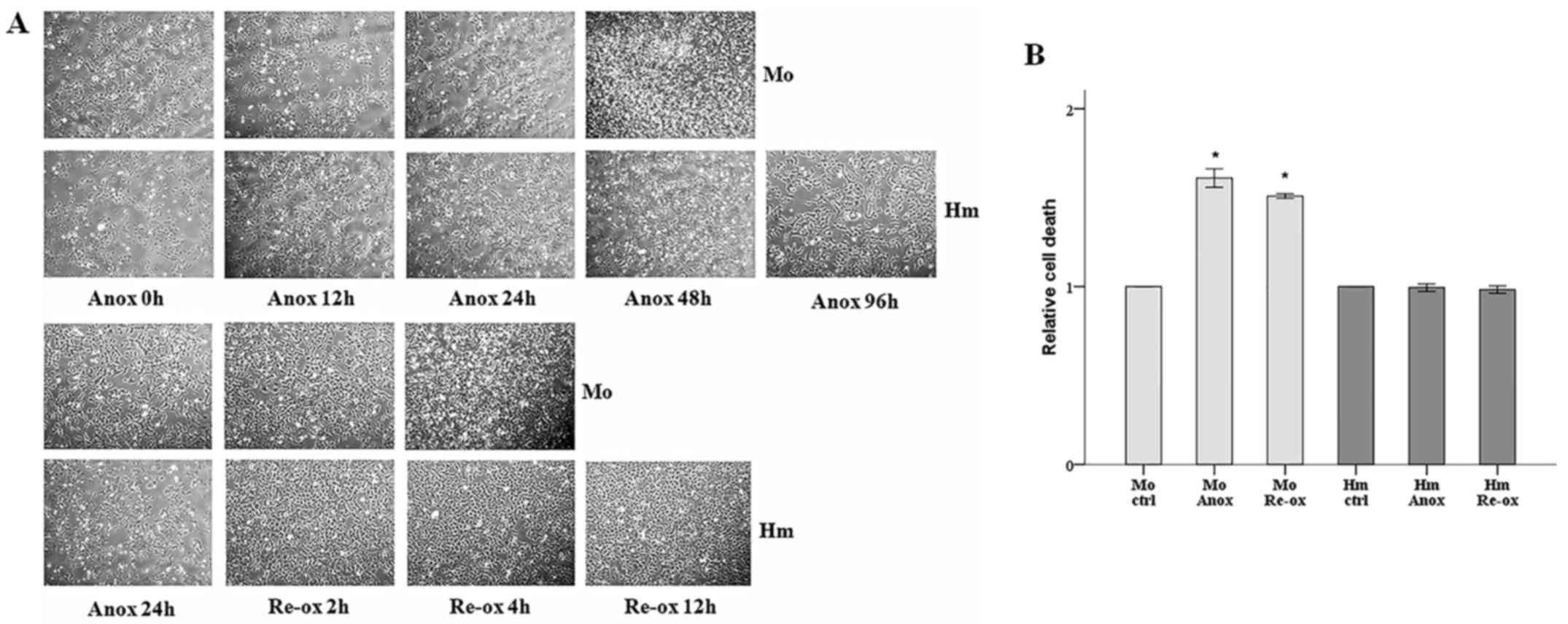
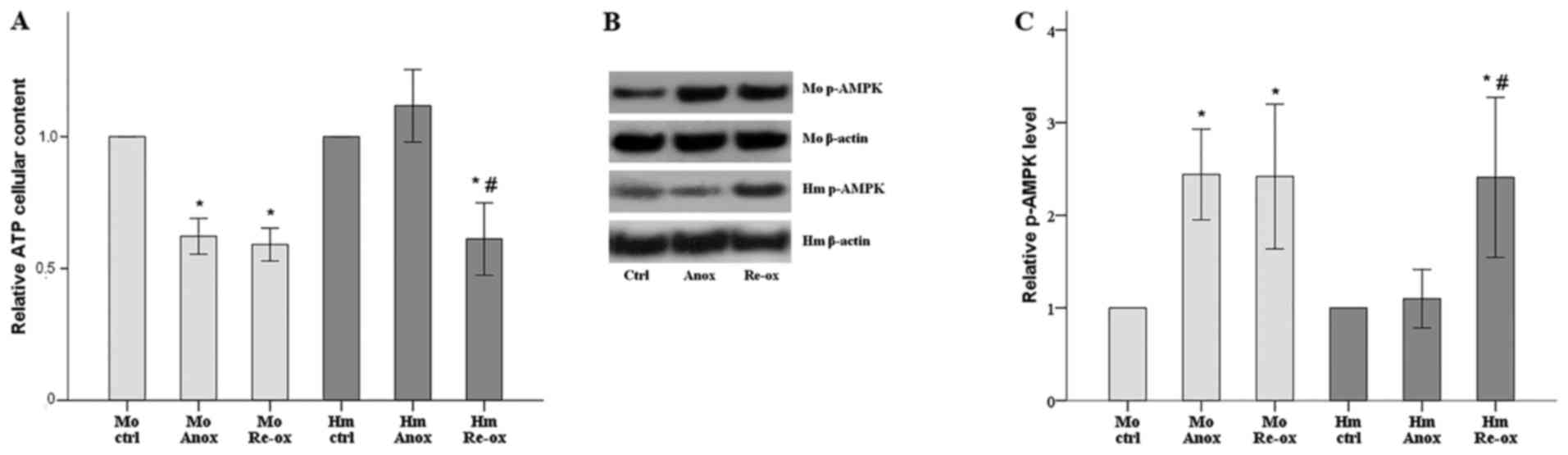
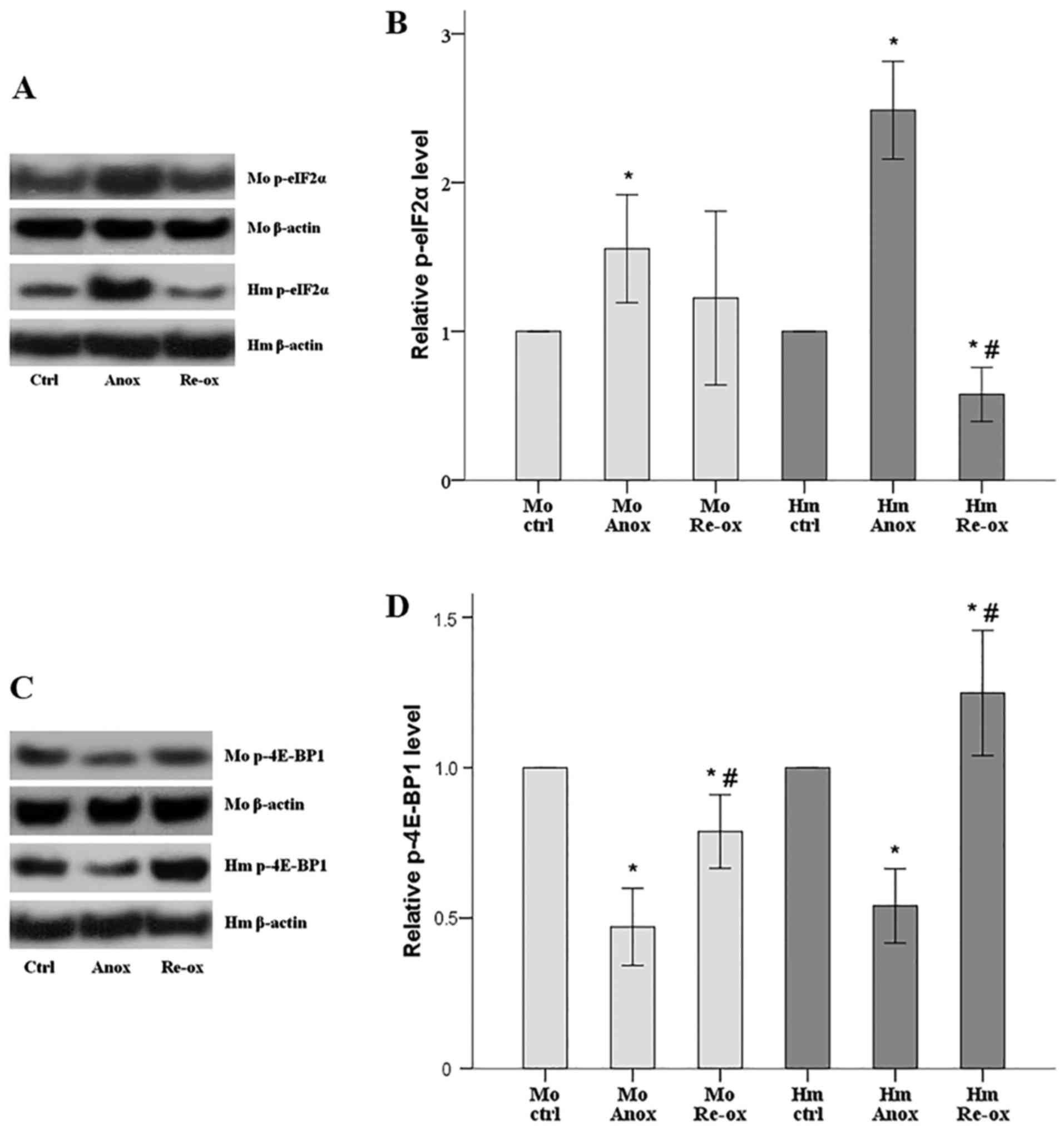
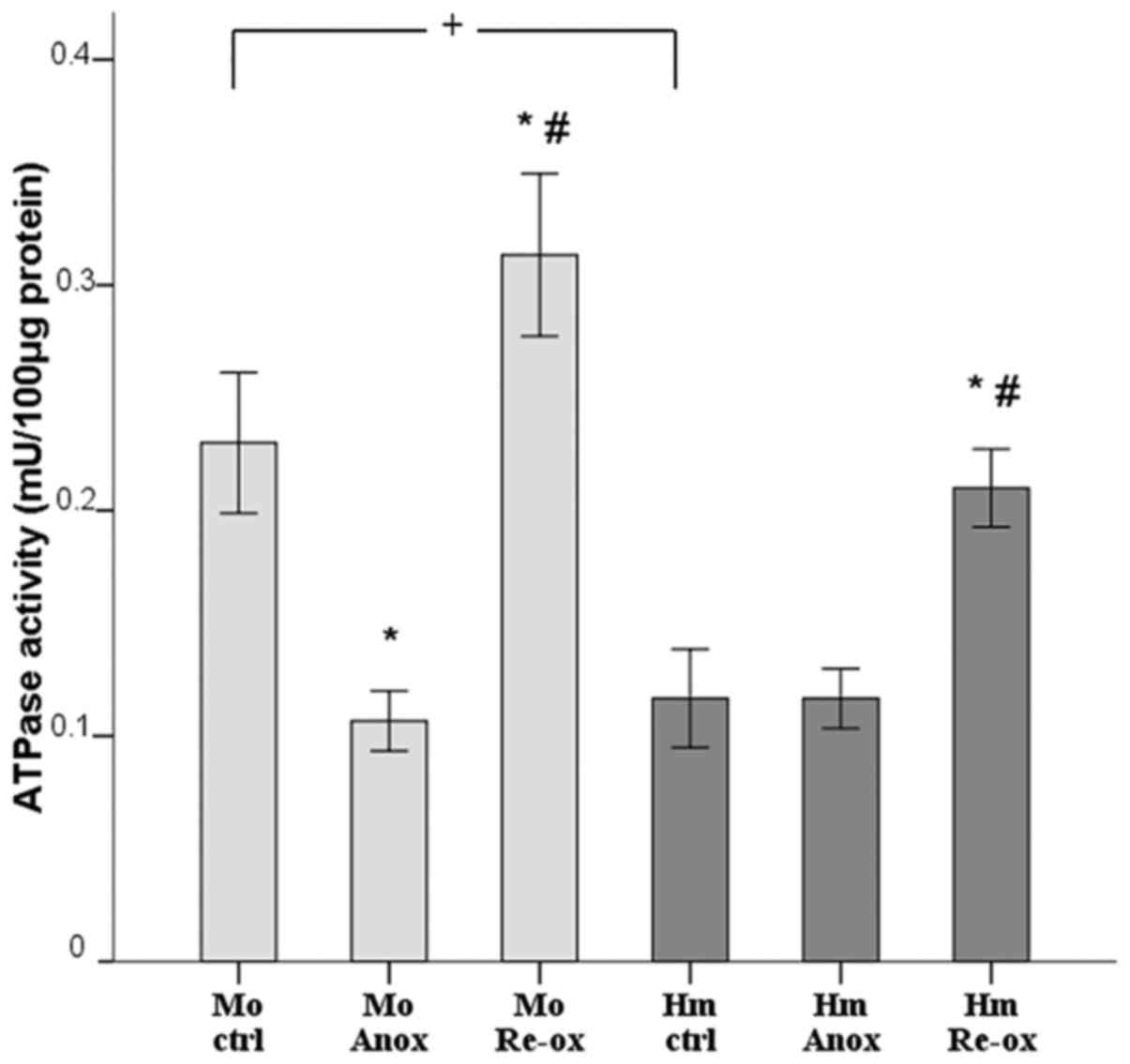
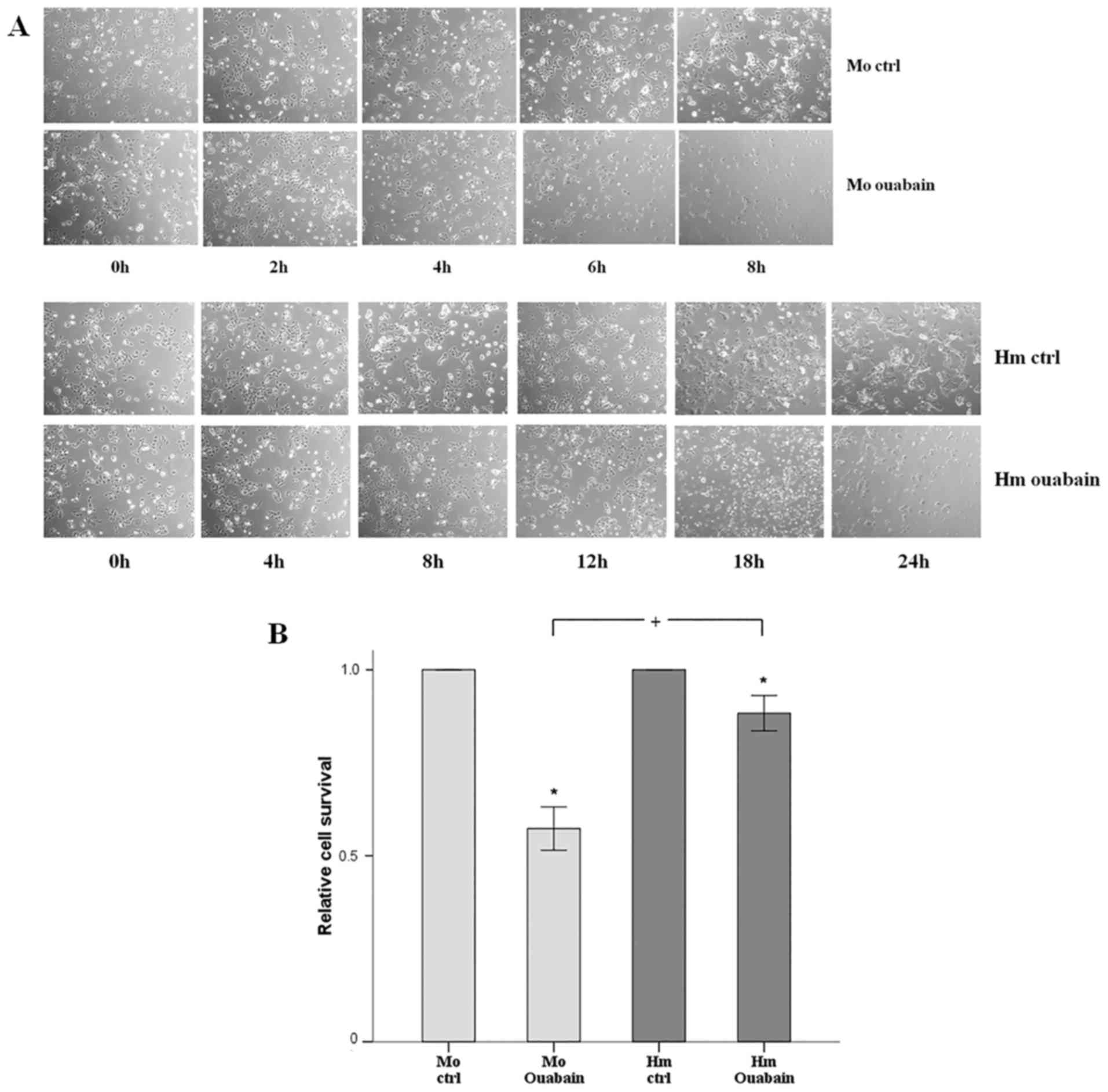
|
1
|
Neri M, Riezzo I, Pascale N, Pomara C and
Turillazzi E: Ischemia/reperfusion injury following acute
myocardial infarction: A critical issue for clinicians and forensic
pathologists. Mediators Inflamm. 2017:70183932017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bakthavachalam P and Shanmugam PS:
Mitochondrial dysfunction - Silent killer in cerebral ischemia. J
Neurol Sci. 375:417–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsukamoto T, Chanthaphavong RS and Pape
HC: Current theories on the pathophysiology of multiple organ
failure after trauma. Injury. 41:21–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonventre JV and Yang L: Cellular
pathophysiology of ischemic acute kidney injury. J Clin Invest.
121:4210–4221. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lieberthal W and Nigam SK: Acute renal
failure. I. Relative importance of proximal vs. distal tubular
injury. Am J Physiol. 275:F623–F631. 1998.PubMed/NCBI
|
|
6
|
Carey HV, Andrews MT and Martin SL:
Mammalian hibernation: Cellular and molecular responses to
depressed metabolism and low temperature. Physiol Rev.
83:1153–1181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Storey KB and Storey JM: Metabolic rate
depression: The biochemistry of mammalian hibernation. Adv Clin
Chem. 52:77–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dave KR, Prado R, Raval AP, Drew KL and
Perez-Pinzon MA: The arctic ground squirrel brain is resistant to
injury from cardiac arrest during euthermia. Stroke. 37:1261–1265.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quinones QJ, Zhang Z, Ma Q, Smith MP,
Soderblom E, Moseley MA, Bain J, Newgard CB, Muehlbauer MJ,
Hirschey M, et al: Proteomic profiling reveals adaptive responses
to surgical myocardial ischemia-reperfusion in hibernating arctic
ground squirrels compared to rats. Anesthesiology. 124:1296–1310.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levin E, Plotnik B, Amichai E, Braulke LJ,
Landau S, Yom-Tov Y and Kronfeld-Schor N: Subtropical mouse-tailed
bats use geothermally heated caves for winter hibernation. Proc
Biol Sci. 282:201427812015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dausmann KH, Glos J, Ganzhorn JU and
Heldmaier G: Physiology: Hibernation in a tropical primate. Nature.
429:825–826. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rolfe DF and Brown GC: Cellular energy
utilization and molecular origin of standard metabolic rate in
mammals. Physiol Rev. 77:731–758. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Venugopal J and Blanco G: On the many
actions of Ouabain: Pro-cystogenic effects in autosomal dominant
polycystic kidney disease. Molecules. 22:222017.
|
|
14
|
Weinberg JM, Davis JA, Abarzua M, Smith RK
and Kunkel R: Ouabain-induced lethal proximal tubule cell injury is
prevented by glycine. Am J Physiol. 258:F346–F355. 1990.PubMed/NCBI
|
|
15
|
Liu J, Periyasamy SM, Gunning W, Fedorova
OV, Bagrov AY, Malhotra D, Xie Z and Shapiro JI: Effects of cardiac
glycosides on sodium pump expression and function in LLC-PK1 and
MDCK cells. Kidney Int. 62:2118–2125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cherniavsky-Lev M, Golani O, Karlish SJ
and Garty H: Ouabain-induced internalization and lysosomal
degradation of the Na+/K+-ATPase. J Biol
Chem. 289:1049–1059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao AY, Wei L, Xia S, Rothman S and Yu
SP: Ionic mechanism of ouabain-induced concurrent apoptosis and
necrosis in individual cultured cortical neurons. J Neurosci.
22:1350–1362. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kilberg MS, Shan J and Su N:
ATF4-dependent transcription mediates signaling of amino acid
limitation. Trends Endocrinol Metab. 20:436–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nat Rev Mol Cell Biol.
10:307–318. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frerichs KU, Smith CB, Brenner M, DeGracia
DJ, Krause GS, Marrone L, Dever TE and Hallenbeck JM: Suppression
of protein synthesis in brain during hibernation involves
inhibition of protein initiation and elongation. Proc Natl Acad Sci
USA. 95:14511–14516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Breukelen F, Sonenberg N and Martin
SL: Seasonal and state-dependent changes of eIF4E and 4E-BP1 during
mammalian hibernation: Implications for the control of translation
during torpor. Am J Physiol Regul Integr Comp Physiol.
287:R349–R353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koumenis C, Naczki C, Koritzinsky M,
Rastani S, Diehl A, Sonenberg N, Koromilas A and Wouters BG:
Regulation of protein synthesis by hypoxia via activation of the
endoplasmic reticulum kinase PERK and phosphorylation of the
translation initiation factor eIF2alpha. Mol Cell Biol.
22:7405–7416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arsham AM, Howell JJ and Simon MC: A novel
hypoxia-inducible factor-independent hypoxic response regulating
mammalian target of rapamycin and its targets. J Biol Chem.
278:29655–29660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Charnock JS and Simonson LP: Seasonal
variations in the renal cortical (Na+ +
K+)-ATPase and Mg2+-ATPase of a hibernator,
the ground squirrel (Spermophilus richardsonii). Comp Biochem
Physiol B. 60:433–439. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hochachka PW: Defense strategies against
hypoxia and hypothermia. Science. 231:234–241. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boutilier RG: Mechanisms of cell survival
in hypoxia and hypothermia. J Exp Biol. 204:3171–3181.
2001.PubMed/NCBI
|