Introduction
Cushing's syndrome (CS) describes a group of
metabolic disorders including Cushing's disease (CD) that are
caused by oversecretion of adrenocortical steroids (1). Corticotropic adenomas secreting
adrenocorticotropic hormone (ACTH) are the most common cause of
endogenous CS (1). Oversecretion of
cortisol stimulated by ACTH leads to a variety of signs and
symptoms of CD including acne, red cheeks, moon face, ‘buffalo
hump’, thin skin, weight gain, a pendulous abdomen and swelling of
the feet/legs (2). A population-based
study in Denmark reported the annual incidence of CD to be 1.2–1.7
cases per million of the population (3). However, it is possible that there are
many undiagnosed cases of CS/CD with poorly controlled diabetes,
arterial hypertension and/or osteoporosis, particularly among
younger individuals (2). CS has been
associated with increased risk of fatal cardiovascular disease and
infection (4). Despite the
established benefits of surgical treatment in CS/CD, the frequency
of obesity-related metabolic disorders (hypertension, diabetes,
impaired glucose tolerance, hypercholesterolemia) and the mortality
rate of CD patients remain high following surgery (5,6). The
current report describes a case of CD that was difficult to treat
for body weight and insulin resistance following surgery. Therapy
with canagliflozin, a sodium-glucose cotransporter 2 (SGLT2)
inhibitor, reduced body weight and the daily insulin dose
requirement of the patient. SGLT2 inhibitors may reduce the daily
insulin requirement in diabetic patients but there is little
information on the clinical course of patients that exhibit
successful reduction of daily insulin dose. This is the first
reported case of CD-associated diabetes that presented improvement
in body weight and insulin resistance following therapy with an
SGLT2 inhibitor.
Case report
The patient was admitted to Mie University Hospital,
Tsu, Japan, in October 2014 for treatment of obesity and diabetes.
The patient was a 47-year-old obese woman with a 20-year history of
type 2 diabetes mellitus associated with hypertension and
dyslipidemia. In the previous hospital (Mie National Hospital, Tsu,
Japan), the subject exhibited 414 mg/dl blood glucose in a random
glucose test [non-diabetic level: <200 mg/dl (7)] that were difficult to control requiring
high doses of insulin. The patient exhibited no diabetic
nephropathy or retinopathy and was under a strict diet (1,000
kcal/day) and bicycle exercise therapy (30 min/day). The treatment
that the patient was receiving was as follows: Insulin aspart 30
U/day (Novo Nordisk Pharma Ltd., Tokyo, Japan), insulin glargine 36
U/day (Eli Lilly Japan K.K., Kobe, Japan), metformin 1,500 mg/day
(Sumitomo Dainippon Pharma Co., Ltd., Tokyo, Japan), sitagliptin 50
mg/day (MSD K.K., Tokyo, Japan), voglibose 0.9 mg/day (Towa
Pharmaceutical Co., Ltd., Osaka, Japan), valsartan 80 mg/day
(Novartis Pharma K.K.) and rosuvastatin 5 mg/day (AstraZeneca K.K.,
Osaka, Japan). The clinical findings on examination were as
follows: Height, 155 cm; body weight, 87.5 kg, body mass index 36.4
kg/m2 (definition of central obesity by International
Diabetic Federation: >30 kg/m2) (8); blood pressure, 119/79 mmHg [target blood
pressure level in diabetic patients: <130/80 mmHg (9)]; heart rate, 103 beats/min; body
temperature, 36.4°C. Physical examination identified central
obesity with waist circumference 126 cm (cut-off value of
International Diabetic Federation: 80 cm) (8) and whitish striae. Laboratory data on
admission including the following: Glycated hemoglobin (HbA1c),
11.2% [reference range: 4.6–6.2% (10)]; fasting blood glucose, 338 mg/dl
[non-diabetic level: <126 mg/dl (7)]; plasma ACTH, 49.5 pg/ml [reference
range: 7.2–63.3 pg/ml (11)]; plasma
cortisol, 21.1 µg/dl [reference range 6.4–21.0 µg/dl (11)]; urinary free cortisol, 295.4 µg/day
[reference range: 11.2–80.3 µg/day (11)] (Table
I). There was no diurnal variation in plasma cortisol
concentration and no suppression of plasma cortisol concentration
during an overnight 0.5 mg dexamethasone suppression test (DST)
performed for the diagnosis of CS (12). An overnight DST using a high dose of
dexamethasone (8 mg) demonstrated suppression of the plasma
cortisol levels to less than half of the basal level. The plasma
ACTH levels did not exhibit a >50% increase at any time
following 100 µg corticotropin releasing hormone (CRH)
administration. In a thyrotropin-releasing hormone test, thyroid
stimulating hormone exhibited a peak value of 10.34 µU/ml at 30 min
[normal response: increase of >5 µU/ml (13)]. In a luteinizing hormone-releasing
hormone test, luteinizing hormone and follicle stimulation hormone
exhibited peak values of 92.6 mU/ml (normal response: increase of
>10 mU/ml) and 94.4 mU/ml (normal response: increase of >2
mU/ml) (13), respectively. In a
growth hormone-releasing protein 2 test, growth hormone exhibited a
peak value of 9.421 ng/ml at 15 min (criteria for severe adult
growth hormone deficiency: values of <9 ng/ml) (14) (Table I).
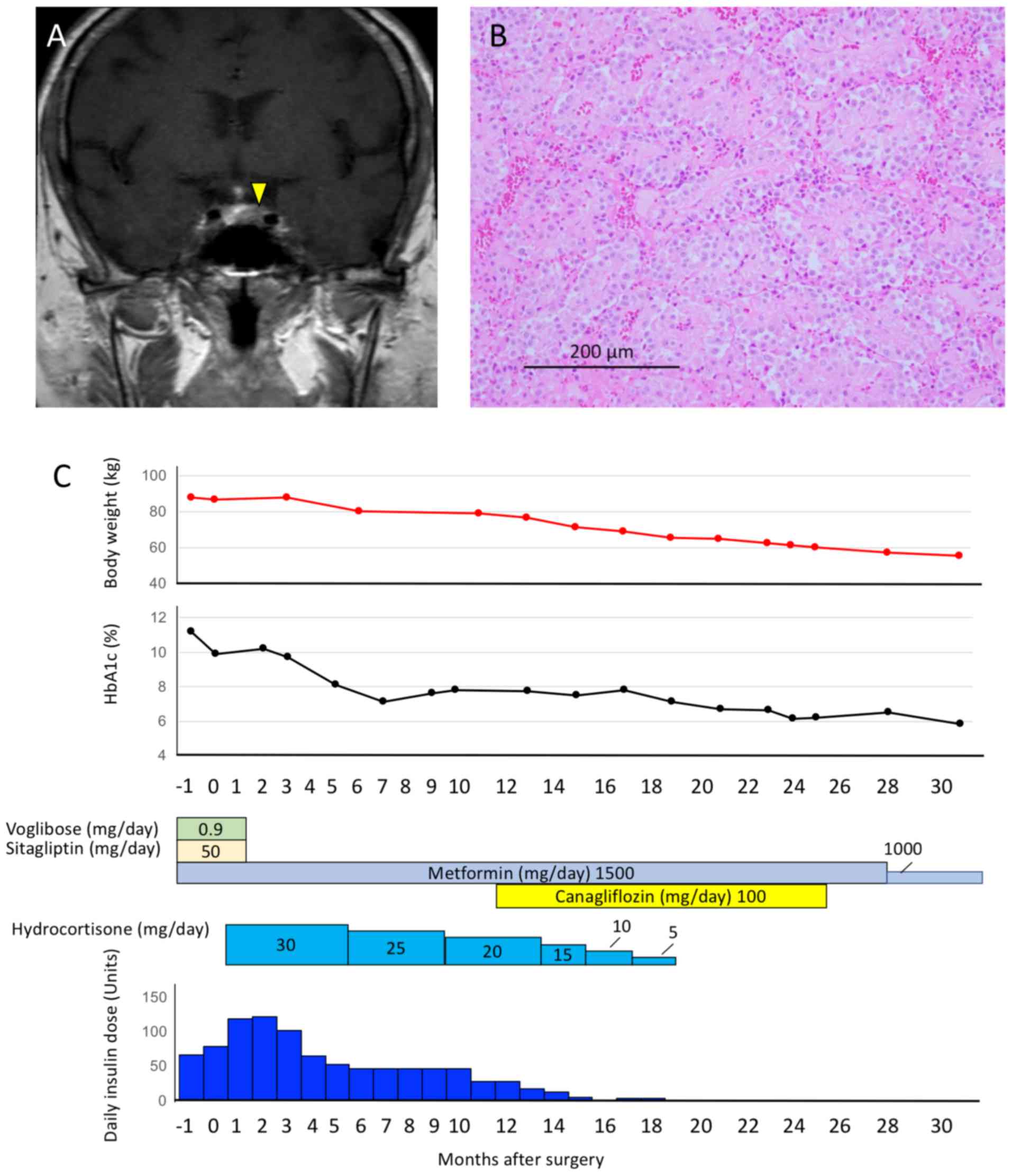
Magnetic resonance imaging (MRI) revealed a 12-mm pituitary tumor
with delayed enhancement in T1-weighted MRI (Fig. 1A). Based on the presence of central
obesity, diabetes mellitus, hypertension, pituitary tumor on MRI
and abnormal findings in endocrinal tests, excluding the results of
the CRH test, a diagnosis of CD was made. The reported low
sensitivity of the CRH test may explain why the test was negative
(15). The patient was then remitted
to the Neurosurgery Department for trans-sphenoidal pituitary
surgery. The patient's hyperglycemia was treated with 112 U/day
insulin the day before surgery and improved following the
trans-sphenoidal pituitary surgery. The pathological findings of
the resected specimen were consistent with the diagnosis of benign
pituitary adenoma as evaluated by histological study and
hematoxylin and eosin staining (Fig.
1B). The serum levels of ACTH and cortisol decreased 9 days
after surgery (to 9.8 pg/ml and 2.4 µg/dl, respectively).
 | Table I.Laboratory data on admission and
results of endocrinal tests. |
Table I.
Laboratory data on admission and
results of endocrinal tests.
| Blood cell
parameters | Biochemical
examination |
|
|
|
|
|
|
|---|
| White blood cell
count | 7,720/µl | HbA1c | 11.2% | 0.5 mg DST | Before | After |
|
|
|
|
Neutrophil | 74% | Glucose | 338 mg/dl | Cortisol (µg/dl) | 21.1 | 25.8 |
|
|
|
|
Lymphocyte | 20.2% | Total protein | 6.5 g/dl | 8 mg DST | Before | After |
|
|
|
|
Monocyte | 5.1% | Albumin | 4.0 g/dl | Cortisol (µg/dl) | 21.1 | 3.8 |
|
|
|
|
Eosinophil | 0.4% | BUN | 10 mg/dl |
|
|
|
|
|
|
|
Basophil | 0.3% | Creatinine | 0.75 mg/dl | Diurnal
variation | Midnight | Morning |
|
|
|
| Red blood
cell count |
500×104/µl | Uric acid | 5.4 mg/dl | Cortisol (µg/dl) | 20.7 | 21.1 |
|
|
|
|
Hemoglobin | 13.4 g/dl | Na | 137 mEq/l | ACTH (pg/ml) | 54.2 | 49.5 |
|
|
|
|
Hematocrit | 41.1% | K | 4.7 mEq/l |
|
|
|
|
|
|
| MCV | 82.2 fl | Cl | 102 mEq/l | CRH test | 0 min | 30 min | 60 min | 90 min |
|
| MCH | 26.8 pg | Ca | 9.4 mg/dl | ACTH (pg/ml) | 49.5 | 50.3 | 38.1 | 20.2 |
|
|
Platelet |
27.3×104/µl | P | 3.0 mg/dl | Cortisol (µg/dl) | 21.1 | 43.2 | 30.9 | 27.4 |
|
|
|
| AST | 23 U/l |
|
|
|
|
|
|
| Urinalysis |
| ALT | 40 U/l | TRH test | 0 min | 30 min | 60 min | 90 min |
|
|
Specific gravity | 1.030 | LDH | 224 U/l | TSH (µU/ml) | 0.89 | 10.34 | 8.06 | 6.01 |
|
| pH | 5 | γ-GTP | 69 U/l | PRL (ng/ml) | 18.8 | 64.3 | 45.3 | 34.7 |
|
|
Glucose | (4+) | ALP | 260 U/l |
|
|
|
|
|
|
|
Protein | (−) | T-Bil | 0.3 mg/dl | LHRH test | 0 min | 30 min | 60 min | 90 min |
|
| Ketone
body | (1+) | Triglyceride | 136 mg/dl | LH (mU/ml) | 29.4 | 72.1 | 86.8 | 92.6 |
|
|
Blood | (−) | T-CHO | 129 mg/dl | FSH (mU/ml) | 69.0 | 85.5 | 82.1 | 94.4 |
|
|
|
| LDL-C | 63 mg/dl |
|
|
|
|
|
|
| Endocrinal
measurement |
| HDL-C | 45 mg/dl | GHRP test | 0 min | 15 min | 30 min | 45 min | 60 min |
|
TSH | 0.89 mU/ml | CRP | 0.13 mg/dl | GH (pg/ml) | 0.196 | 9.421 | 5.025 | 2.308 | 1.199 |
| Free
T3 | 1.6 pg/ml |
|
|
|
|
|
|
|
|
| Free
T4 | 1.06 ng/dl |
|
|
|
|
|
|
|
|
| Serum
C-peptide | 3.8 ng/ml |
|
|
|
|
|
|
|
|
| Serum
renin activity | 2.9 ng/ml/h |
|
|
|
|
|
|
|
|
| Plasma
aldosterone | 216 pg/ml |
|
|
|
|
|
|
|
|
|
DHEA-S | 463 µg/dl |
|
|
|
|
|
|
|
|
| GH | 0.346 ng/ml |
|
|
|
|
|
|
|
|
| Urine
free cortisol | 295.4 µg/day |
|
|
|
|
|
|
|
|
Following surgery, the patient was treated with 30
mg/day hydrocortisone with gradual tapering over 19 months. The
dose of insulin was gradually reduced to avoid hypoglycemia via
self-monitoring of blood glucose levels; the dose was reduced by
4–10 units/week at the discretion of the patient. Sitagliptin and
voglibose were withdrawn following surgery. After 6 months the
level of HbA1c was <7.0% and patient body weight decreased from
85.9 to 80.0 kg. The daily insulin dose was 46 U/day during the
postoperative period under self-monitoring and outpatient
follow-up. The patient's body weight and daily insulin dose
remained stable over the following 5 months. Canagliflozin was
administrated 11 months after surgery. Following the administration
of 100 mg/day canagliflozin the patient exhibited loss of body
weight (to 69.5 kg) and required less daily insulin dose (to 0
U/day). Therapy with insulin was withdrawn 19 months after the
surgical procedure when HbA1c level was <7.0%. The dose of
valsartan was decreased (from 80 to 40 mg/day) 10 months after and
was withdrawn 12 months after starting therapy with canagliflozin.
Remission of the CD was confirmed 19 months after the pituitary
adenoma resection. In the absence of hydrocortisone, the plasma
level of ACTH was 9.0 pg/ml and that of cortisol was 4.9 µg/dl. The
body weight of the patient was 55.3 kg and the HbA1c level was 5.8%
31 months after the surgical procedure. This overall clinical
course is depicted in Fig. 1C.
Discussion
CS including CD is characterized by metabolic
disorders such as obesity and insulin resistance that are caused by
overproduction of steroid hormones (2). CD is associated with a high rate of
mortality caused by ischemic coronary artery disease and/or
infectious diseases; a recent review article reported that
standardized mortality ratios of CD ranged from 1.84 to 4.25
including global retrospective analysis (16). Surgical resection of pituitary adenoma
is the first-line treatment for CD but not all patients with CS
exhibit improvement in metabolic function following surgical
treatment (17). Obesity and diabetes
are particularly difficult to control, and the amount of
inter-muscular adipose tissue remains unchanged following surgery
(5,18). Nevertheless, the present case
exhibited loss of body weight and required lower daily insulin dose
in the immediate postoperative period, and these signs remained
stable 6 months after surgery.
Management of metabolic disorders in the
postoperative period for patients with CD undergoing steroid
therapy is a challenge. The present case required a relatively long
period for withdrawal of steroid administration following surgery.
Weight gain is accelerated during steroid therapy as
glucocorticoids promote glucose production by the liver, peripheral
glucose uptake in muscle and adipose tissues, and inhibit insulin
production and secretion in pancreatic β-cells (19,20). Body
weight and the daily insulin dose of the current patient remained
stable with no improvement between the sixth and eleventh month of
the postoperative period. Initiation of canagliflozin, a sodium
glucose co-transporter inhibitor, led to decreased body weight and
lower requirement of insulin dose; complete insulin withdrawal and
a body weight loss of 24.7 kg were observed 8 months after
initiating therapy with canagliflozin.
To the best of our knowledge, this is the first
report on the therapeutic effect of a sodium glucose co-transporter
inhibitor in a CD patient post-surgery. The markedly beneficial
effect of canagliflozin on body weight and diabetes in the
postoperative period of the present patient whilst undergoing
steroid therapy may be explained by the accelerated excretory
activity of carbohydrates induced by the drug. Although data
supporting the beneficial effects of SGLT2 inhibitor in
glucocorticoid-related metabolic disorders is limited, studies on
the mechanism of action of the drug suggest its potential favorable
activity (21–23). Glucocorticoids increase the expression
of sodium-dependent glucose cotransporters 1 and 2 by promoting the
activity of serum- and glucocorticoid-inducible kinases;
canaglifozin may suppress the effects of both sodium-dependent
glucose cotransporters (21). An
alternative explanation for the beneficial effect of canagliflozin
is its stimulatory activity on the AMP-activated protein kinase,
which may increase insulin sensitivity by activating intracellular
signal pathways and decrease body weight by promoting energy
consumption in adipose tissues (22).
Therefore, it is conceivable that canagliflozin counteracted the
inhibitory effect of glucocorticoids on AMP-activated protein
kinase in the adipose tissues of the current CD patient undergoing
steroid therapy during the postoperative period (23).
It is noteworthy that the use of SGLT2 inhibitor in
the early post-operative period may be detrimental since it may
cause profound diuresis, which eventually may result in increased
predisposition for deep vein thrombosis and hypotension due to
decreased intravascular volume (24–26).
Therefore, careful monitoring of the patient condition is required
when SGLT2 inhibitor is used after surgery.
The present report demonstrates the potential of
canagliflozin for the management of diabetic patients receiving
steroids following surgical therapy for CD. However, it is
noteworthy that other agents including glucagon-like peptide-1
agonists may also have similar beneficial effects on weight
reduction in CD (27). The use of
HbA1c alone and the lack of a glucose tolerance test to confirm the
diabetic condition are limitations of the present case report. The
blood glucose levels of the patient were measured during
consultation at the outpatient department. However, the blood
glucose levels measured during follow-up at the outpatient
department are usually variable depending on the time from the last
meal. Pasireotide has become available for the treatment of CD
caused by pituitary adenoma (28).
Pasireotide was not used in the present case since this medication
has not been officially approved in Japan and the tumor was
completely removed.
Despite the beneficial effects of canagliflozin
observed in the current report, further data are required to
demonstrate the effectiveness of SGLT2 inhibitor in CS patients
with diabetes.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Patient data and the clinical course were retrieved
from electronic medical records.
Authors' contributions
KN was responsible for clinical treatment,
follow-up, and preparation of the first draft of the manuscript.
NF, KM, YO, RH, YH, MU, TY, TS and YY were responsible for clinical
treatment, follow-up and interpretation of the data. CDG and ECG
were responsible for interpretation of the data and intellectual
contribution in the preparation of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of clinical details and images.
Competing interests
The authors declare no competing interests.
References
|
1
|
Newell-Price J, Bertagna X, Grossman AB
and Nieman LK: Cushing's syndrome. Lancet. 367:1605–1617. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colao A, Boscaro M, Ferone D and Casanueva
FF: Managing Cushing's disease: The state of the art. Endocrine.
47:9–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lindholm J, Juul S, Jørgensen JO, Astrup
J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jørgensen J, Kosteljanetz
M, Kristensen L, et al: Incidence and late prognosis of Cushing's
syndrome: A population-based study. J Clin Endocrinol Metab.
86:117–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieman LK, Biller BM, Findling JW, Murad
MH, Newell-Price J, Savage MO and Tabarin A; Endocrine Society, :
Treatment of Cushing's Syndrome: An Endocrine Society Clinical
Practice Guideline. J Clin Endocrinol Metab. 100:2807–2831. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chanson P and Salenave S: Metabolic
syndrome in Cushing's syndrome. Neuroendocrinology. 92 Suppl
1:96–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hassan-Smith ZK, Sherlock M, Reulen RC,
Arlt W, Ayuk J, Toogood AA, Cooper MS, Johnson AP and Stewart PM:
Outcome of Cushing's disease following transsphenoidal surgery in a
single center over 20 years. J Clin Endocrinol Metab. 97:1194–1201.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
American Diabetes Association, . 2.
Classification and Diagnosis of Diabetes: Standards of Medical Care
in Diabetes-2018. Diabetes Care. 41 Suppl 1:S13–S27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alberti KG, Zimmet P and Shaw J: Metabolic
syndrome - a new world-wide definition. A Consensus Statement from
the International Diabetes Federation. Diabet Med. 23:469–480.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kai H: Blood pressure management in
patients with type 2 diabetes mellitus. Hypertens Res. 40:721–729.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Little RR and Rohlfing CL: The long and
winding road to optimal HbA1c measurement. Clin Chim Acta.
418:63–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwayama H, Hirase S, Nomura Y, Ito T,
Morita H, Otake K, Okumura A and Takagi J: Spontaneous
adrenocorticotropic hormone (ACTH) normalisation due to tumour
regression induced by metyrapone in a patient with ectopic ACTH
syndrome: Case report and literature review. BMC Endocr Disord.
18:192018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kageyama K, Oki Y, Sakihara S, Nigawara T,
Terui K and Suda T: Evaluation of the diagnostic criteria for
Cushing's disease in Japan. Endocr J. 60:127–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Melmed S and Jameson JL:
HypopituitarismHarrison's Principles of Internal Medicine. 19th
edition. McGraw-Hill Education; New York, NY: pp. 2255–2261.
2015
|
|
14
|
Fukuda I, Hizuka N, Nuraoka T and Ichihara
A: Adult growth hormone deficiency: Current concepts. Neurol Med
Chir (Tokyo). 54:599–605. 2014. View Article : Google Scholar
|
|
15
|
Newell-Price J, Morris DG, Drake WM,
Korbonits M, Monson JP, Besser GM and Grossman AB: Optimal response
criteria for the human CRH test in the differential diagnosis of
ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab.
87:1640–1645. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pivonello R, De Martino MC, De Leo M,
Simeoli C and Colao A: Cushing's disease: The burden of illness.
Endocrine. 56:10–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pivonello R, De Leo M, Cozzolino A and
Colao A: The Treatment of Cushing's Disease. Endocr Rev.
36:385–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geer EB, Shen W, Strohmayer E, Post KD and
Freda PU: Body composition and cardiovascular risk markers after
remission of Cushing's disease: A prospective study using
whole-body MRI. J Clin Endocrinol Metab. 97:1702–1711. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bouclaous C, Torbay N, Nassar C and Hwalla
N: Modification of glucocorticoid effects on body weight gain,
plasma lipids by changes in diet composition. Nutr Res.
23:1105–1115. 2003. View Article : Google Scholar
|
|
20
|
Tamez-Pérez HE, Quintanilla-Flores DL,
Rodríguez-Gutiérrez R, González-González JG and Tamez-Peña AL:
Steroid hyperglycemia: Prevalence, early detection and therapeutic
recommendations: A narrative review. World J Diabetes. 6:1073–1081.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sopjani M, Alesutan I, Wilmes J,
Dërmaku-Sopjani M, Lam RS, Koutsouki E, Jakupi M, Föller M and Lang
F: Stimulation of Na+/K+ ATPase activity and
Na+ coupled glucose transport by β-catenin. Biochem Biophys Res
Commun. 402:467–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hawley SA, Ford RJ, Smith BK, Gowans GJ,
Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR and Hardie DG:
The Na+/glucose cotransporter inhibitor canagliflozin
activates AMPK by inhibiting mitochondrial function and increasing
cellular AMP Levels. Diabetes. 65:2784–2794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kola B, Christ-Crain M, Lolli F, Arnaldi
G, Giacchetti G, Boscaro M, Grossman AB and Korbonits M: Changes in
adenosine 5-monophosphate-activated protein kinase as a mechanism
of visceral obesity in Cushing's syndrome. J Clin Endocrinol Metab.
93:4969–4973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kelly J, Hunt BJ, Lewis RR, Swaminathan R,
Moody A, Seed PT and Rudd A: Dehydration and venous thromboembolism
after acute stroke. QJM. 97:293–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yasui A, Lee G, Hirase T, Kaneko T,
Kaspers S, von Eynatten M and Okamura T: Empagliflozin induces
transient diuresis without changing long-term overall fluid balance
in Japanese patients with type 2 diabetes. Diabetes Ther.
9:863–871. 2018.PubMed/NCBI
|
|
26
|
Gelbenegger G, Buchtele N, Schoergenhofer
C, Roeggla M and Schwameis M: Severe hypernatraemic dehydration and
unconsciousness in a care-dependent inpatient treated with
empagliflozin. Drug Saf Case Rep. 4:172017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andersen A, Lund A, Knop FK and Vilsbøll
T: Glucagon-like peptide 1 in health and disease. Nat Rev
Endocrinol. 14:390–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cuevas-Ramos D, Lim DST and Fleseriu M:
Update on medical treatment for Cushing's disease. Clin Diabetes
Endocrinol. 2:162016. View Article : Google Scholar : PubMed/NCBI
|