Introduction
D-dimer is the cleavage product of cross-linked
fibrin that is formed by activation of the coagulation system,
which signals hyperfibrinolysis in response to clot activation and
fibrin formation (1). Elevated levels
of D-dimer have been detected in patients exhibiting diffuse
intravascular coagulation (2),
vascular occlusion crisis in sickle cell disease (3), thromboembolic events (4,5) and
myocardial infarction (6). D-dimer is
a widely used biomarker for indicating the activation of
coagulation and fibrinolysis (7,8).
Coagulation disorders are among the most common complications in
cancer patients (7,9). D-dimer levels are elevated in the plasma
of patients with various solid cancers, including of the prostate
(10–12), cervix (13–15) and
esophageal squamous cells (16).
However, the association of D-dimer levels and cancer progression
remains to be a focus of study.
In the present retrospective study, the plasma
levels of D-dimer in patients with breast, gastric, pancreatic,
colon and rectal cancers and in healthy controls were firstly
measured. Subsequently, associations between the D-dimer levels and
clinical features were assessed. The results suggested that the
plasma level of D-dimer was notably associated with the extent of
tumor metastasis and tumor stage in cancer patients.
Materials and methods
Study population
Enrolled patients had been diagnosed with breast
cancer (n=86; age range, 35–79 years; all female), gastric cancer
(n=317; age range, 32–83 years; male: female, 208:109), pancreatic
cancer (n=37; age range, 34–82 years; male: female, 22:15), colon
cancer (n=153; age range, 26–90 years; male: female 73:80) or
rectal cancer (n=137; age range, 44–79 years; male: female, 81:56)
at the Affiliated Changzhou No. 2 People's Hospital of Nanjing
Medical University, Changzhou, China, from January 2010 to December
2014. A total of 92 healthy volunteers (age range, 23–69 years;
male: female, 43:49) undergoing physical examination at Changzhou
No. 2 People's Hospital in January 2016 were also enrolled. The
cancers were defined according to pathological findings. Other
inclusion criteria were measurable disease by magnetic resonance
imaging. Disease clinical staging (I–IV) depended on the systems
recommended by the National Comprehensive Cancer Network Clinical
Practice Guidelines in Oncology (17–21).
Exclusion criteria were intravascular disseminated coagulation,
which was diagnosed on the parameters issued by the Chinese
Hematology Society (22–24). The present retrospective study was
approved by the Ethics Committee of Changzhou No. 2 People's
Hospital, and written informed consent from all participants was
obtained following full disclosure of the research aims and
procedures.
D-dimer level assays
D-dimer expression levels were detected prior to and
following treatment in cancer patients. In the healthy controls
D-dimer was detected during a fasting (for 8 h prior) physical
examination. A total of 3 ml whole blood was drawn from the
antecubital vein of each subject and collected in 3.8% sodium
citrate vacutainer collection tubes (Greiner Bio-One GmbH,
Frickenhausen, Germany). Plasma D-dimer levels were analyzed with
an INNOVANCE®D-Dimer Calibrator (Siemens AG, Munich,
Germany). All samples were run in duplicate according to
manufacturer's recommendations. The D-dimer cross-linkage region
has a stereosymmetrical structure, meaning the epitope for the
monoclonal antibody occurs twice. D-dimer levels >0.55 mg/l were
considered to be elevated, since this was the upper limit of the
90% confidence interval for the average value determined in the
healthy volunteers. The D-dimer levels in tumor patients can be
affected following surgery (25,26),
chemotherapy (27,28) and adjuvant therapy (29). Therefore, the data analyzed herein is
the D-dimer expression level of all patients prior to
treatment.
Statistical analysis
D-dimer levels were presented as the median and
range. Statistically significant differences between healthy
volunteers and cancer patients were determined using unpaired
Student's t-tests. Cases were divided into two groups, high or low,
according to the median D-dimer level as a cutoff. Associations of
D-dimer level with clinicopathological characteristics were
analyzed by using the χ2 test. P<0.05 was considered
to indicate statistical significance. The statistical analysis and
graphing of data were performed with GraphPad Prism version 7.00
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
D-dimer levels are significantly
increased in patients with cancer
To investigate the role of D-dimer in the
development of cancer, an INNOVANCE®D-Dimer Calibrator
was used to analyze the D-dimer levels in patients with breast,
gastric, pancreatic, colon and rectal cancers and in healthy
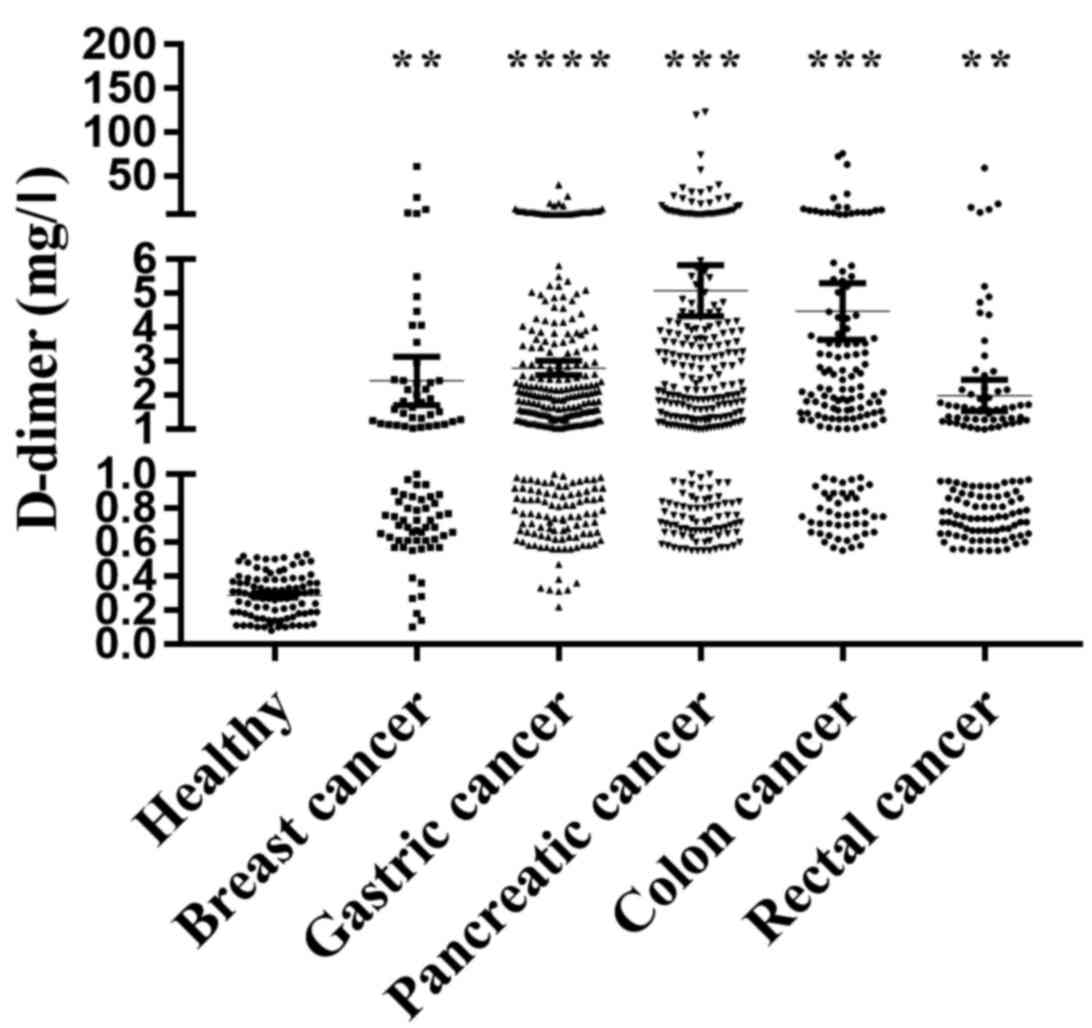
controls. The results demonstrated that D-dimer levels were
significantly higher in the patients with breast cancer (2.61±0.77
mg/l; n=86; P=0.0022), gastric cancer (2.85±0.21 mg/l; n=317;
P<0.0001), pancreatic cancer (5.65±1.39 mg/l; n=37; P=0.0003),
colon cancer (4.48±0.83 mg/l; n=153; P=0.0001) and rectal cancer
(2.00±0.46 mg/l; n=137; P=0.0028), compared with in healthy
volunteers (0.29±0.01 mg/l, n=92; Fig.
1). These results indicated that D-dimer is expressed to a high
level and may serve important roles during cancer development.
D-dimer levels are associated with the
clinicopathological characteristics of patients with cancer
Additionally, associations of D-dimer level with the
clinicopathological characteristics of patients were assessed.
Specimens were divided into two groups according to the expression
level of D-dimer (≤ or >0.55 mg/l). The data indicated that the
D-dimer plasma level differed significantly according to metastasis
(P=0.0181) and TNM stage (P=0.0101) in patients with breast cancer
(Table I); metastasis (P<0.0001)
and TNM stage (P=0.0063) in patients with gastric cancer (Table II); TNM stage (P=0.0395) in patients
with pancreatic cancer (Table III);
metastasis (P=0.0120), TNM stage (P=0.0039) and sex (P=0.0145) in
patients with colon cancer (Table
IV); and metastasis (P=0.0104) and TNM stage (P=0.0002) in
patients with rectal cancer (Table
V). No differences were identified between D-dimer level and
other clinical features (Tables
I–V). These data suggested that
the plasma level of D-dimer may be used as a marker in cancer
progression.
 | Table I.Clinical association between D-dimer
level and clinicopathological variables of breast cancer
patients. |
Table I.
Clinical association between D-dimer
level and clinicopathological variables of breast cancer
patients.
|
|
| Cases with low and
high D-dimer level, n (%) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases (n=86) | Low | High | P-value |
|---|
| Age, years |
|
|
| 1 |
| ≥60 | 46 | 35 (76.1) | 11 (23.9) |
|
|
<60 | 40 | 31 (77.5) | 9 (22.5) |
|
| Metastasis |
|
|
| 0.0181 |
|
Positive | 70 | 20 (28.6) | 50 (71.4) |
|
|
Negative | 16 | 10 (62.5) | 6 (37.5) |
|
| TNM stage |
|
|
| 0.0101 |
|
I&II | 17 | 11 (64.7) | 6 (35.3) |
|
|
III&IV | 69 | 20 (28.9) | 49 (71.1) |
|
 | Table II.Clinical association between D-dimer
level and clinicopathological variables of gastric cancer
patients. |
Table II.
Clinical association between D-dimer
level and clinicopathological variables of gastric cancer
patients.
|
|
| Cases with low and
high D-dimer level, n (%) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases (n=317) | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.5337 |
|
≥60 | 171 | 119 (60.0) | 52 (40.0) |
|
|
<60 | 146 | 107 (69.4) | 39 (30.6) |
|
| Gender |
|
|
| 0.4341 |
|
Male | 208 | 145 (69.7) | 63 (30.7) |
|
|
Female | 109 | 81 (74.3) | 28 (25.7) |
|
| Metastasis |
|
|
| <0.0001 |
|
Positive | 215 | 117 (54.5) | 98 (45.6) |
|
|
Negative | 102 | 79 (77.5) | 23 (22.5) |
|
| TNM stage |
|
|
| 0.0063 |
|
I&II | 32 | 26 (84.2) | 6 (15.8) |
|
|
III&IV | 285 | 174 (61.1) | 111 (38.9) |
|
 | Table III.Clinical association between D-dimer
level and clinicopathological variables of pancreatic cancer
patients. |
Table III.
Clinical association between D-dimer
level and clinicopathological variables of pancreatic cancer
patients.
|
|
| Cases with low and
high D-dimer level, n (%) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases (n=37) | Low | High | P-value |
|---|
| Age, years |
|
|
| 1 |
|
≥60 | 23 | 10 (43.5) | 13 (56.5) |
|
|
<60 | 14 | 6 (42.9) | 8 (57.1) |
|
| Gender |
|
|
| 1 |
|
Male | 22 | 10 (45.5) | 12 (54.5) |
|
|
Female | 15 | 6 (40.0) | 9 (60.0) |
|
| Metastasis |
|
|
| 0.5541 |
|
Positive | 34 | 13 (38.2) | 21 (61.8) |
|
|
Negative | 3 | 2 (66.7) | 1 (33.3) |
|
| TNM stage |
|
|
| 0.0395 |
|
I&II | 14 | 8 (57.1) | 6 (42.9) |
|
|
III&IV | 23 | 5 (21.7) | 18 (78.3) |
|
 | Table IV.Clinical association between D-dimer
level and clinicopathological variables of colon cancer
patients. |
Table IV.
Clinical association between D-dimer
level and clinicopathological variables of colon cancer
patients.
|
|
| Cases with low and
high D-dimer level, n (%) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases (n=153) | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.7127 |
|
≥60 | 113 | 64 (56.6) | 49 (43.4) |
|
|
<60 | 40 | 21 (52.5) | 19 (47.5) |
|
| Gender |
|
|
| 0.0145 |
|
Male | 73 | 48 (65.8) | 25 (34.2) |
|
|
Female | 80 | 36 (45.0) | 44 (55.0) |
|
| Metastasis |
|
|
| 0.0120 |
|
Positive | 119 | 51 (42.9) | 68 (57.1) |
|
|
Negative | 34 | 23 (67.7) | 11 (32.3) |
|
| TNM stage |
|
|
| 0.0039 |
|
I&II | 17 | 15 (88.2) | 2 (11.8) |
|
|
III&IV | 136 | 70 (51.5) | 66 (48.5) |
|
 | Table V.Clinical association between D-dimer
level and clinicopathological variables of rectal cancer
patients. |
Table V.
Clinical association between D-dimer
level and clinicopathological variables of rectal cancer
patients.
|
|
| Cases with low and
high D-dimer level, n (%) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases (n=137) | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.8477 |
|
≥60 | 100 | 48 (48.0) | 52 (52.0) |
|
|
<60 | 37 | 19 (51.4) | 18 (48.6) |
|
| Gender |
|
|
| 1 |
|
Male | 81 | 40 (49.4) | 41 (50.6) |
|
|
Female | 56 | 27 (48.2) | 29 (51.8) |
|
| Metastasis |
|
|
| 0.0104 |
|
Positive | 109 | 47 (43.1) | 62 (56.9) |
|
|
Negative | 28 | 20 (71.4) | 8 (28.6) |
|
| TNM stage |
|
|
| 0.0002 |
|
I&II | 44 | 30 (68.2) | 14 (31.8) |
|
|
III&IV | 93 | 32 (34.4) | 61 (65.6) |
|
Discussion
In the present study, it was demonstrated that
elevated plasma levels of D-dimer were associated with clinical
cancer stages and metastasis in patients with breast, gastric,
colon and rectal cancers. It was also identified that D-dimer
plasma level was associated with cancer stages in patients with
pancreatic cancer and with gender in patients with colon cancer.
These findings suggest that D-dimer level has a potential use in
predicting the likelihood of metastasis and progression in various
cancers.
Distant metastasis is the main cause of poor
prognosis and leads to inefficacy in treatments in cancer patients
(30). D-dimer is a widely used
biomarker for indicating the activation of coagulation and
fibrinolysis. It is established that activated coagulation, which
is common in malignancy, plays an important role in cancer
metastasis (31). The
coagulation/fibrinolytic system is activated in cancer patients and
may contribute to cancer progression (14). Thus, tumor-related degradation
products of the coagulation and fibrinolytic system have been
proposed to predict tumor load and prognosis (14,32).
Plasma D-dimer is a pro-coagulation factor that may reflect the
presence of micro-metastases or circulating tumor cells, which may
be responsible for tumor recurrence (30,33,34).
Recent studies have reported a positive association between
circulating tumor cells and plasma D-dimer levels in patients with
metastatic breast cancer (35,36). Diao
et al (37) observed that
plasma D-dimer levels were markedly increased in gastric cancer
patients with distant metastases, particularly in patients with
visceral metastases. In fact, they suggested D-dimer to be a
promising predictor of clinical stage in the gastric cancer
patients (37). Lee et al
(38) reported that increased plasma
D-dimer and fibrinogen degradation product levels were
significantly associated with TNM stage in colorectal cancer
patients. Furthermore, preoperative plasma D-dimer levels have been
associated with larger tumor size and lymph node metastasis in
colorectal cancer patients (39).
Other recent studies have shown that plasma D-dimer values may
predict overall survival and progression free survival in patients
with pancreatic cancer (40,41). In accordance with the above studies,
the present study confirmed a positive association between D-dimer
level and tumorigenesis. It was further identified that D-dimer
level was associated with cancer stages but not with metastasis in
patients with pancreatic cancer. Among the patients with pancreatic
cancer, the majority presented with metastasis (34/37), and
fibrinogen and carbohydrate antigen 19-9 (CA19-9) were
significantly elevated in the patients with metastatic pancreatic
cancer, compared with patients with non-metastatic cancer (data not
shown), suggesting that a combination of D-dimer, fibrinogen and
CA19-9 may be used for predicting metastasis of pancreatic
cancer.
In conclusion, the present retrospective study
demonstrated that D-dimer plasma level was significantly higher in
patients with cancer and associated with clinical stages and
metastasis. The current study was limited in that it only
identified the association of D-dimer with tumor stage and
metastasis. We will further investigate the sensitivity and
specificity of D-dimer as an indicator of tumorigenesis, and the
correlation between D-dimer and the survival and prognosis of
cancer patients, to clarify whether D-dimer level may be considered
as an important factor in the prognosis of cancer patients.
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from the
Project of Jiangsu Provincial Medical Youth Talent (grant no.
QNRC2016267 to TF) and the Projects of Nanjing Medical University
Science and Technology Development Fund Key (grant no.
2016NJMUZD103 to PZ).
Availability of data and materials
All data generated or analyzed in this study are
included in this published article.
Authors' contributions
PZ, HD and TF designed the study. HZ, YS, ZX and SW
performed the experiments and collected and analyzed data. PZ, HD
and TF wrote, reviewed and revised the manuscript. All authors read
and approved the final version to be published.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Changzhou No. 2 People's Hospital of Nanjing Medical
University, Changzhou, China, and written informed consent
permitting the use of patient samples in the current study was
obtained prior to treatment.
Consent for publication
All patients consented to the publication of any
relevant data on the basis of anonymization of all personal
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang X, Mei X, Wu H and Chen X: D-dimer
level is related to the prognosis of patients with small cell lung
cancer. Ann Transl Med. 5:3942017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi T, Kajiki M, Nihashi K and Honda
G: Surveillance of the safety and efficacy of recombinant human
soluble thrombomodulin in patients with obstetrical disseminated
intravascular coagulation. Thromb Res. 159:109–115. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmed S, Siddiqui AK, Iqbal U, Sison CP,
Shahid RK, Sheth M, Patel DV and Russo LA: Effect of low-dose
warfarin on D-dimer levels during sickle cell vaso-occlusive
crisis: A brief report. Eur J Haematol. 72:213–216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lowe GD, Haverkate F, Thompson SG, Turner
RM, Bertina RM, Turpie AG and Mannucci PM: Prediction of deep vein
thrombosis after elective hip replacement surgery by preoperative
clinical and haemostatic variables: The ECAT DVT Study. European
Concerted Action on Thrombosis. Thromb Haemost. 81:879–886. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwietniak M, Al-Amawi T, Błaszkowski T,
Sulżyc-Bielicka V and Kładny J: The usefulness of D-dimer in
diagnosis and prediction of venous thromboembolism in patients with
abdominal malignancy. Pol Przegl Chir. 89:27–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Qiu B, Zhang Y, Cao Y, Zhang X,
Wu Z, Wang S and Mei L: The Value of Pre-Infarction Angina and
Plasma D-Dimer in Predicting No-Reflow After Primary Percutaneous
Coronary Intervention in ST-Segment Elevation Acute Myocardial
Infarction Patients. Med Sci Monit. 24:4528–4535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horowitz NA, Blevins EA, Miller WM, Perry
AR, Talmage KE, Mullins ES, Flick MJ, Queiroz KC, Shi K, Spek CA,
et al: Thrombomodulin is a determinant of metastasis through a
mechanism linked to the thrombin binding domain but not the
lectin-like domain. Blood. 118:2889–2895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Im JH, Fu W, Wang H, Bhatia SK, Hammer DA,
Kowalska MA and Muschel RJ: Coagulation facilitates tumor cell
spreading in the pulmonary vasculature during early metastatic
colony formation. Cancer Res. 64:8613–8619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang W and Kim HR: Dynamic regulation of
platelet-derived growth factor D (PDGF-D) activity and
extracellular spatial distribution by matriptase-mediated
proteolysis. J Biol Chem. 290:9162–9170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamzah AB, Choo YM, Hassali MA, Saleem F
and Verma AK: Disseminated Intravascular Coagulation and Excessive
Fibrinolysis (DIC XFL) Syndrome in Prostate Cancer: A Rare
Complicated Disorder. J Clin Diagn Res. 11:XD01–XD02.
2017.PubMed/NCBI
|
|
11
|
Hong SK, Ko DW, Park J, Kim IS, Doo SH,
Yoon CY, Park H, Lee WK, Kim DS, Jeong SJ, et al: Alteration of
Antithrombin III and D-dimer Levels in Clinically Localized
Prostate Cancer. Korean J Urol. 51:25–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khoury JD, Adcock DM, Chan F, Symanowski
JT, Tiefenbacher S, Goodman O, Paz L, Ma Y, Ward DC, Vogelzang NJ,
et al: Increases in quantitative D-dimer levels correlate with
progressive disease better than circulating tumor cell counts in
patients with refractory prostate cancer. Am J Clin Pathol.
134:964–969. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo YL, Chi PD, Zheng X, Zhang L, Wang XP
and Chen H: Preoperative D-dimers as an independent prognostic
marker in cervical carcinoma. Tumour Biol. 36:8903–8911. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Satoh T, Matsumoto K, Tanaka YO, Akiyama
A, Nakao S, Sakurai M, Ochi H, Onuki M, Minaguchi T, Sakurai H, et
al: Incidence of venous thromboembolism before treatment in
cervical cancer and the impact of management on venous
thromboembolism after commencement of treatment. Thromb Res.
131:e127–e132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun YH, Cui L, Chen J, Wang M, Liu JJ, Liu
XX and Huang XE: Analysis of Relationships Between Prethrombotic
States and Cervical Cancer. Asian Pac J Cancer Prev. 16:6163–6166.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng JF, Yang X, Chen S, Zhao Q and Chen
QX: Prognostic Value of Plasma D-dimer in Patients with Resectable
Esophageal Squamous Cell Carcinoma in China. J Cancer. 7:1663–1667.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carlson RW, Allred DC, Anderson BO,
Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Forero A,
Giordano SH, et al: National Comprehensive Cancer Network: Invasive
breast cancer. J Natl Compr Canc Netw. 9:136–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ettinger DS, Akerley W, Bepler G, Blum MG,
Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Govindan R,
et al: National Comprehensive Cancer Network: Thymic malignancies.
J Natl Compr Canc Netw. 8:1302–1315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romanus D, Weiser MR, Skibber JM, Ter Veer
A, Niland JC, Wilson JL, Rajput A, Wong YN, Benson AB III, Shibata
S, et al: Concordance with NCCN Colorectal Cancer Guidelines and
ASCO/NCCN Quality Measures: An NCCN institutional analysis. J Natl
Compr Canc Netw. 7:895–904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benson AB III, Arnoletti JP, Bekaii-Saab
T, Chan E, Chen YJ, Choti MA, Cooper HS, Dilawari RA, Engstrom PF,
Enzinger PC, et al: National Comprehensive Cancer Network: Colon
cancer. J Natl Compr Canc Netw. 9:1238–1290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Engstrom PF, Arnoletti JP, Benson AB III,
Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS,
Enzinger PC, et al: National Comprehensive Cancer Network: NCCN
Clinical Practice Guidelines in Oncology: Rectal cancer. J Natl
Compr Canc Netw. 7:838–881. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma J and Qiu LG: Guidelines for treatment
of myeloma bone disease. Zhonghua Xue Ye Xue Za Zhi. 32:721–723.
2011.(In Chinese). PubMed/NCBI
|
|
23
|
Lymphoma Working Party of Chinese Society
of Hematology, . Chinese guidelines for the diagnosis and
management of chronic lymphocytic leukemia. Zhonghua Xue Ye Xue Za
Zhi. 32:498–501. 2011.(In Chinese). PubMed/NCBI
|
|
24
|
Shen ZX; Chinese Society of Hematology, ;
Chinese Medical Association, : Chinese guidelines for the diagnosis
and treatment of acute promyelocytic leukemia. Zhonghua Xue Ye Xue
Za Zhi. 32:885–886. 2011.(In Chinese). PubMed/NCBI
|
|
25
|
Shi A, Huang J, Wang X, Li M, Zhang J,
Chen Y and Huang Y: Postoperative D dimer predicts venous
thromboembolism in patients undergoing urologic tumor surgery. Urol
Oncol. 36:307 e315 307 e3212018. View Article : Google Scholar
|
|
26
|
Yao Y, Shen H, Zhou Y, Yang Z and Huang H:
Efficacy of thoracoscopic surgery in the treatment of lung cancer
in the perioperative period and its effects on serum D-dimer. Oncol
Lett. 15:4397–4403. 2018.PubMed/NCBI
|
|
27
|
Zhang B, Liu G, Liu X, Zheng S, Dong K and
Dong R: Circulating D-dimer level correlates with disease
characteristics in hepatoblastoma patients. Medicine (Baltimore).
96:e87982017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pace A, Mandoj C, Antenucci A, Villani V,
Sperduti I, Casini B, Carosi M, Fabi A, Vidiri A, Koudriavtseva T,
et al: A predictive value of von Willebrand factor for early
response to Bevacizumab therapy in recurrent glioma. J Neurooncol.
138:527–535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khangarot SS, Gupta N, Goswami B, Hadke
NS, Lal P, Gupta N and Khurana N: Correlation of D dimer and factor
VIII levels with histopathology in patients with breast carcinoma.
Cancer Biomark. 7:305–314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thaler J, Ay C, Mackman N, Bertina RM,
Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, et
al: Microparticle-associated tissue factor activity, venous
thromboembolism and mortality in pancreatic, gastric, colorectal
and brain cancer patients. J Thromb Haemost. 10:1363–1370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M and Degen JL:
Platelets and fibrin(ogen) increase metastatic potential by
impeding natural killer cell-mediated elimination of tumor cells.
Blood. 105:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palumbo JS, Kombrinck KW, Drew AF, Grimes
TS, Kiser JH, Degen JL and Bugge TH: Fibrinogen is an important
determinant of the metastatic potential of circulating tumor cells.
Blood. 96:3302–3309. 2000.PubMed/NCBI
|
|
33
|
Batschauer AP, Figueiredo CP, Bueno EC,
Ribeiro MA, Dusse LM, Fernandes AP, Gomes KB and Carvalho MG:
D-dimer as a possible prognostic marker of operable hormone
receptor-negative breast cancer. Ann Oncol. 21:1267–1272. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Blackwell K, Haroon Z, Broadwater G, Berry
D, Harris L, Iglehart JD, Dewhirst M and Greenberg C: Plasma
D-dimer levels in operable breast cancer patients correlate with
clinical stage and axillary lymph node status. J Clin Oncol.
18:600–608. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cui LN, Li N, Fu S, Zhang X, Wang X and
Wang RT: Combination of preoperative D-dimer and mean platelet
volume predicts postoperative deep venous thrombosis in breast
cancer patients. Cancer Biomark. 21:909–913. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mego M, Zuo Z, Gao H, Cohen EN, Giordano
A, Tin S, Anfossi S, Jackson S, Woodward W, Ueno NT, et al:
Circulating tumour cells are linked to plasma D-dimer levels in
patients with metastatic breast cancer. Thromb Haemost.
113:593–598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Diao D, Cheng Y, Song Y, Zhang H, Zhou Z
and Dang C: D-dimer is an essential accompaniment of circulating
tumor cells in gastric cancer. BMC Cancer. 17:562017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee S, Huh SJ, Oh SY, Koh MS, Kim SH, Lee
JH, Han JY, Choi HJ, Kim SJ and Kim HJ: Clinical significance of
coagulation factors in operable colorectal cancer. Oncol Lett.
13:4669–4674. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stender MT, Larsen TB, Lundbye-Christensen
S, Yilmaz MK and Thorlacius-Ussing O: Haemostatis activity in
rectal cancer patients exposed to preoperative radiotherapy: A
clinical prospective cohort study. Blood Coagul Fibrinolysis.
20:276–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Durczynski A, Skulimowski A, Hogendorf P,
Szymanski D, Kumor A, Marski K, Juliebø SØ, Poznanska G and
Strzelczyk J: The concentration of D-dimers in portal blood
positively correlates with overall survival in patients with
non-resectable pancreatic cancer. World J Surg Oncol. 15:2232017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao J, Fu Z, Gao L, Wang X, Cheng S, Wang
X and Ren H: Evaluation of serum D-dimer, fibrinogen, and CA19-9
for postoperative monitoring and survival prediction in resectable
pancreatic carcinoma. World J Surg Oncol. 15:482017. View Article : Google Scholar : PubMed/NCBI
|