Introduction
Ischemia-reperfusion (I-R) injury is the consequence
of anoxia due to ischemia of a tissue resulting from an obstructed
artery or circulation collapse and the marked production of
reactive oxygen species following re-canalization of the blocked
artery or restoration of effective blood volume. Therefore, I-R
injury is important in the pathogenesis of several human diseases,
including coronary heart disease, cerebral ischemia and multiple
organ failure (1–3). The kidney is particularly prone to I-R
injury as the partial pressure of oxygen is relatively low in this
organ and renal tubular epithelial cells require large amounts of
energy to preserve water, acid-base and electrolyte homeostasis
(4,5).
Several mammals hibernate during the winter months
in order to cope with a scarcity of food. Hibernation is
characterized by prolonged periods of deep torpor with a rapid fall
in body temperature, heart rate and breathing, with the whole
organism being in an ischemic state. Deep torpor is interrupted by
short periods of arousal when the animals rewarm themselves back to
euthermia and restore heart rate and breathing for several hours,
setting the organism in a state of reperfusion. Intriguingly, these
animals survive without signs of I-R injury in the brain, heart,
kidneys or other organs (6,7).
Classically, mammalian hibernation is considered to
represent a state of resistance to cold I-R injury, although body
temperature is restored during interbout arousals (6,7). However,
studies have demonstrated that these mammals resist warm I-R injury
more than other phylogenetically related species that are unable to
hibernate (8,9). Evidence has revealed hibernation in high
ambient and body temperatures, a phenomenon observed even in
primates (10,11). Therefore, besides resistance to cold
I-R injury, resistance to warm I-R injury also occurs in certain
hibernators, some of which are phylogenetically close to humans.
These data indicate that, under certain circumstances, human cells
may also be able to become resistant to warm I-R injury, making the
investigation of this phenomenon interesting from a clinical point
of view.
One of the events that requires further
investigation is the preservation of energy homeostasis in
hibernators during I-R. For this purpose, the present study
compared the effects of warm I-R on two of the most energy
demanding cellular processes, protein translation and the activity
of the Na+-K+-ATPase pump. Of the 80% of
oxygen consumption coupled to ATP synthesis, 25–30% is used for
protein synthesis and 19–28% is used by
Na+-K+-ATPase. Experiments in rats have shown
that the percentage of ATP consumed for the
Na+-K+-ATPase function in the mammalian
kidney tissue is even higher (12).
In the present study, primary renal proximal tubular
epithelial cells (RPTECs), which are sensitive to hypoxia (4,5), of mouse
or native hibernator Syrian hamster origin were cultured at 37°C
under normoxia, anoxia or anoxia followed by reoxygenation.
Investigating the mechanisms that offer mammalian hibernators
resistance to I-R injury may reveal novel therapeutic strategies
for attenuating I-R injury in humans.
Materials and methods
Cell culture conditions
Primary Syrian hamster RPTECs (cat. no. HM-6015) and
primary C57BL/6 Mouse RPTECs (cat. no. C57-6015) were cultured with
the Complete Epithelial Cell Medium/w kit (cat. no. M6621) (all
from Cell Biologics, Chicago, IL, USA), supplemented with
epithelial cell growth supplement (insulin-transferrin-selenium and
epidermal growth factor), antibiotic-antimycotic solution
(penicillin, streptomycin and amphotericin B) and 2% fetal bovine
serum (all from Cell Biologics). The cells were seeded in 6-well
plates at a density of 300,000 cells per well, in 12-well plates at
a density of 100,000 cells per well or in 96-well plates at a
density of 10,000 cells per well for 16 h prior to the onset of
anoxic conditions. The GasPak™ EZ Anaerobe Container System with
Indicator (cat. no. 26001; BD Biosciences, Franklin Lakes, NJ, USA)
was used to reduce oxygen levels <1%. Cells within the anaerobe
container were cultured at 37°C. These anoxic conditions imitate
warm ischemia. The reoxygenation experiments started following 24 h
of anoxia. In the experimental group exposed to reoxygenation, the
cells were washed with Dulbecco's phosphate buffer saline
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), fresh supplemented
culture medium was added, and the cells were incubated at 37°C in a
humidified atmosphere containing 5% CO2. These
reoxygenation conditions imitate warm reperfusion. Whenever cell
manipulation under anoxic conditions was necessary, this was
performed within an anoxic chamber. All the experiments were
performed nine times.
Evaluation of cell death, cellular ATP
content and ATPase activity
Cell imaging was performed to assess the sensitivity
of mouse and hamster RPTECs to warm anoxia or reoxygenation in
cells cultured in 12-well plates. For this purpose, an inverted
microscope (Axiovert 40C; Carl Zeiss AG, Göttingen, Germany) and a
digital camera with the related software (3MP USB2.0; Microscope
Digital Camera, Amscope, Irvine, CA, USA) were used. The cells
under anoxic conditions were monitored within their anoxic
container, which was transparent permitting live image capture. As
cell staining was not possible in living cells within the anoxic
container, cell death was also evaluated biochemically. Cell death
was assessed using the Cell Death Detection ELISA Plus kit (cat.
no. 11774425001 ver. 11; Roche Diagnostics, Indianapolis, IN, USA),
which relies on the detection of cytoplasmic histone-associated DNA
fragments. The RPTECs were cultured in 6-well plates for 24 h in
anoxia, and for another 2 h they were reoxygenated in the case of
the reoxygenation procedure. The respective time points were
selected according to the results obtained by cell imaging.
Cellular ATP content was assessed using the ATP
Colorimetric/Fluorometric Assay kit (cat. no. MAK190,
Sigma-Aldrich; Merck KGaA) in RPTECs cultured in 96-well
plates.
Cellular ATPase activity was assessed using the
ATPase Activity Assay kit (colorimetric; cat. no. K417-100;
BioVision, Inc., Milpitas, CA, USA) in RPTECs cultured in 6-well
plates. The principle of this assay lies in the detection of a
stable chromophore generated by the release of a free phosphate ion
derived from ATP hydrolysis. The activity is expressed as free
phosphate production as nmol/min (or mU)/100 µg of cellular
protein.
Evaluation of proteins that control
protein translation and activated AMP-activated protein kinase
(AMPK)
The cells were cultured in 6-well plates as
described above. The cells were then lysed using T-PER tissue
protein extraction reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with protease and phosphatase
inhibitors (Sigma-Aldrich; Merck KGaA and Roche Diagnostics,
respectively). Protein was quantified using a Bradford assay
(Sigma-Aldrich; Merck KGaA) and 10 µg from each sample was used for
western blotting. Protein samples were electrophoresed in a 4–12%
bis-tris acrylamide gel (NuPAGE 4–12% Bis-Tris Gel 1.0 mm × 15
well; cat. no. NP0323BOX, Invitrogen; Thermo Fisher Scientific,
Inc.) at 180V constant for 30 min. Blotting of the electrophoresed
gel proteins on the polyvinylidene difluoride (PVDF) membrane was
performed via electroporation at 30V constant for 1 h. Skimmed milk
in tris-buffered saline with Tween-20 was used for blocking. The
blots were incubated with primary antibody against phosphorylated
Ser51 eukaryotic translation initiation factor 2α (p-eIF2α;
dilution 1:500; cat. no. 9721), phosphorylated Thr37/46 eukaryotic
translation initiation factor 4E-binding protein 1 (p-4E-BP1;
dilution 1:1,000; cat. no. 2855), phosphorylated Thr172α subunit of
AMPK (p-AMPK; dilution 1:1,000; cat. no. 2535) and β-actin
(dilution 1:2000; cat. no. 4967) (all from Cell Signaling
Technology, Inc., Danvers, MA, USA) for 16 h at 4°C. This was
followed by 30 min of incubation at room temperature with the
secondary antibody (anti-rabbit IgG, HRP-linked; dilution 1:1,000;
cat. no. 7074, Cell Signaling Technology, Inc.). In reprobing the
PVDF blots, the previous primary and secondary antibodies were
removed with Restore Western Blot Stripping Buffer (Thermo Fisher
Scientific, Inc.). Densitometric analysis of the western blot bands
was performed using ImageJ software (National Institute of Health,
Bethesda, MD, USA).
Evaluation of resistance to
ouabain-induced cell death
The resistance of the mouse or hamster RPTECs to
ouabain-induced cell death was assessed by cell imaging in cells
cultured in 12-well plates under normoxic conditions (37°C in a
humidified atmosphere containing 5% CO2) with or without
the presence of ouabain (cat. no. O3125, Sigma-Aldrich; Merck KGaA)
at a concentration of 2 mM. Ouabain is a well-known
Na+-K+-ATPase inhibitor. It exerts its effect
mainly by disrupting the ion gradient of the cell. However,
evidence supports that Na+-K+-ATPase may also
be a receptor and signal transducer, and that its stabilization by
ouabain exerts additional, but less profound effects (13). Various concentrations of ouabain have
been used in several studies showing a varying degree of
Na+-K+-ATPase inhibition (14–16). In
the present study, the high concentration of 2 mM was selected to
achieve the highest possible inhibition of
Na+-K+-ATPase. For this purpose, images were
captured hourly using an inverted microscope (Axiovert 40C; Carl
Zeiss AG) and a digital camera with the related software (3MP
USB2.0, Microscope Digital Camera; AmScope, Irvine, CA, USA).
In addition to cell imaging, cell survival was
assessed biochemically in cultures of RPTECs in 96-well plates
under normoxic conditions, with or without the presence of 2 mM
ouabain, for 4 h. This time point was selected according to the
results obtained by cell imaging. For this purpose, the TACS XTT
assay kit (cat. no. 4891025K; Trevigen, Inc., Gaithersburg, MD,
USA) was used according to the protocol provided by the
manufacturer.
Statistical analysis
The normality of the evaluated variables was
assessed and confirmed using a one-sample Kolmogorov-Smirnov test.
For comparison of means, an unpaired t-test or one-way analysis of
variance followed by Bonferroni's correction test was used. For
statistical analysis of the cell imaging results, the Mann-Whitney
U test was used as these results did not fit the normal
distribution. The results are expressed as the mean ± standard
deviation and a P<0.05 was considered to indicate a
statistically significant difference. Although the original data
were analyzed statistically, for reader's convenience, in the
majority of cases, the results are shown following normalization of
means for the control group. Statistical analysis was performed
with IBM SPSS Statistics for Windows, version 20 (IBM Corp.,
Armonk, NY, USA).
Results
In contrast to mouse cells, hamster
cells are resistant to cell death due to warm anoxia or
reoxygenation
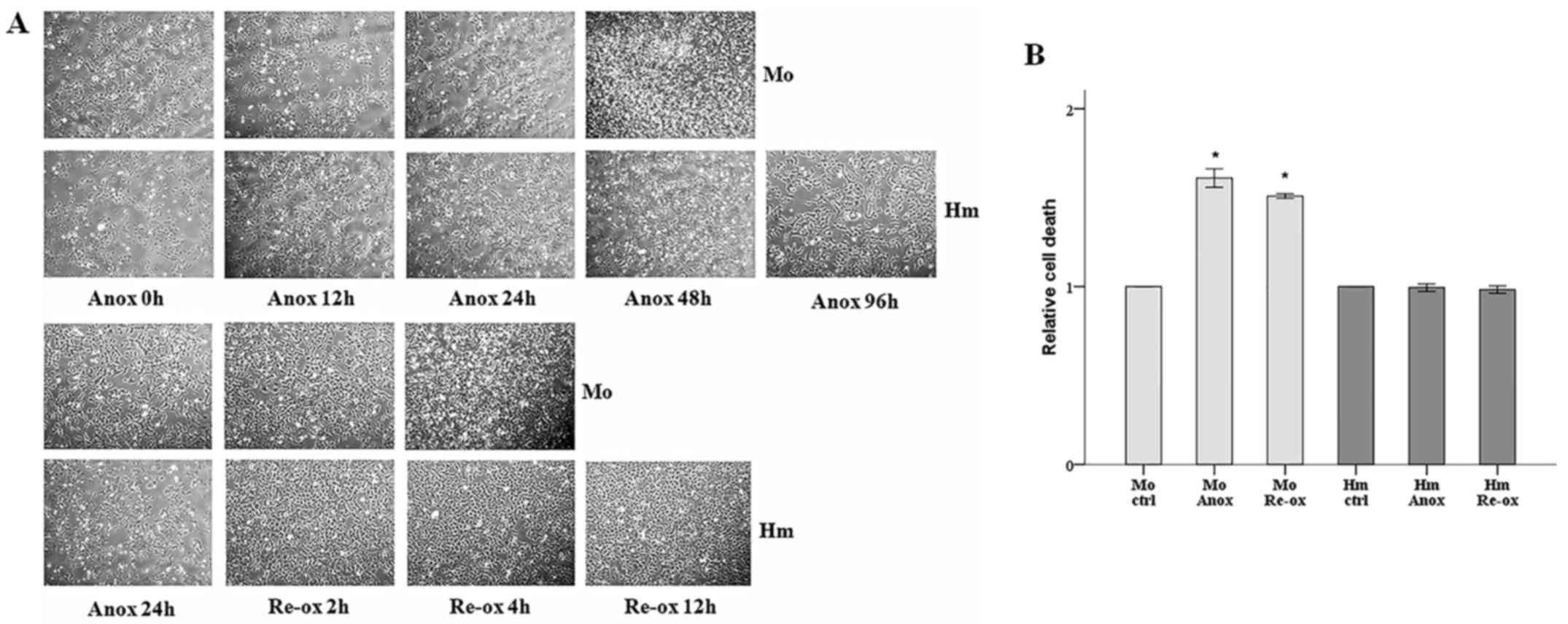
Cell imaging revealed that the mouse RPTECs died
after 48 h under anoxic conditions, whereas the hamster RPTECs
remained viable even after 96 h. The mouse RPTECs were also more
sensitive to reoxygenation, as their morphology deteriorated
significantly after 4 h, exhibiting condensation and loss of
adherence on a large scale, whereas the hamster cells exhibited
none of these signs after 12 h (Fig.
1A).
The cell imaging results were also confirmed
biochemically. The hamster RPTECs resisted 24 h of warm anoxia and
2 h of reoxygenation. By contrast, the cell death assay revealed
that mouse RPTECs were susceptible to both deleterious conditions,
as cell death increased by 61% during anoxia and 51% during
reoxygenation (Fig. 1B).
In mouse cells, cellular ATP content
decreases under warm anoxia or reoxygenation but remains constant
in hamster cells under anoxia
Compared with the control, cellular ATP content in
the mouse RPTECs decreased by 38% following 24 h of anoxia and
remained decreased, by 41%, following 2 h of reoxygenation. In the
hamster RPTECs, cellular ATP content did not alter significantly
under anoxia but decreased by 39% following reoxygenation (Fig. 2A).
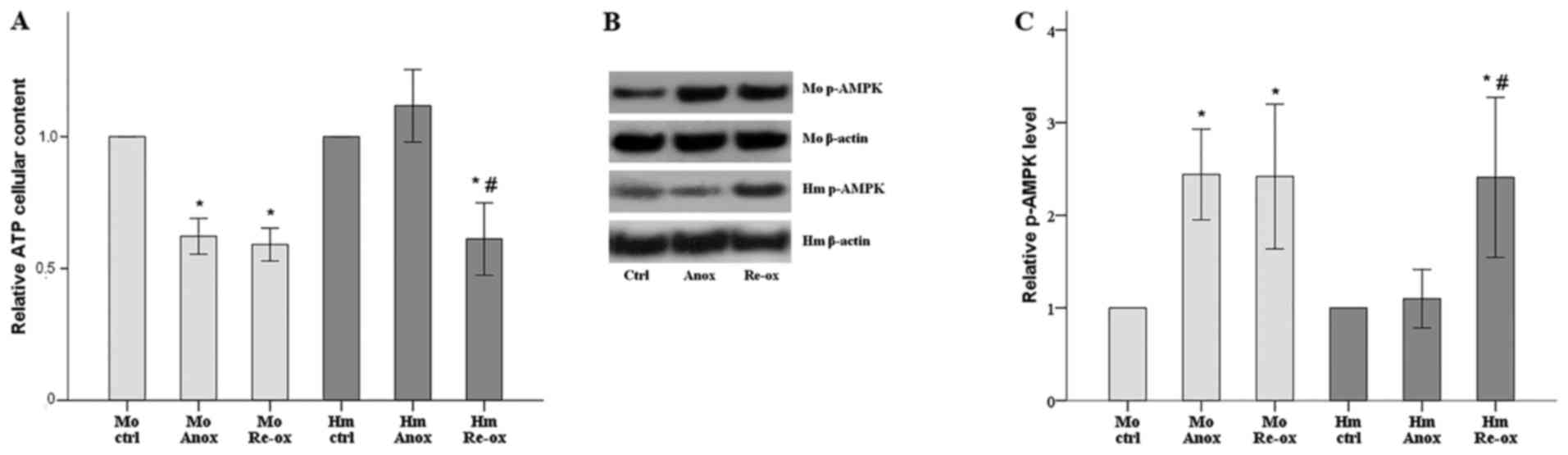 | Figure 2.Cellular ATP content following warm
anoxia or reoxygenation in mouse and hamster renal proximal tubular
epithelial cells. (A) ATP assay revealed that, in mouse cells,
cellular ATP content decreased under warm anoxia or reoxygenation.
In hamster cells, ATP remained constant under anoxia and decreased
following reoxygenation. (B) To confirm the results of the ATP
assay, western blotting was performed to assess the activation
status of the cellular sensor of energy AMPK; results of one of the
nine performed experiments are presented. (C) Alterations in the
levels of activated p-AMPK were in an opposite direction to the
alterations of cellular ATP content. All experiments were performed
nine times. *P<0.05 vs. ctrl; #P<0.05 vs. Anox.
Error bars correspond to the mean ± standard deviation. Mo, mouse;
Hm, hamster; Anox, anoxia; ctrl, control culture under normoxia;
Re-ox, reoxygenation; AMPK, AMP-activated protein kinase; p-AMPK,
phosphorylated AMPK. |
The levels of activated p-AMPK also confirmed the
ATP content results as this enzyme is a sensor and regulator of
cellular energy. In the mouse RPTECs, anoxia and reoxygenation
increased the level of p-AMPK to 244 and 242% of the control level,
respectively. In the hamster RPTECs, p-AMPK remained stable under
anoxia but increased to 241% of the control level following
reoxygenation (Fig. 2B and C).
Activated regulators of the protein
translation eIF2α and 4E-BP1 follow the same trend in mouse and
hamster cells under warm anoxia, but are overcorrected in hamster
cells following reoxygenation
In the mouse RPTECs, the level of p-eIF2α increased
by 56% following anoxia and returned to the level of the normoxic
conditions following reoxygenation. In the hamster RPTECs, anoxia
increased the level of p-eIF2α to 249% of the control, whereas
reoxygenation decreased p-eIF2α by 42% (Fig. 3A and B).
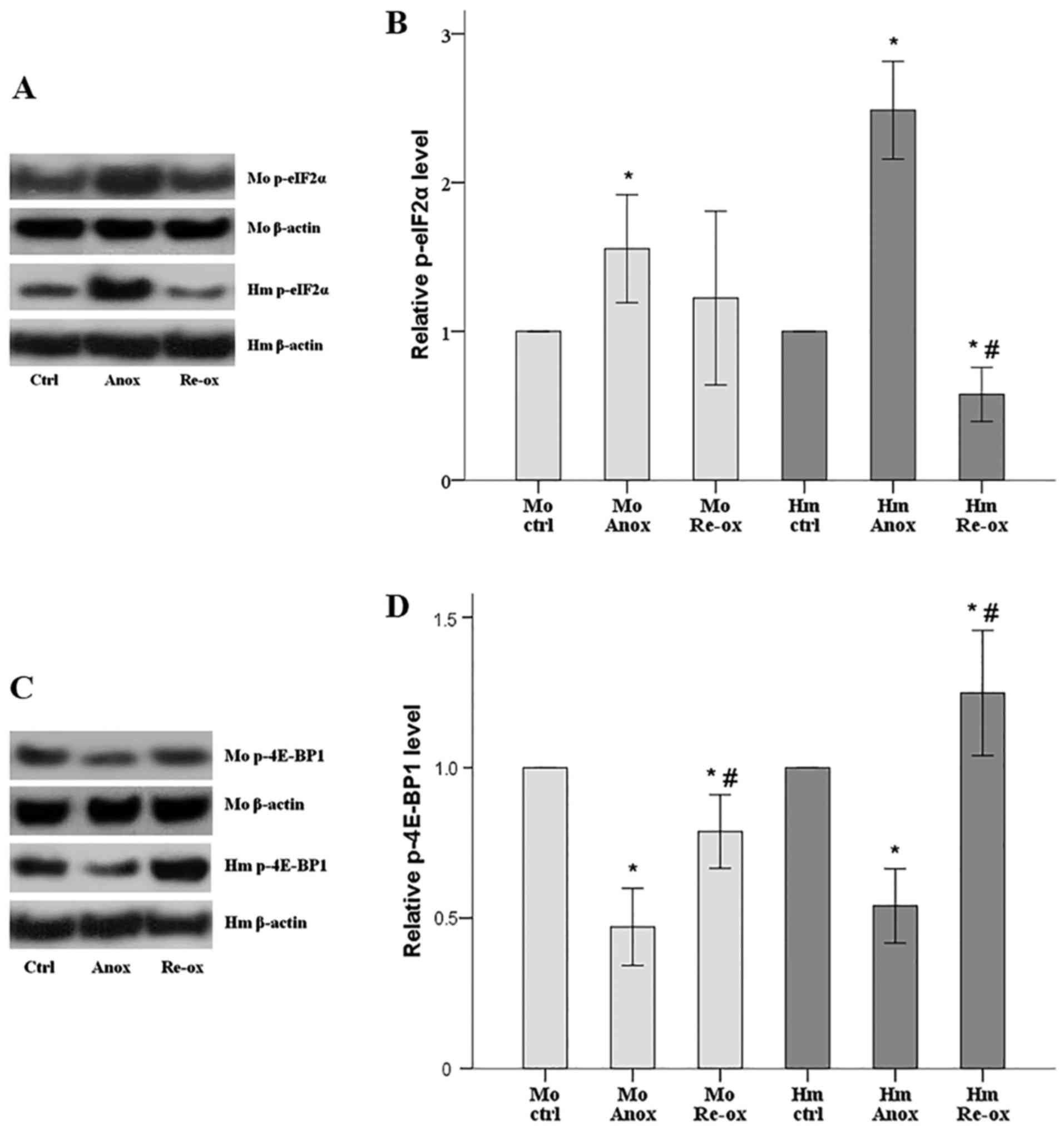 | Figure 3.Activation status of protein
translation regulators eIF2α and 4E-BP1 following warm anoxia or
reoxygenation in mouse and hamster RPTECs. (A) Western blot
analysis was performed to assess the level of p-eIF2α, and the
results of one of the nine experiments are shown. (B) In mouse
RPTECs, the level of p-eIF2α increased following anoxia and
returned towards the level measured in normoxic conditions
following reoxygenation. In hamster RPTECs, p-eIF2α increased
following anoxia but overcorrected and was decreased following
reoxygenation. (C) Levels of p-4E-BP1 from one of the nine
performed experiments are shown. (D) In mouse RPTECs, the level of
p-4E-BP1 decreased following anoxia and increases following
reoxygenation, but remained lower compared with the level in
normoxic conditions. In hamster RPTECs, the level of p-4E-BP1
decreased following anoxia but overcorrected and increased
following reoxygenation. All experiments were performed nine times.
*P<0.05 vs. ctrl; #P<0.05 vs. Anox. Error bars
correspond to the mean ± standard deviation. RPTECs, renal proximal
tubular epithelial cells; Mo, mouse; Hm, hamster; Anox, anoxia;
ctrl, control culture under normoxia; Re-ox, reoxygenation;
p-eIF2α, phosphorylated eukaryotic translation initiation factor
2α; p-4E-BP1, phosphorylated eukaryotic translation initiation
factor 4E-binding protein 1. |
In the mouse RPTECs, the level of p-4E-BP1 decreased
by 53% following anoxia and increased following reoxygenation, but
remained 21% lower than the level in normoxia. In the hamster
RPTECs, the level of p-4E-BP1 decreased by 46% following anoxia but
increased by 25% following reoxygenation (Fig. 3C and D).
ATPase activity decreases in mouse
cells and remains stable in hamster cells under anoxia, whereas
ATPase activity increases in mouse and hamster cells during
reoxygenation
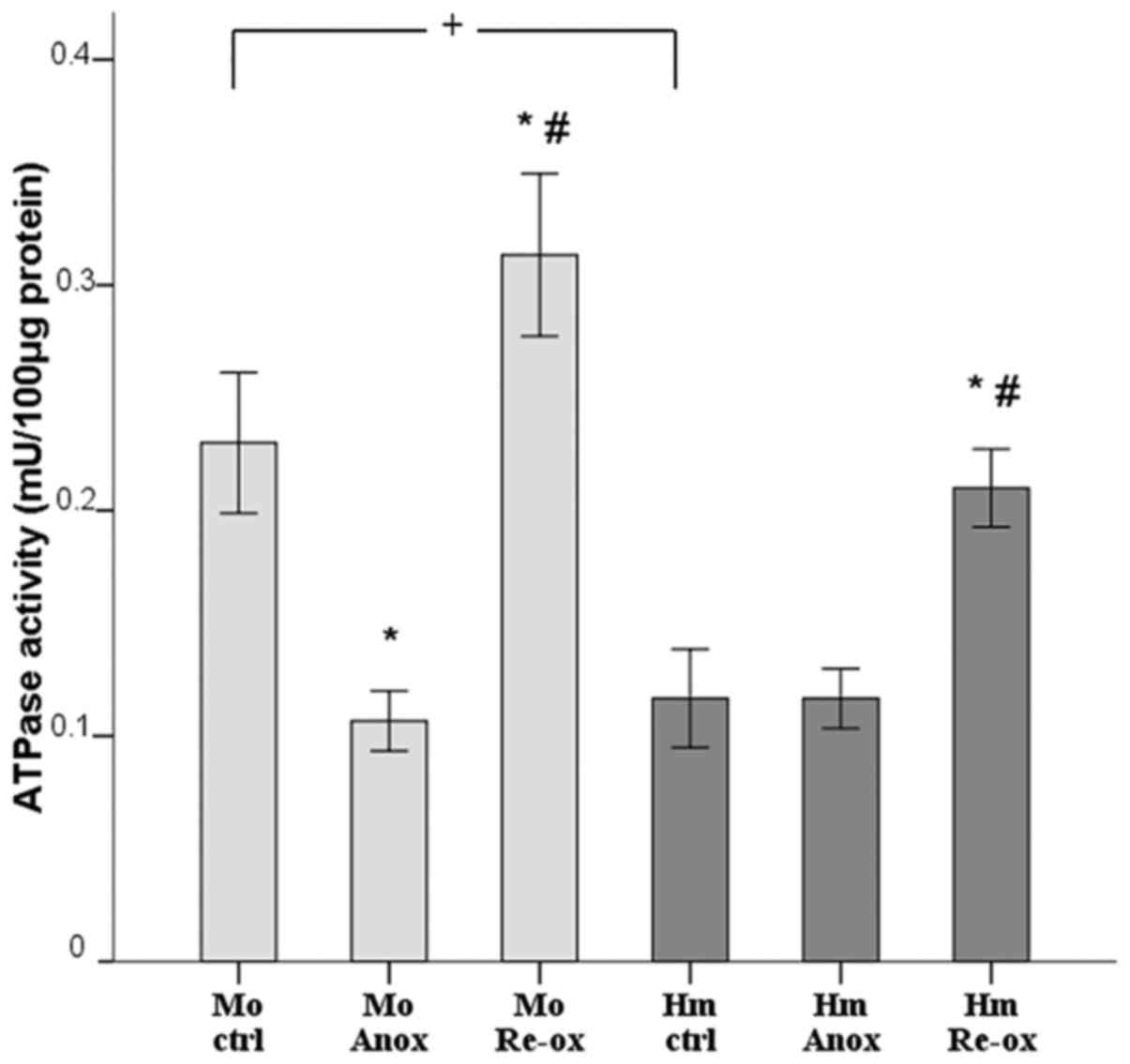
In the mouse RPTECs, ATPase activity decreased from
0.23±0.03 mU/100 µg protein under normoxic conditions to 0.11±0.01
mU/100 µg protein under anoxia, whereas it increased to 0.31±0.04
mU/100 µg protein following reoxygenation. Compared with normoxia,
the activity of ATPase in the hamster RPTECs did not alter during
anoxia (0.12±0.02 vs. 0.12±0.01 mU/100 µg protein), but increased
to 0.21±0.02 mU/100 µg protein following reoxygenation. Of note,
under normoxic conditions, the activity of ATPase in the hamster
cells was half of that observed in the mouse cells (Fig. 4).
Under normoxic conditions, hamster
cells are less sensitive to ouabain-induced cell death than mouse
cells
It has been noted in RPTECs that the majority of the
activity of ATPase corresponds to
Na+-K+-ATPase activity (12). Therefore, the observation that ATPase
activity was ~50% lower in hamster than mouse RPTECs under normoxic
conditions may reflect the lower energy required for adequate
Na+-K+-ATPase maintenance of a normal ion
gradient in hamster cells. As the
Na+-K+-ATPase pump preserves the
Na+ and K+ ion gradient against passive
leakage through ion channels, the lower energy demand of this pump
may be the result of fewer ion channels in the hamster cell
membrane. Once Na+-K+-ATPase pump activity is
inhibited, deregulation of the intracellular ion concentration
leads to cell death (17). The more
ion channels there are, the faster the intracellular ion
deregulation and cell death. Therefore, by inhibiting the
Na+-K+-ATPase pump with a high concentration
of ouabain and measuring the time span until cell death, the number
or the functionality of ion channels can be assessed.
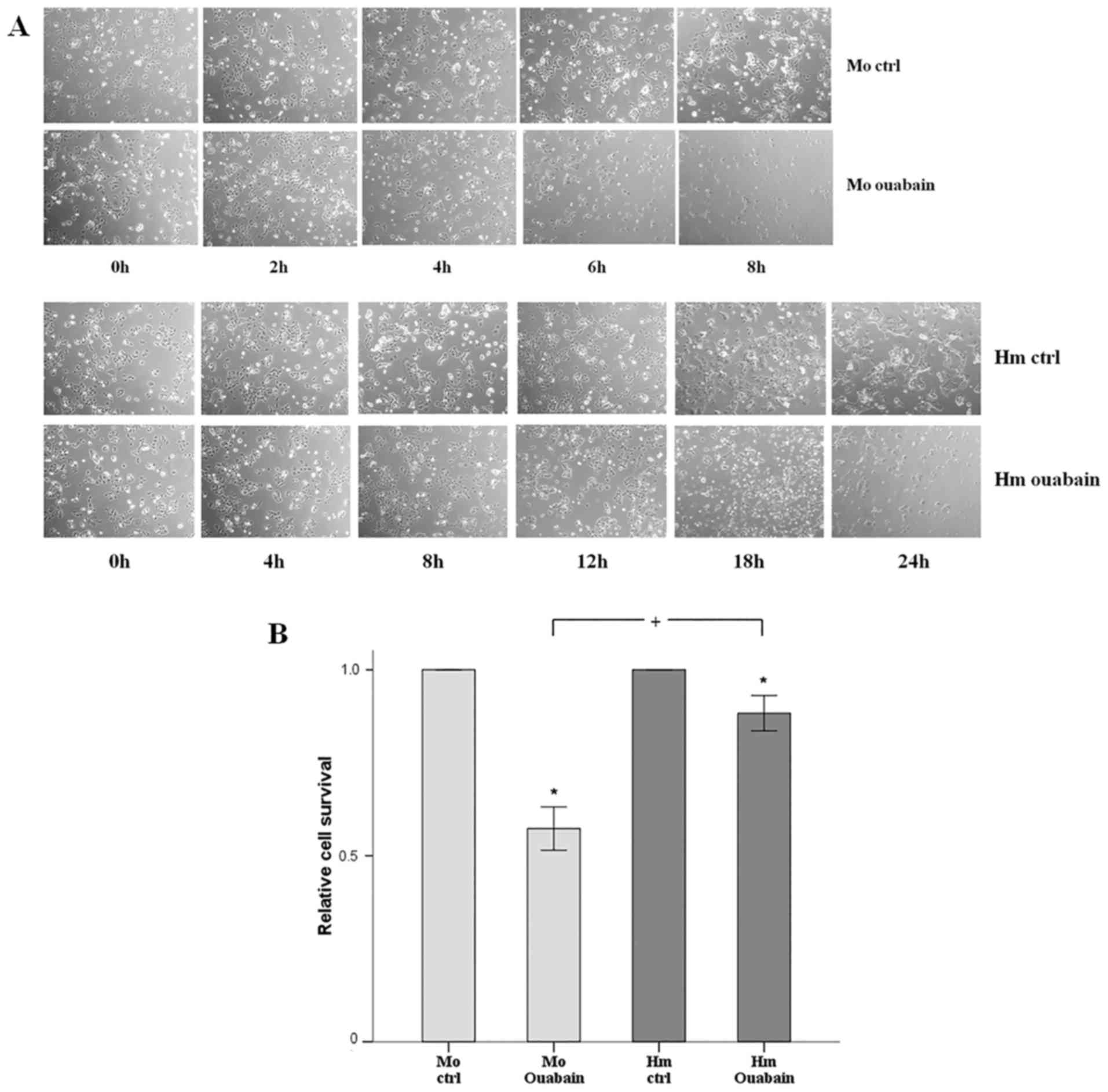
Cell imaging revealed that, under normoxic
conditions, the ouabain-treated mouse RPTECs deteriorated
significantly after 6 h and cell death occurred after 8 h. The
hamster RPTECs were less sensitive to ouabain as they declined
considerably after 18 h and died after 24 h (Fig. 5A). The cell imaging results were
confirmed biochemically. Following treatment for 4 h with ouabain,
88% of the hamster RPTECs survived, whereas only 57% of mouse
RPTECs survived (Fig. 5B).
Discussion
Defining the mechanisms of resistance to I-R injury
in mammalian hibernators may reveal novel therapeutic strategies
against several human diseases, including coronary heart disease,
cerebral ischemia, acute kidney injury and multiple organ failure
(1–5).
Using RPTEC cultures, in consensus with previous
studies (8,9), the present study corroborated that, in
contrast to the non-hibernator mouse, cells from the native
hibernator Syrian hamster resisted cell death caused by warm anoxia
or reoxygenation. One of the problems that mammalian hibernators
have to cope with is cellular energy deprivation during periods of
low tissue perfusion, as anaerobic glycolysis is less efficient
than oxidative phosphorylation in ATP production. As expected, the
experiments in the present study showed that cellular ATP content
in the mouse RPTECs decreased during anoxia. By contrast, ATP
remained stable in hamster RPTECs under anoxic conditions,
indicating the existence of adaptive mechanisms which lower the
energy requirements for cell survival. Cellular ATP content
decreased in the RPTECs of both species following 2 h of
reoxygenation, possibly as a result of the energetically demanding
processes of cell repair following anoxia, including protein
translation, correction of ion gradient disturbances and
degradation of misfolded proteins. Confirming the above results, it
was found that the alterations in the levels of activated AMPK were
the inverse of changes in cellular ATP content, which is expected
as AMPK is a sensor of cellular ATP and is activated in cases of
low ATP levels (18).
In an attempt to clarify the mechanisms of ATP
preservation in hamster RPTECs under anoxia, the present study
evaluated the most energy demanding cellular processes. Protein
translation is one such process (12). Two main factors that regulate protein
translation are eIF2α and 4E-BP1. Once eIF2α is phosphorylated, it
downregulates protein translation in general (19). In terms of 4E-BP1, it is
phosphorylated by mammalian target of rapamycin complex 1 (mTORC1).
Unphosphorylated 4E-BP1 inhibits protein translation (20). In hibernating squirrels, increased
phosphorylation status of eIF2α has been detected (21), whereas 4E-BP1 is hypophosphorylated
(22). This sugestes that during low
tissue perfusion mammalian hibernators can save energy by
inhibiting global protein translation.
The experiments in the present study showed that
warm anoxia increased p-eIF2α and decreased p-4E-BP1 in mouse and
hamster RPTECs. Therefore, protein translation was inhibited in
cells from the two species, indicating that this adaptive mechanism
is not specific for hibernators. In line with these results, other
studies in cells from non-hibernators have also shown that, under
hypoxia, eIF2α is phosphorylated by endoplasmic reticulum-resident
eIF2 kinase during the unfolded protein response (23), whereas mTORC1 is inhibited (24). When the cells were reoxygenated, the
levels of p-eIF2a and p-4E-BP1 shifted towards the normoxic levels
in mouse cells, but an overcorrection was observed in hamster
cells. This may represent an adaptive mechanism for a faster
recovery from anoxia-induced injury in hamster cells. Such an
overcorrection has also been detected in p-4E-BP1 during interbout
arousals in squirrels (22).
As protein synthesis decreased during anoxia in the
RPTECs of the two species examined, the lower ATP content observed
in anoxic mouse cells cannot be attributed solely to this. By
contrast, the increased protein translation during reoxygenation in
the mouse and hamster cells may contribute to the observed decrease
in cellular ATP content. Therefore, in addition to protein
translation, the present study examined the other most energy
demanding cellular process, the function of the
Na+-K+-ATPase pump. In the mammalian kidney,
Na+-K+-ATPase consumes up to 40–70% of the
produced ATP (12). Therefore, the
majority of the ATPase activity measured in the present study
corresponded to the function of the
Na+-K+-ATPase pump. The experiments revealed
that the activity of ATPase decreased during warm anoxia in the
mouse RPTECs but remained stable in the hamster RPTECs. Under
reoxygenation, the activity of ATPase was increased in cells from
the two species, possibly in the context of energy consuming
reparative processes. The latter contributes to the low cellular
ATP content detected following 2 h of reoxygenation in the two cell
types, despite the restoration of oxidative phosphorylation.
Under normoxia, the activity of ATPase in the mouse
RPTECs was 2-fold higher than that in the hamster RPTECs. This may
reflect lower energy requirements for the function of
Na+-K+-ATPase to be sufficient to preserve a
normal ion gradient in hamster RPTECs. Therefore, it is likely
that, under anoxic conditions, the net gain of ATP from the
decreased protein synthesis in hamster RPTECs is sufficient for
retaining a normal Na+-K+-ATPase function and
cell survival. This agrees with the lower
Na+-K+-ATPase activity observed in the renal
cortex of active ground squirrels when compared with
non-hibernating rabbits (25).
Regarding the curtailed energy demand for
Na+-K+-ATPase function in hamster RPTECs, the
channel arrest theory states that hibernators are less sensitive to
anoxia, as they downregulate cell membrane Na+ and
K+ ion channels under anoxia. Therefore, despite the
decreased function of the Na+-K+-ATPase pump
due to the reduced production of ATP, the decreased passive leakage
of the Na+ and K+ ions allows the
preservation of a normal ion gradient and cell survival under
anoxia (26,27). However, the experiments in the present
study raise questions as to whether the predictions of the channel
arrest theory are well-founded during normoxia. In such a case, the
resistance of hamster RPTECs to anoxia pre-exists the encountering
of anoxic conditions.
Once Na+-K+-ATPase activity is
inhibited, deregulation of the intracellular ion concentration
leads to cell death (17). The more
ion channels there are, the faster the intracellular ion
deregulation and cell death. In the present study, under normoxic
conditions, more hamster cells survived than mouse cells following
treatment with the Na+-K+-ATPase inhibitor
ouabain, indicating that hamster RPTECs have fewer Na+
and K+ ion channels in the cell membrane than mouse
RPTECs, and consequently less energy is necessary for adequate
function of the Na+-K+-ATPase pump.
Therefore, hamster RPTECs are prepaired for survival against an
anoxic assault.
In conclusion, under anoxia, hamster RPTECs
preserved energy homeostasis more efficiently than mouse RPTECs as
a result of lower levels of ATP required for adequate
Na+-K+-ATPase maintenance of a normal ion
gradient; this is possibly due to fewer cell membrane
Na+ and K+ ion channels. The increase in ATP
from the depression of other energy-demanding processes, including
protein synthesis, is sufficient for sustaining
Na+-K+-ATPase function in hamster cells. The
low energy required for Na+-K+-ATPase
function and the faster recovery during reoxygenation contribute to
the resistance of the native hibernator Syrian hamster RPTECs to
anoxia or reoxygenation. Defining how mammalian hibernators cope
with the repeated cycles of I-R may reveal possible molecular
targets in humans for preventing warm I-R injury and potential
novel therapeutic strategies against several I-R injury-induced
human diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
TE designed the study; GP and TE performed the
experiments; TE, GP, GA, SG, VL and IS analyzed the results; TE
wrote the manuscript with assistance from GP; IS supported all
stages.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neri M, Riezzo I, Pascale N, Pomara C and
Turillazzi E: Ischemia/reperfusion injury following acute
myocardial infarction: A critical issue for clinicians and forensic
pathologists. Mediators Inflamm. 2017:70183932017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bakthavachalam P and Shanmugam PS:
Mitochondrial dysfunction - Silent killer in cerebral ischemia. J
Neurol Sci. 375:417–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsukamoto T, Chanthaphavong RS and Pape
HC: Current theories on the pathophysiology of multiple organ
failure after trauma. Injury. 41:21–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonventre JV and Yang L: Cellular
pathophysiology of ischemic acute kidney injury. J Clin Invest.
121:4210–4221. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lieberthal W and Nigam SK: Acute renal
failure. I. Relative importance of proximal vs. distal tubular
injury. Am J Physiol. 275:F623–F631. 1998.PubMed/NCBI
|
|
6
|
Carey HV, Andrews MT and Martin SL:
Mammalian hibernation: Cellular and molecular responses to
depressed metabolism and low temperature. Physiol Rev.
83:1153–1181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Storey KB and Storey JM: Metabolic rate
depression: The biochemistry of mammalian hibernation. Adv Clin
Chem. 52:77–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dave KR, Prado R, Raval AP, Drew KL and
Perez-Pinzon MA: The arctic ground squirrel brain is resistant to
injury from cardiac arrest during euthermia. Stroke. 37:1261–1265.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quinones QJ, Zhang Z, Ma Q, Smith MP,
Soderblom E, Moseley MA, Bain J, Newgard CB, Muehlbauer MJ,
Hirschey M, et al: Proteomic profiling reveals adaptive responses
to surgical myocardial ischemia-reperfusion in hibernating arctic
ground squirrels compared to rats. Anesthesiology. 124:1296–1310.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levin E, Plotnik B, Amichai E, Braulke LJ,
Landau S, Yom-Tov Y and Kronfeld-Schor N: Subtropical mouse-tailed
bats use geothermally heated caves for winter hibernation. Proc
Biol Sci. 282:201427812015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dausmann KH, Glos J, Ganzhorn JU and
Heldmaier G: Physiology: Hibernation in a tropical primate. Nature.
429:825–826. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rolfe DF and Brown GC: Cellular energy
utilization and molecular origin of standard metabolic rate in
mammals. Physiol Rev. 77:731–758. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Venugopal J and Blanco G: On the many
actions of Ouabain: Pro-cystogenic effects in autosomal dominant
polycystic kidney disease. Molecules. 22:222017.
|
|
14
|
Weinberg JM, Davis JA, Abarzua M, Smith RK
and Kunkel R: Ouabain-induced lethal proximal tubule cell injury is
prevented by glycine. Am J Physiol. 258:F346–F355. 1990.PubMed/NCBI
|
|
15
|
Liu J, Periyasamy SM, Gunning W, Fedorova
OV, Bagrov AY, Malhotra D, Xie Z and Shapiro JI: Effects of cardiac
glycosides on sodium pump expression and function in LLC-PK1 and
MDCK cells. Kidney Int. 62:2118–2125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cherniavsky-Lev M, Golani O, Karlish SJ
and Garty H: Ouabain-induced internalization and lysosomal
degradation of the Na+/K+-ATPase. J Biol
Chem. 289:1049–1059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao AY, Wei L, Xia S, Rothman S and Yu
SP: Ionic mechanism of ouabain-induced concurrent apoptosis and
necrosis in individual cultured cortical neurons. J Neurosci.
22:1350–1362. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kilberg MS, Shan J and Su N:
ATF4-dependent transcription mediates signaling of amino acid
limitation. Trends Endocrinol Metab. 20:436–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nat Rev Mol Cell Biol.
10:307–318. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frerichs KU, Smith CB, Brenner M, DeGracia
DJ, Krause GS, Marrone L, Dever TE and Hallenbeck JM: Suppression
of protein synthesis in brain during hibernation involves
inhibition of protein initiation and elongation. Proc Natl Acad Sci
USA. 95:14511–14516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Breukelen F, Sonenberg N and Martin
SL: Seasonal and state-dependent changes of eIF4E and 4E-BP1 during
mammalian hibernation: Implications for the control of translation
during torpor. Am J Physiol Regul Integr Comp Physiol.
287:R349–R353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koumenis C, Naczki C, Koritzinsky M,
Rastani S, Diehl A, Sonenberg N, Koromilas A and Wouters BG:
Regulation of protein synthesis by hypoxia via activation of the
endoplasmic reticulum kinase PERK and phosphorylation of the
translation initiation factor eIF2alpha. Mol Cell Biol.
22:7405–7416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arsham AM, Howell JJ and Simon MC: A novel
hypoxia-inducible factor-independent hypoxic response regulating
mammalian target of rapamycin and its targets. J Biol Chem.
278:29655–29660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Charnock JS and Simonson LP: Seasonal
variations in the renal cortical (Na+ +
K+)-ATPase and Mg2+-ATPase of a hibernator,
the ground squirrel (Spermophilus richardsonii). Comp Biochem
Physiol B. 60:433–439. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hochachka PW: Defense strategies against
hypoxia and hypothermia. Science. 231:234–241. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boutilier RG: Mechanisms of cell survival
in hypoxia and hypothermia. J Exp Biol. 204:3171–3181.
2001.PubMed/NCBI
|