affinity for
binding to ER compared with tamoxifen (12,13).
However, ~30% of patients receiving tamoxifen therapy do not
benefit from this therapeutic approach (14). The CYP2D6 gene is highly polymorphic,
and metabolism in the CYP2D6 pathway exhibits marked variation
between different racial and ethnic populations (15). The genetic polymorphisms leading to
diminished enzymatic activity result in low levels of the main
active metabolite endoxifen, which may lead to a poor response to
tamoxifen (16).
To date, 105 allelic variants of CYP2D6 have been
described (17). These alleles can be
classified into three categories, namely functional alleles,
reduced-function alleles and nonfunctional alleles, which in turn
determine the CYP2D6 phenotypes of poor metabolizers (PMs),
intermediate metabolizers (IMs), extensive metabolizers (EMs) and
ultra-rapid metabolizers (18).
Four major mutated alleles of CYP2D6 including
CYP2D6*3, CYP2D6*4, CYP2D6*10 and CYP2D6*17 are among the most
frequent polymorphic alleles in humans (19,20). These
alleles have been described as the most frequent mutant alleles
throughout different Asian countries (18,21–23).
CYP2D6*3 and CYP2D6*4 are associated with PM phenotype, and
CYP2D6*10 and CYP2D6*17 are associated with IM phenotype.
It is notable that there are controversies among
studies, regarding the association between CYP2D6 genetic
polymorphisms and alterations in the efficacy of tamoxifen in
patients with breast cancer. To have an improved insight into the
polymorphism of CYP2D6 alleles in a cohort of patients with breast
cancer, the present study was conducted to determine the CYP2D6
genotyping in a group of patients with breast cancer treated with
adjuvant tamoxifen in the north of Iran.
Materials and methods
Patients
The present study included 84 female patients with
hormone-sensitive breast cancer, with or without disease recurrence
and taking adjuvant tamoxifen for at least four weeks. The patients
who had presented to Tooba Clinic, a university-affiliated clinic
in the north of Iran, were prospectively enrolled into the study
from June to November 2017. Written informed consent was obtained
from each patient prior to enrolment. The study population
consisted of all women aged 18 years or higher with a diagnosis of
breast cancer. The Internal Review Board of Mazandaran University
of Medical Sciences (Sari, Iran) approved the study protocol under
the number IR.MAZUMS.REC.1397.90.
Sample collection and DNA
extraction
From each patient, 5 ml peripheral blood was drawn
and stored in a tube containing EDTA anticoagulant. The samples
were stored at −20°C. Genomic DNA was extracted from 300 µl of the
peripheral blood using a column-based DNA isolation kit (Denazist
Asia Co., Mashhad, Iran) according to the manufacturer's protocol.
The concentration and purity of the extracted DNA was measured
using a NanoDrop spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Immunohistochemical (IHC)
analysis
IHC of HER-2/neu protein was performed on
paraffin-embedded breast tissue sections, which were fixed in 10%
neutral-buffered formalin at 25°C for 24 h. Thereafter, the fixed
tissues were deparaffinized in xylene and then rehydrated in a
graded ethanol series (100, 96, 80 and 70%), the samples were cut
into 3 to 4-µm sections and mounted on Poly-L-Lysine-coated slides.
Next, deparaffinization and blocking of endogenous peroxidase
activity with 0.3% hydrogen peroxide in methanol at 25°C for 15 min
was performed. This step is important to prevent detachment of
tissues from the slides during the antigen retrieval process. Next,
antigen retrieval or the high-temperature heating method were used
to recover antigenic-sites hidden in tissue samples. Slides were
placed in plastic Coplin jars filled with Trizma base solution with
pH:9 and then placed in the center of the microwave oven. The jars
were heated for 5 min in high temperature 900 W. Next, the Coplin
jars were removed from the oven to cool for 15 min. At the end,
slides were rinse in TBS and distilled water for 20 min.
Treated slides were stained as follows: HER-2/neu
immunostaining was performed using rabbit anti-human c-erbB-2
oncoprotein as a primary antibody (cat. no. RMAB008; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) for 1 h at 25°C (1:100
dilution). Binding of the primary antibody was checked by Dako
Quick-Staining, Labelled Streptavidin-Biotin system (Dako; Agilent
Technologies, Inc.). Following washing with TBS solution, membranes
were incubated with 50 µl secondary antibody (cat. no. K5007; Dako;
Agilent Technologies, Inc.) at 25°C for 30 min. The detection
reagent in the kit (cat. no. K5007; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) consisted of a dextran backbone to
which a large number of horseradish peroxidase molecule and
secondary antibody molecule had been coupled in order to react to a
mouse/rabbit primary antibody, followed by the addition of
diaminobenzidine (DAB) as a chromogen (10 min at 25°C). Each slide
was scored in a blinded fashion by two pathologists using a light
microscope (magnification, ×400), according to the manufacturer's
protocol. The immunostaining was read in a semiquantitative manner
and graded as follows: 0, 1+, 2+ and 3+. In this study we
considered 2+ or higher as HER-2/neu positive (24).
Polymerase chain reaction (PCR)
A PCR-based restriction fragment-length polymorphism
analysis was performed to determine the CYP2D6 alleles (CYP2D6*3,
CYP2D6*4, CYP2D6*10 and CYP2D6*17). PCR was performed using 300 ng
of the template DNA, 10 µl Taq DNA polymerase 2× master mix RED
(Ampliqon A/S, Odense, Denmark) containing 150 mM Tris-HCl pH 8.5,
40 mM (NH4)2SO4, 3 mM
MgCl2, 0.2% Tween-20, 0.4 mM of each dNTP and 0.2 U/µl
Ampliqon Taq DNA polymerase, along with 0.015 pM appropriate
primers and 3 µl nuclease-free water.
The PCR program consisted of an initial denaturation
step at 95°C for 5 min followed by 35 cycles of denaturation at
95°C for 30 sec, annealing at 62°C for 40 sec (63°C for CYP2D6*10),
extension at 72°C for 30 sec, and a final step of extension at 72°C
for 5 min. Following confirmation of the amplified fragments of the
expected size on agarose gel containing DNA green fluorescent dye
(Pars Tous Biotechnology, Mashhad, Iran), the PCR products were
restriction-digested with 10 U/µl MspI, BstNI and
HphI (Fermentas; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C (55°C for CYP2D6*10) for 16 h. The DNA fragments
were electrophoresed through a 3% agarose gel containing DNA green
fluorescent dye for CYP2D6*3, *10 and *17 and through a 2% agarose
gel for CYP2D6*4 as in other studies (25,26).
Table I presents a summary of the
single nucleotide polymorphisms, primers, product lengths and
restriction enzymes. The expected digested fragments are shown in
Table II.
 | Table I.CYP2D6 variant primers, product
lengths and enzymes used for polymerase chain reaction-restriction
fragment length polymorphism analysis. |
Table I.
CYP2D6 variant primers, product
lengths and enzymes used for polymerase chain reaction-restriction
fragment length polymorphism analysis.
| Variant | rs no. | Primers, 5–3 | Product length,
bp | Enzyme | Refs. |
|---|
| CYP2D6*3 | rs35742686 |
F-ATGAGCTGCTAACTGAGCCC | 270 | MspI | (43) |
|
|
|
R-CCGAGAGCATACTCGGGAC |
|
|
|
| CYP2D6*4 | rs3892097 |
F-TGCCGCCTTCGCCAACCACT | 309 | BstNI | (26) |
|
|
|
R-TCGCCCTGCAGAGACTCCTC |
|
|
|
| CYP2D6*10 | Rs1065852 |
F-GTGCTGAGAGTGTCCTGCC | 344 | HphI | (26) |
|
|
|
R-CACCCACCATCCATGTTTGC |
|
|
|
| CYP2D6*17 | rs28371706 |
F-CGGTGGTCGTGCCTCAATG | 167 | HphI | (44) |
|
|
|
R-CCCGGGTCCCACGGAAATCT |
|
|
|
 | Table II.The length of products and products
of digestion were shown. |
Table II.
The length of products and products
of digestion were shown.
| Polymorphisms | Product length
(bp) | Enzyme | Products of
digestion length | Refs. |
|---|
| CYP2D6*3 | 270 | MspI | Wild-type
(AA):188/82 | (43) |
|
|
|
| Heterozygote (A/-):
188/168/20 |
|
|
|
|
| Mutant (−/-):
168/20 |
|
| CYP2D6*4 | 309 | BstNI | Wild-type (GG):
201/108 | (26) |
|
|
|
| Heterozygote (GA):
309/201/108 |
|
|
|
|
| Mutant (AA):
309 |
|
| CYP2D6*10 | 344 | HphI | Wild-type (CC):
282/62 | (26) |
|
|
|
| Heterozygote (CT):
282/182/100/62 |
|
|
|
|
| Mutant (TT):
182/100/62 |
|
| CYP2D6*17 | 167 | HphI | Wild-type (TT):
167 | (44) |
|
|
|
| Heterozygote (CT):
167/88/55 |
|
|
|
|
| Mutant (CC):
88/55 |
|
Statistical analysis
Considering the non-interventional characteristics
of the study, only descriptive statistics (e.g., mean, standard
deviation, frequency and percent) were computed and reported using
SPSS 25.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
Baseline characteristics of the patients (Table III) and also clinical and
pathological features of the patients (Table IV) were presented. The mean age was
45.4±6.8 years (range, 29–63 years). Almost half of patients had a
positive family history of breast cancer. Most of patients were in
the premenopausal state (89.3%). HER-2 negative state was found in
~55% of the patients. Majority of patients (~93%) were
hormone-positive. Regarding staging, most patients (54.8%) were
assigned as Stage 2 disease. More than 80% of patient did not have
any evidence of metastasis. Most patients (about 85%) were
undergone adjuvant chemoradiation.
 | Table III.Baseline characteristics of
patients. |
Table III.
Baseline characteristics of
patients.
| Variable | n | % |
|---|
| Number of
patients | 84 | 100.0 |
| Mean age, years
(standard deviation) | 45.4 (6.8) |
|
| Family history |
|
|
|
Negative | 43 | 51.2 |
|
Positive | 41 | 48.8 |
| Marital status |
|
|
|
Married | 73 | 86.9 |
|
Unmarried | 11 | 13.1 |
| Menopausal
status |
|
|
|
Premenopausal | 75 | 89.3 |
|
Post-menopausal | 9 | 10.7 |
 | Table IV.Clinical characteristics and
pathological features of patients. |
Table IV.
Clinical characteristics and
pathological features of patients.
| Variable | n | % |
|---|
| HER-2/neu |
|
|
| 0 | 38 | 45.2 |
| 1+ | 20 | 23.8 |
| 2+ | 9 | 10.7 |
| 3+ | 17 | 20.2 |
|
Estrogen/progesterone receptor status |
|
|
|
Positive/positive (+/+) | 78 | 92.9 |
|
Positive/negative (+/−) | 6 | 7.1 |
| Recurrence |
|
|
|
Positive | 15 | 17.9 |
|
Negative | 69 | 82.1 |
| TNM stage |
|
|
| I | 10 | 11.9 |
| II | 46 | 54.8 |
|
III | 14 | 16.7 |
| IV | 14 | 16.7 |
| Lymph node
involvement |
|
|
|
Positive | 66 | 78.6 |
|
Negative | 18 | 21.4 |
| Metastasis |
|
|
|
Negative | 69 | 82.1 |
|
Bone | 11 | 13.1 |
|
Liver | 3 | 3.6 |
| Bone
and liver | 1 | 1.2 |
| History of
resection |
|
|
|
Positive | 82 | 97.6 |
|
Negative | 2 | 2.4 |
| Type of
resection |
|
|
| Radical
mastectomy | 1 | 1.2 |
|
Modified radical
mastectomy | 49 | 59.8 |
|
Conservative | 32 | 39.0 |
| Previous
chemotherapy |
|
|
|
Positive | 80 | 95.2 |
|
Negative | 4 | 4.8 |
| Type of
chemotherapy |
|
|
|
Adjuvant | 70 | 87.5 |
|
Neo-adjuvant | 10 | 12.5 |
| Concurrent
diseases |
|
|
|
Positive | 37 | 44.1 |
|
Negative | 47 | 55.9 |
| History of
radiotherapy |
|
|
|
Positive | 71 | 84.5 |
|
Negative | 13 | 15.5 |
| History of oral
contraceptive pill use |
|
|
|
Positive | 44 | 52.4 |
|
Negative | 40 | 47.6 |
Genotypes of patients
The allele and genotype frequency distributions of
the CYP2D6*3 (A2549del), *4 (G1846A), *10 (C100T) and *17 (C1023T)
variants were analyzed in the blood samples of 84 patients with
ER-positive breast cancer.
The figures present the alleles in the pattern of
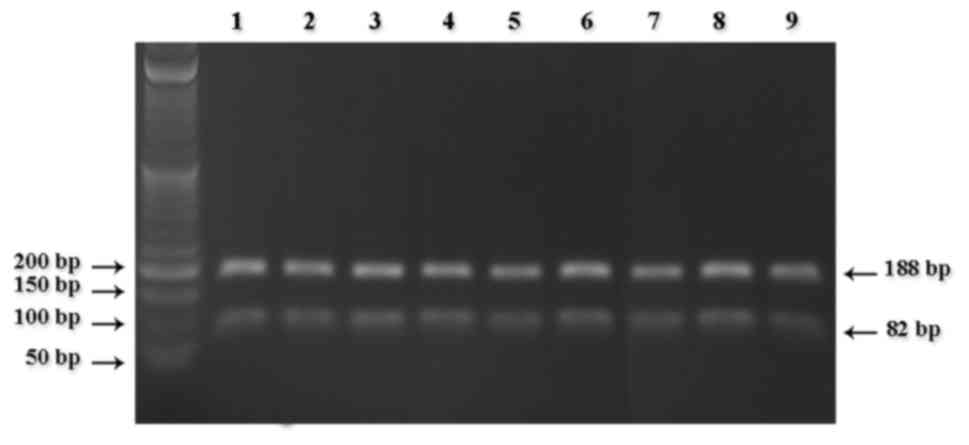
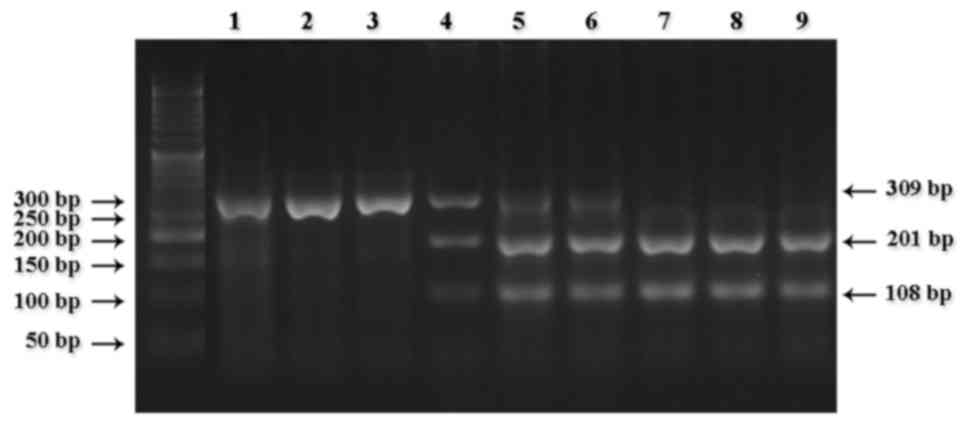
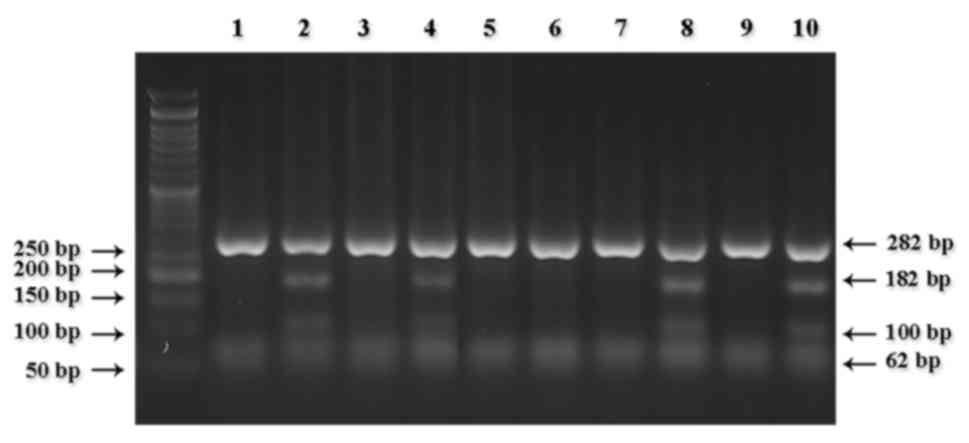
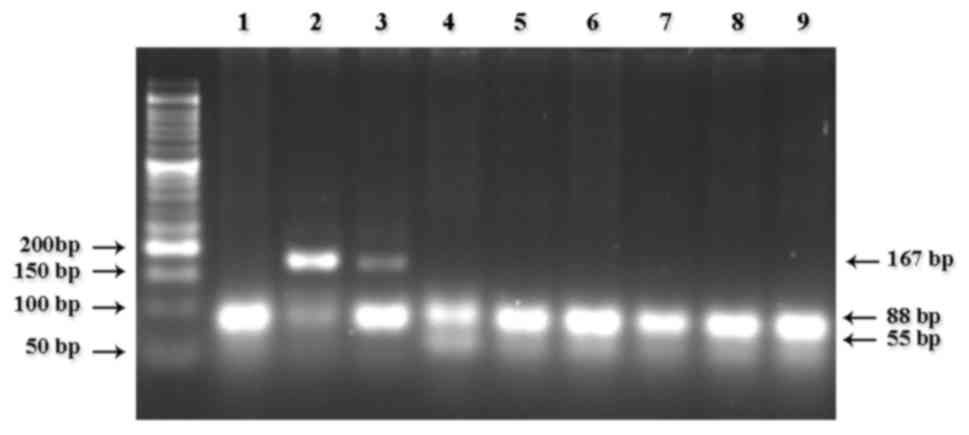
the fragments digested for the detection of CYP2D6*3 (Fig. 1), CYP2D6*4 (Fig. 2), CYP2D6*10 (Fig. 3) and CYP2D6*17 (Fig. 4).
No mutant CYP2D6*3 heterozygous (A/-)
or homozygous (−/-) alleles were observed in the study population;
however, mutant CYP2D6*10 heterozygous alleles (C/T) were present
in 22 cases (26.2%)
Regarding the metabolizing phenotype, CYP2D6
activity was classified as poor, intermediate or extensive, as
presented in Table V. Of the four
genotype groups, 3 (3.6%) and 41 (48.8%) of the patients were PMs
(CYP2D6*4 homozygote, A/A) and IMs (CYP2D6*4 heterozygote, G/A),
respectively. Variant *17 was less common and was detected only as
heterozygous (C/T) in 3 patients (3.6%), classified as IMs.
 | Table V.CYP2D6 genotype and metabolizer
category. |
Table V.
CYP2D6 genotype and metabolizer
category.
| Frequency, %
(n) | Genotype | Activity | Category |
|---|
| PM | None | Two null alleles
(CYP2D6 *4) | *4(AA): 3.6
(3) |
| IM | Reduced | One null allele
(CYP2D6 *4, *10, *17) | *4(GA): 48.8
(41) |
|
|
|
| *10(CT): 26.2
(22) |
|
|
|
| *17(CT): 3.6
(3) |
| EM | Normal | Two
wild-type/normal alleles | *3(AA): 100.0
(84) |
|
|
| (CYP2D6 *3, *4,
*10, *17) | *4(GG): 47.6
(40) |
|
|
|
| *10(CC): 73.8
(62) |
|
|
|
| *17(CC): 96.4
(81) |
Discussion
Genetic polymorphism of CYP2D6 in Iranian breast
cancer patients treated with tamoxifen was the focus of the present
study. There has been numerous studies performed on this topic with
conflicting results, particularly when examining the clinical
impact of this variation on patient outcome and response to
hormonal therapy (23,27,28). Of
the four CYP2D6 alleles evaluated in the present study, the most
common alleles were CYP2D6*4 and CYP2D6*10, followed by CYP2D6*17.
The CYP2D6*10 allele has been described as the most frequently
mutated gene in various Asian countries, including China, Japan and
Korea (29–31); however, the CYP2D6*4 allele is
reportedly the most common among Caucasian populations (32,33).
Table VI presents the frequencies of
the CYP2D6 nonfunctional alleles *3, *4, *10 and *17 in different
populations.
 | Table VI.Frequency of four CYP2D6
non-functional alleles in different populations. |
Table VI.
Frequency of four CYP2D6
non-functional alleles in different populations.
|
|
|
|
| Allele frequency
(%) |
|
|---|
|
|
|
|
|
|
|
|---|
| Population | Year | N | Patient Status | *3 | *4 | *10 | *17 | Refs. |
|---|
| Iranian | 2018 | 84 | BCP | Nd | 52.4 | 26.2 | 3.6 | Present study |
| Iranian | 2009 | 100 | HP | – | 12.5 | 9.0 | Nd | (26) |
| Iranian | 2011 | 100 | HP | 0.5 | 9.0 | – | – | (46) |
| Iranian | 2015 | 101 | BCP | – | 36.6 | – | – | (37) |
| Japanese | 2000 | 412 | HP | – | 0.2 | 38.1 | – | (22) |
| Chinese | 2009 | 100 | HP | – | 1.0 | 49.0 | – | (21) |
| Spanish | 2006 | 105 | HP | 0.95 | 13.8 | – | – | (39) |
| Brazilian | 2016 | 80 | BCP | – | 13.75 | 21.25 | 10.0 | (40) |
| Pakistani | 2016 | 232 | BCP | – | – | 7.0 | – | (35) |
| South-Indian | 2006 | 447 | HP | Nd | 7.3 | 10.2 | Nd | (36) |
| Malaysian
Indian | 2016 | 80 | BCP | – | 28.6 | 21.4 | – | (18) |
| Malaysian
Malay | 2016 | 80 | BCP | – | 2.9 | 54.8 | – | (18) |
| Malaysian
Chinese | 2016 | 80 | BCP | – | Nd | 71.4 | – | (18) |
| Malaysian
Malay | 2001 | 107 | HP | – | 2.8 | 49.5 | 0.5 | (42) |
| Korean | 2006 | 400 | HP | – | – | 45.0 | – | (34) |
| Thai | 2012 | 67 | BCP | – | – | 51.0 |
| (23) |
| Turkish | 2009 | 100 | HP | – | 28.0 | – | – | (38) |
| Turkish | 2005 | 140 | HP | 2.5 | 13.9 | – | – | (47) |
| Saudi Arabians | 1997 | 101 | HP | – | 3.5 | 3.0 | 3.0 | (20) |
| USA | 2006 | 158 | BCP | 0.013 | 0.161 | 0.035 | – | (41) |
Genotype combinations were used to categorize CYP2D6
metabolism phenotypes as PM, IM and EM. The three major mutated
alleles of CYP2D6 (*3, *4 and *5) account for the majority of PM
alleles (19). Asians have exhibited
a high prevalence for the IM allele CYP2D6*10, which may lead to a
reduced activity of the enzyme and lower serum concentrations of
endoxifen (31). This finding may
explain the poor survival outcome in Asian women with breast cancer
receiving adjuvant tamoxifen therapy (32).
The allele frequency of CYP2D6*10 was 26.2% in the
present study, which is markedly less than that observed in Korea
[45.0% (34)] and other eastern
countries, including Thailand (51.0%) (18), Malaysia (54.8% in Malays and 71.4% in
Malaysian-Chinese) (23) and China
(49.0%) (21), but higher than in
Pakistan (7.0%) (35) and South India
(10.2%) (36). Previous study
reported the prevalence of CYP2D6*10 as ~9.0% in Iranian
Azerbaijanis (26), but another study
of Iranians of different ethnicities reported the prevalence of the
allele to be 39.3% (24.3% for homozygous T/T and 15.0% for
heterozygous C/T) (25).
The frequency of CYP2D6*4 varies across different
races, and frequencies of 36.6, 28.6, 28.0, 13.8, 13.8, 7.3 and
0.2% have been reported in Iranians, Malaysian Indians, Turks,
Spanish subjects, Brazilians, South-Indians and North Americans,
respectively (18,36–41).
Conversely, the frequency of CYP2D6*4 varies from 0.2 to 3.5% among
Japanese, Chinese and Saudi Arabians, which is considered
relatively low (20–22). In the present study, a notably higher
prevalence of 52.4% was observed for the CYP2D6*4 allele.
According to the present findings, the low frequency
or absence of the CYP2D6*3 and *17 alleles in the Iranian
population appears to reflect a general similarity (20,36,39,42).
However, a major limitation of the present study was
the relatively small number of patients. Although the results may
provide clinical indication and rational for the administration of
tamoxifen as first-line hormone therapy, the findings require
confirmation in a larger group of patients with breast cancer.
Furthermore, the effect of the genetic polymorphisms in terms of
treatment efficacy of adjuvant tamoxifen warrants further
studies.
To conclude, the present study demonstrated that the
CYP2D6 nonfunctional alleles *4 and *10 were relatively frequent in
an Iranian population of breast cancer patients. This finding may
affect the selection of an optimal hormone therapy, as patients
with low CYP2D6 pathway activity may not sufficiently convert
tamoxifen to its active metabolite endoxifen.
Acknowledgements
This article is derived from the thesis Evaluation
of CYP2D6*3, *4, *10 and *17 polymorphisms on the Tamoxifen
Pharmacokinetics in Breast Cancer Patients, Mazandaran, Iran
supervised by Professor Ebrahim Salehifar and submitted by Dr
Fatemeh Saghafi to the Faculty of Pharmacy of Mazandaran University
of Medical Sciences, Sari, Iran, in partial fulfillment of the
Requirements for the Degree of Doctor of Philosophy (Ph.D.) in
Clinical Pharmacy.
Funding
The manuscript was financially supported by a grant
from the Research and Technology Department of Mazandaran
University of Medical Sciences (grant no. 1397.90), Sari, Iran.
Data sharing is not applicable to this article, as no datasets were
generated or analyzed during the current study.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
FS was involved in collecting data and performing
the genotyping. ES designed the study and supervised all aspects of
the study. GJ and EZ aided in the recruitment of the patients and
in the collection of clinical data. AHO and OA performed the DNA
extraction and RFLP PCR experiments. SM was involved in the design
of the study and data analysis. All authors have read the
manuscript and its revisions and confirmed the contents of the
manuscript.
Ethics approval and consent to
participate
All patients signed an informed consent form prior
to participation in the study. The study was approved by the Ethics
Committee of Mazandaran University of Medical Sciences, Sari, Iran
(approval no. IR.MAZUMS.REC.1397.90).
Patient consent for publication
Consent for publication was agreed upon in the
written consent forms signed by the patients.
Competing interests
The authors declare that they have no competing
interests regarding the publication of this paper.
References
|
1
|
Fitzmaurice C, Allen C, Barber RM,
Barregard L, Bhutta ZA, Brenner H, et al: Global, regional, and
national cancer incidence, mortality, years of life lost, years
lived with disability, and disability-adjusted life-years for 32
cancer groups, 1990 to 2015: A systematic analysis for the global
burden of disease study. JAMA Oncol. 3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolahdoozan S, Sadjadi A, Radmard AR and
Khademi H: Five common cancers in Iran. Arch Iran Med. 13:143–146.
2010.PubMed/NCBI
|
|
5
|
Mousavi SM, Montazeri A, Mohagheghi MA,
Jarrahi AM, Harirchi I, Najafi M and Ebrahimi M: Breast cancer in
Iran: An epidemiological review. Breast J. 13:383–391. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karimi A, Shandiz FH, Sharifzadeh GR,
Zoubin F, Fatemeh T and Rahmani S: Breast cancer in Iran. Life Sci
J. 10:2013.PubMed/NCBI
|
|
7
|
Kumar G: Symptom Clusters and Quality of
Life Trajectories in Breast Cancer Patients Before and After
Chemotherapy. University of Nebraska Medical Center. 2018.
|
|
8
|
Motaghed M: Cytotoxic, cytostatic and
anti-estrogenic effect of Thymoquinone on estrogen
receptor-positive breast cancer MCF7 cell line. American Journal of
Life Sciences. 3:7–14. 2015. View Article : Google Scholar
|
|
9
|
Hoskins JM, Carey LA and McLeod HL: CYP2D6
and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer.
9:576–586. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taylor R and Taguchi K: Tamoxifen for
breast cancer chemoprevention: Low uptake by high-risk women after
evaluation of a breast lump. Ann Fam Med. 3:242–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar KS and Kumar MM: Antiestrogen
therapy for Breast Cancer: An overview. Cancer Ther. 6:2008.
|
|
12
|
Kiyotani K, Mushiroda T, Sasa M, Bando Y,
Sumitomo I, Hosono N, Kubo M, Nakamura Y and Zembutsu H: Impact of
CYP2D6*10 on recurrence-free survival in breast cancer patients
receiving adjuvant tamoxifen therapy. Cancer Sci. 99:995–999. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morrow PK, Serna R, Broglio K, Pusztai L,
Nikoloff DM, Hillman GR, Fontecha M, Li R, Michaud L, Hortobagyi G,
et al: Effect of CYP2D6 polymorphisms on breast cancer recurrence.
Cancer. 118:1221–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Group EBCTC: Tamoxifen for early breast
cancer: An overview of the randomised trials. Early Breast Cancer
Trialists' Collaborative Group. Lancet. 351:1451–1467. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bertilsson L, Dahl ML, Dalén P and
Al-Shurbaji A: Molecular genetics of CYP2D6: Clinical relevance
with focus on psychotropic drugs. Br J Clin Pharmacol. 53:111–122.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Schaik RH: Cancer treatment and
pharmacogenetics of cytochrome P450 enzymes. Invest New Drugs.
23:513–522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai DP, Geng PW, Wang SH, Cai J, Hu LM,
Nie JJ, Hu JH, Hu GX and Cai JP: In vitro functional assessment of
22 newly identified CYP2D6 allelic variants in the Chinese
population. Basic Clin Pharmacol Toxicol. 117:39–43. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chin FW, Chan SC, Rahman Abdul S, Noor
Akmal S and Rosli R: CYP2D6 genetic polymorphisms and phenotypes in
different ethnicities of Malaysian breast cancer patients. Breast
J. 22:54–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yagihashi T, Mizuno M, Chino B, Sato Y,
Sakuma K, Takebayashi T, Takao T and Kosaki K: Effects of the
CYP2D6*10 alleles and co-medication with CYP2D6-dependent drugs on
risperidone metabolism in patients with schizophrenia. Hum
Psychopharmacol. 24:301–308. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
McLellan RA, Oscarson M, Seidegård J,
Evans DA and Ingelman-Sundberg M: Frequent occurrence of CYP2D6
gene duplication in Saudi Arabians. Pharmacogenetics. 7:187–191.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Q, Yu XM, Lin HB, Wang L, Yun QZ, Hu
SN and Wang DM: Genetic polymorphism, linkage disequilibrium,
haplotype structure and novel allele analysis of CYP2C19 and CYP2D6
in Han Chinese. Pharmacogenomics J. 9:380–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishida Y, Fukuda T, Yamamoto I and Azuma
J: CYP2D6 genotypes in a Japanese population: Low frequencies of
CYP2D6 gene duplication but high frequency of CYP2D6*10.
Pharmacogenetics. 10:567–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rungwanonchai P, Ayudhya DPN, Areepium N,
Voravud N and Satthaporn S: Prevalence of CYP2D6* 10 Genotype in
Thai Breast Cancer Patients. Wētchasān phǣt Thahān Bok. 65:113–118.
2012.
|
|
24
|
Roepman P, Horlings HM, Krijgsman O, Kok
M, Bueno-de-Mesquita JM, Bender R, Linn SC, Glas AM, van de Vijver
MJ, et al: Microarray-based determination of estrogen receptor,
progesterone receptor, and HER2 receptor status in breast cancer.
Clin Cancer Res. 15:7003–7011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bagheri A, Kamalidehghan B, Haghshenas M,
Azadfar P, Akbari L, Sangtarash MH, Vejdandoust F, Ahmadipour F,
Meng GY and Houshmand M: Prevalence of the CYP2D6*10 (C100T), *4
(G1846A), and *14 (G1758A) alleles among Iranians of different
ethnicities. Drug Des Devel Ther. 9:2627–2634. 2015.PubMed/NCBI
|
|
26
|
Kouhi H, Hamzeiy H, Barar J, Asadi M and
Omidi Y: Frequency of five important CYP2D6 alleles within an
Iranian population (Eastern Azerbaijan). Genet Test Mol Biomarkers.
13:665–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou LP, Luan H, Dong XH, Jin GJ, Man DL
and Shang H: Genetic variants of CYP2D6 gene and cancer risk: A
HuGE systematic review and meta-analysis. Asian Pac J Cancer Prev.
13:3165–3172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gutman G, Morad T, Peleg B, Peretz C,
Bar-Am A, Safra T and Grisaru D: CYP1A1 and CYP2D6 gene
polymorphisms in Israeli Jewish women with cervical cancer. Int J
Gynecol Cancer. 19:1300–1302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teh LK and Bertilsson L: Pharmacogenomics
of CYP2D6: Molecular genetics, interethnic differences and clinical
importance. Drug Metab Pharmacokinet. 27:55–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oscarson M: Pharmacogenetics of drug
metabolising enzymes: Importance for personalised medicine. Clin
Chem Lab Med. 41:573–580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramamoorthy Y, Tyndale RF and Sellers EM:
Cytochrome P450 2D6.1 and cytochrome P450 2D6.10 differ in
catalytic activity for multiple substrates. Pharmacogenetics.
11:477–487. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu Y, Sun Y, Yao L, Shi L, Wu Y, Ouyang T,
Li J, Wang T, Fan Z, Fan T, et al: Association between CYP2D6 *10
genotype and survival of breast cancer patients receiving tamoxifen
treatment. Ann Oncol. 19:1423–1429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
LLerena A, Naranjo MEG, Rodrigues-Soares
F, Penas-LLedó EM, Fariñas H and Tarazona-Santos E: Interethnic
variability of CYP2D6 alleles and of predicted and measured
metabolic phenotypes across world populations. Expert Opin Drug
Metab Toxicol. 10:1569–1583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee SY, Sohn KM, Ryu JY, Yoon YR, Shin JG
and Kim JW: Sequence-based CYP2D6 genotyping in the Korean
population. Ther Drug Monit. 28:382–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nazir N, Waheed A, Farhat K, Ismail M and
Mansoor Q: Frequency of CYP2D6*10 genotypes in Pakistani breast
cancer patients taking adjuvant tamoxifen. J Pak Med Assoc.
66:1554–1558. 2016.PubMed/NCBI
|
|
36
|
Naveen AT, Adithan C, Soya SS, Gerard N
and Krishnamoorthy R: CYP2D6 genetic polymorphism in South Indian
populations. Biol Pharm Bull. 29:1655–1658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yazdi MF, Rafieian S, Gholi-Nataj M,
Sheikhha MH, Nazari T and Neamatzadeh H: CYP2D6 Genotype and risk
of recurrence in tamoxifen treated breast cancer patients. Asian
Pac J Cancer Prev. 16:6783–6787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Erden G, Acar FS, Inal EE, Soydas AO,
Ozoran K, Bodur H and Yildirimkaya MM: Frequency of mutated allele
CYP2D6*4 in the Turkish ankylosing spondylitis patients and healthy
controls. Rheumatol Int. 29:1431–1434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Menoyo A, del Rio E and Baiget M:
Characterization of variant alleles of cytochrome CYP2D6 in a
Spanish population. Cell Biochem Funct. 24:381–385. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Ameida Melo M, De Vasconcelos-Valença
RJ, Neto FM, Borges RS, Costa-Silva DR, Da Conceição
Barros-Oliveira M, Borges US, Alencar AP, Silva VC and Da Silva BB:
CYP2D6 gene polymorphisms in Brazilian patients with breast cancer
treated with adjuvant tamoxifen and its association with disease
recurrence. Biomed Rep. 5:574–578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Borges S, Desta Z, Li L, Skaar TC, Ward
BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM and Wu L:
Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen
metabolism: Implication for optimization of breast cancer
treatment. Clin Pharmacol Ther. 80:61–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Teh LK, Ismail R, Yusoff R, Hussein A, Isa
MN and Rahman AR: Heterogeneity of the CYP2D6 gene among Malays in
Malaysia. J Clin Pharm Ther. 26:205–211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Topić E, Stefanović M, Nikolić V, Zoricić
I, Ivanisević AM and Žuntar I: Detection of CYP2D6*3 and 2D6*4
allelic variants by PCR-restriction fragment length polymorphism.
Clin Chem Lab Med. 36:655–658. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Varela N, Quiñones LA, Stojanova J, Garay
J, Cáceres D, Cespedes S, Sasso J and Miranda C: Characterization
of the CYP2D6 drug metabolizing phenotypes of the Chilean mestizo
population through polymorphism analyses. Pharmacol Res.
101:124–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hashemi-Soteh SM, Sarzare F, Merat F,
Salehifar E and Shiran MR: Frequencies of three CYP2D6
nonfunctional alleles (CYP2D6*3, *4, and *6) within an Iranian
population (Mazandaran). Genet Test Mol Biomarkers. 15:821–825.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aydin M, Hatirnaz O, Erensoy N and Ozbek
U: CYP2D6 and CYP1A1 mutations in the Turkish population. Cell
Biochem Funct. 23:133–135. 2005. View Article : Google Scholar : PubMed/NCBI
|