Introduction
Osteoporosis is a common bone disease characterized
by a decrease in bone strength, an increase in fracture risk and
microarchitectural deterioration of the bone tissue (1). A total of >9 million people worldwide
suffer from osteoporosis, and its prevalence is increasing within
aging societies (2). This skeletal
disorder is directly associated with quality of life, as the first
symptoms may be osteoporotic fractures in the vertebral column,
rib, hip or wrist (3).
Several risk factors for osteoporosis, including
estrogen loss, aging, vitamin D deficiency and low dietary calcium,
have been widely observed in humans and other mammals (4). Pharmacological treatment for
osteoporosis, including bisphosphonates, raloxifene and calcitonin
has been suggested to cause side effects including fever, damage to
the kidneys, joint pain and osteonecrosis (5). In addition, calcium supplements have
occasionally demonstrated adverse effects including abdominal gas,
bloating and constipation, although they are used for maintenance
of bone remodeling and the prevention of osteoporosis and other
bone diseases (6). Clinically,
hormone replacement therapy (HRT) has been used to prevent bone
loss in postmenopausal women (7).
However, it has been revealed that HRT has side effects, including
breast cancer, thromboembolic disease, musculoskeletal pain and
gastrointestinal intolerance (8). Due
to these limitations, the development of alternative
anti-osteoporotic treatments is required.
Phlomis umbrosa Turcz (labiatae), a perennial
herbaceous plant in Asia, has been traditionally used for treatment
of bronchitis, colds, bleeding, arthralgia, rheumatic disease and
bone fractures (9). Previous studies
have suggested that P. umbrosa has anti-inflammatory,
anti-nociceptive, anti-allergy and antioxidant activities (9,10).
Notably, P. umbrosa exhibited beneficial effects on
longitudinal bone growth rate in rats (11). However, the anti-osteoporotic effects
of P. umbrosa have not been investigated yet.
The present study evaluated the therapeutic effects
of P. umbrosa on osteoporosis in ovariectomized
(OVX)-induced mice. In addition, the potential mechanisms of action
of P. umbrosa extract were investigated in human
osteoblasts-like Saos-2 cells.
Materials and methods
Preparation of P. umbrosa
P. umbrosa was purchased from Jungdo Herb,
Inc. (Guri, Korea). A total of 100 g P. umbrosa was
extracted with 1 liter distilled water for 24 h at room temperature
(RT) with shaking. Following filtration, the extract was
concentrated under decreased pressure with a rotary evaporator and
lyophilized (yield=31.44%). The obtained powder was termed ‘PU’. A
voucher specimen was deposited at the College of Korean Medicine of
Kyung Hee University (Seoul, Korea).
PU was identified on the basis of its loganin and
sweroside content by high-performance liquid chromatography (HPLC)
with diode-array detection. The extract was dissolved in 70%
methanol and sonicated for 30 min. Following filtration through a
0.2 µm filter membrane, 10 µl of aliquot was subjected to HPLC
Agilent 1100 series (Agilent Technologies, Inc., Santa Clara, CA,
USA). Chromatographic separation was achieved using a C18 column
(250×4.6 mm, 5 µm; Shiseido, Osaka, Japan). Mobile phase A involved
water with 0.1% formic acid, and mobile phase B consisted of
acetonitrile with 0.1% formic acid. The separation temperature was
set at 30°C and a flow rate of 0.45 ml/min. The peak on PU was
synchronized with loganin and sweroside. The concentration of
loganin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
sweroside (Sigma-Aldrich) in PU was 82.32 and 237.65 µg/ml,
respectively.
Animals and treatments
ICR mice were purchased from Raonbio, Inc. (Yongin,
Korea). Female 6-week-old ICR mice were housed at 22±1°C in an
atmosphere with 55±10% humidity in a 12 h light: dark cycle with
ad libitum access to a standard chow diet (Orient Bio.,
Inc., Seongnam, Korea) and water. The animal experiments were
approved by the Institutional Animal Care and Use Committee of
Kyung Hee University Laboratory Animal Center [approval no. KHUASP
(SE)-15-079].
The mice were randomized into 7 groups (n=7;
total=49 mice): Sham-operated mice (Sham group); OVX mice treated
orally with vehicle (OVX group); OVX mice injected
intraperitoneally with 10 µg/kg 17β-estradiol (E2 group); OVX mice
treated orally with 150 mg/kg calcium chloride (Ca group); OVX mice
treated orally with 1 mg/kg PU (PU1 group); OVX mice treated orally
with 10 mg/kg PU (PU10 group); and OVX mice treated orally with 100
mg/kg PU (PU100 group). E2 and Ca were used as positive controls.
All treatments started at 7 weeks following OVX surgery, and lasted
for 6 weeks. At 13 weeks after the experiment began, the animals
were sacrificed, and blood was collected by cardiac puncture. The
right and left femurs were obtained.
Histological analysis
The right femur was fixed in 10% neutralized
formalin for 18 h at RT and demineralized with 0.1 M
ethylenediaminetetraacetic acid aqueous solution for 1 month.
Following demineralization, femur samples were dehydrated by using
xylene and consecutive ethanol concentrations (70, 80, 90, 95 and
100%) at 10 min each. Sagittal sections of the paraffin-embedded
tissues were sliced at a 7 µm thickness. The slides were stained
with hematoxylin for 5 min and eosin solution for 5 sec at RT
according to kit instructions (Sigma-Aldrich). Histological changes
were monitored using the Leica Microscope DML B2/11888111 equipped
with a Leica camera DFC450 (Leica Microsystems, Buffalo Grove, IL,
USA) at ×100 magnification.
Measurement of bone mineral density
(BMD) and bone mineral content (BMC)
Following sacrifice, the left femur was collected
and cleaned by removing the attached muscles and connective
tissues. The sample was stored in 10% neutralized formalin until
use. The levels of BMD and BMC in the left femur were determined by
dual-energy X-ray absorptiometry with an InAlyzer instrument
(Medikors, Seongnam, Korea).
Serum analysis
Samples were prepared from blood collected by
cardiac puncture in heparinized tubes. The collected blood was
centrifuged at 27,000 × g and 22°C for 30 min, and then the
supernatant was stored at −80°C until use. The concentration of
serum calcium was measured using the Calcium Colorimetric Assay kit
(AdipoGen Life Sciences, Shizuoka, Japan) according to the
manufacturer's protocol. The concentration of calcium in the serum
was measured at 570 nm absorbance using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Cell culture
The human osteoblastic Saos-2 cell line (Korean Cell
Line Bank, Seoul, Korea) was routinely grown in Dulbecco's modified
minimal essential medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 1% penicillin and 10%
heat-inactivated fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in an atmosphere containing 5%
CO2 and 95% humidity. The culture medium was changed
every 3–4 days. To confirm the cytotoxicity of PU, Saos-2 cells
were incubated with culture medium containing different
concentrations of PU extract (0.01, 0.1 and 1 µg/ml) for 10 days.
Subsequently, 2 mg/ml MTT solution was added for 4 h. Dimethyl
sulfoxide was then added, and cell viability was measured at an
absorbance of 570 nm.
Mineralized matrix formation
assay
The cells were seeded in 6-well plates at density of
0.8×105 cells/well and stabilized for 24 h. To induce
osteoblast differentiation, 50 µg/ml L-ascorbic acid (AA; Thermo
Fisher Scientific, Inc.) and 10 mM β-glycerophosphate (β-GP;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were added into
osteogenic culture medium for 10 days. The culture medium was
changed every 3–4 days. Then, the cells were fixed in 10% formalin
for 10 min and stained with the 40 mM Alizarin Red-S (pH 4.2;
Sigma-Aldrich; Merck KGaA) for 15 min, all at RT. The plates were
observed under the Leica Microscope DML B2/11888111 equipped with
Leica camera DFC450 at ×100 magnification. For quantification of
Alizarin red S, 500 µl citrate solution containing 20% methanol and
10% acetic acid was added for 20 min at RT, and the absorbance of
supernatants was measured at 570 nm using an ELISA reader
(Molecular Devices, LLC., Downingtown, PA, USA).
Western blot analysis
The Saos-2 cells were lysed with
radioimmunoprecipitation assay lysis buffer (BioPrince, Seoul,
Korea) containing protease inhibitors (Sigma-Aldrich). The Bradford
method was used for quantification of total protein. Subsequently,
20 µg of each sample was resolved using 10% SDS-PAGE and then
transferred onto a polyvinylidene fluoride membrane (Bio-Rad
Laboratories, Hercules, CA, USA). The membrane was blocked with 5%
bovine serum albumin (Sigma-Aldrich) for 1 h at RT and then
incubated with primary antibodies against runt-related
transcription factor 2 (Runx2; 1:700 dilution; cat. no. 12556; Cell
Signaling Technology, Inc., Danvers, MA, USA), transcription factor
Sp7 (osterix; 1:1,000 dilution; cat. no. ab22552; Abcam, Cambridge,
MA, USA) and β-actin (1:1,000 dilution; cat. no. sc-69879; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) diluted in TBS
containing 0.1% Tween-20 (TBS-T) overnight at 4°C. The membrane was
washed and incubated with m-IgG BP-HRP (1:2,000 dilution; cat. no.
sc-516102; Santa Cruz Biotechnology, Inc.) and mouse anti-rabbit
immunoglobulin G-horseradish peroxidase (1:2,000 dilution; cat. no.
sc-2357, Santa Cruz Biotechnology, Inc.) diluted in TBS-T for 2 h
at RT. Following washing, the bands were visualized with enhanced
chemiluminescence (ECL) reagent (Amersham; GE Healthcare, Chicago,
IL, USA). β-actin was used as an internal loading control for Runx2
and osterix. The band intensity was quantified using Image J
software version 1.38e (National Institutes of Health, Bethesda,
MD, USA). All experiments were performed in triplicate.
Statistical analysis
Significance was determined by one-way analysis of
variance and followed by Dunnett's post-hoc test, using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference. All values are expressed as the mean ± standard error
of the mean.
Results
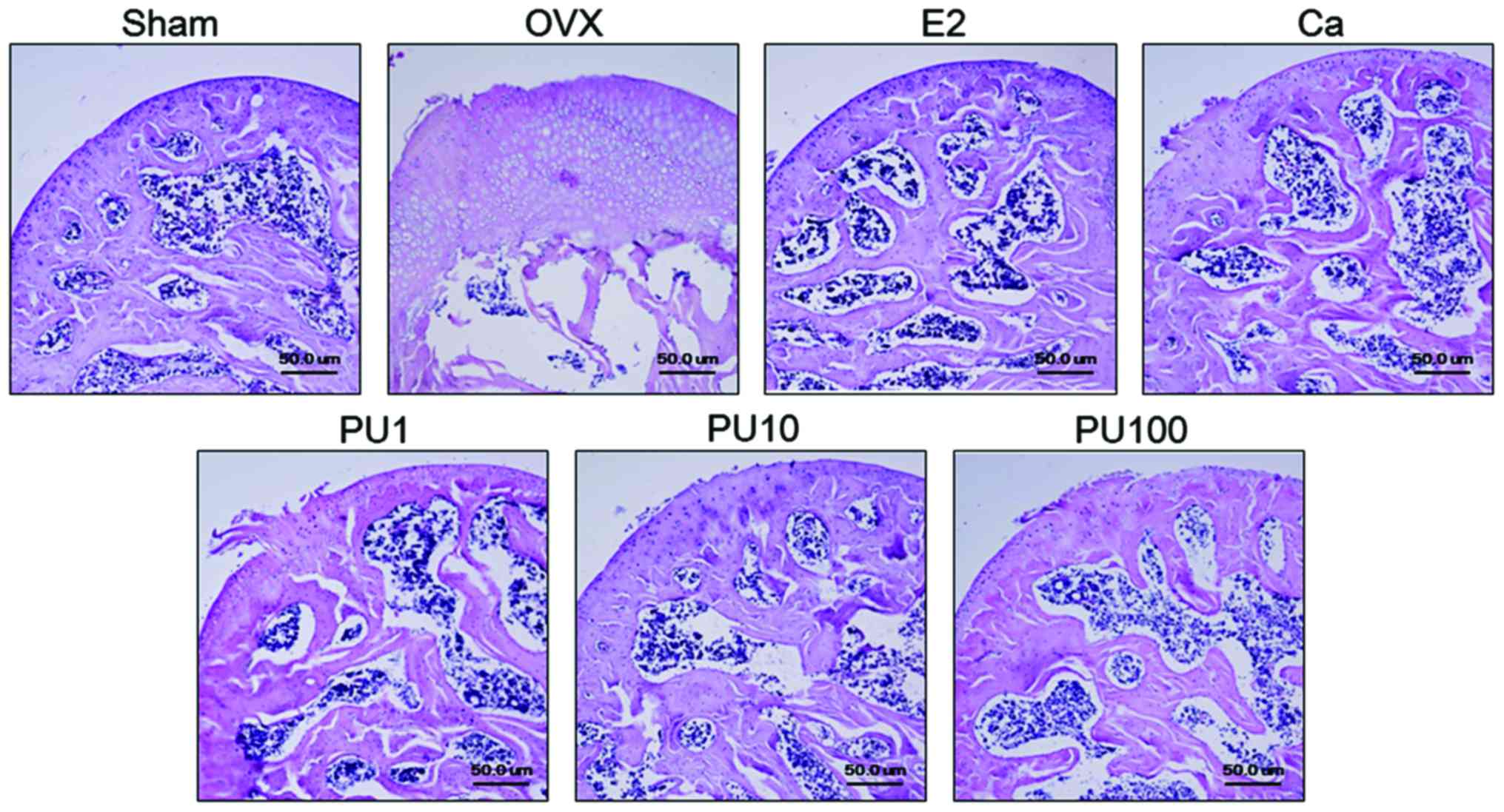
Effect of PU on the growth plate
thickness of femur
The thickness of the epiphyseal plate was
significantly increased in the OVX group compared with the Sham
group. Administration of E2 and Ca decreased the growth plate
thickness compared with OVX group. Similarly, PU-treated mice (1,
10 and 100 mg/kg) exhibited an amelioration of growth plate
hyperplasia compared with the OVX-induced osteoporotic mice
(Fig. 1).
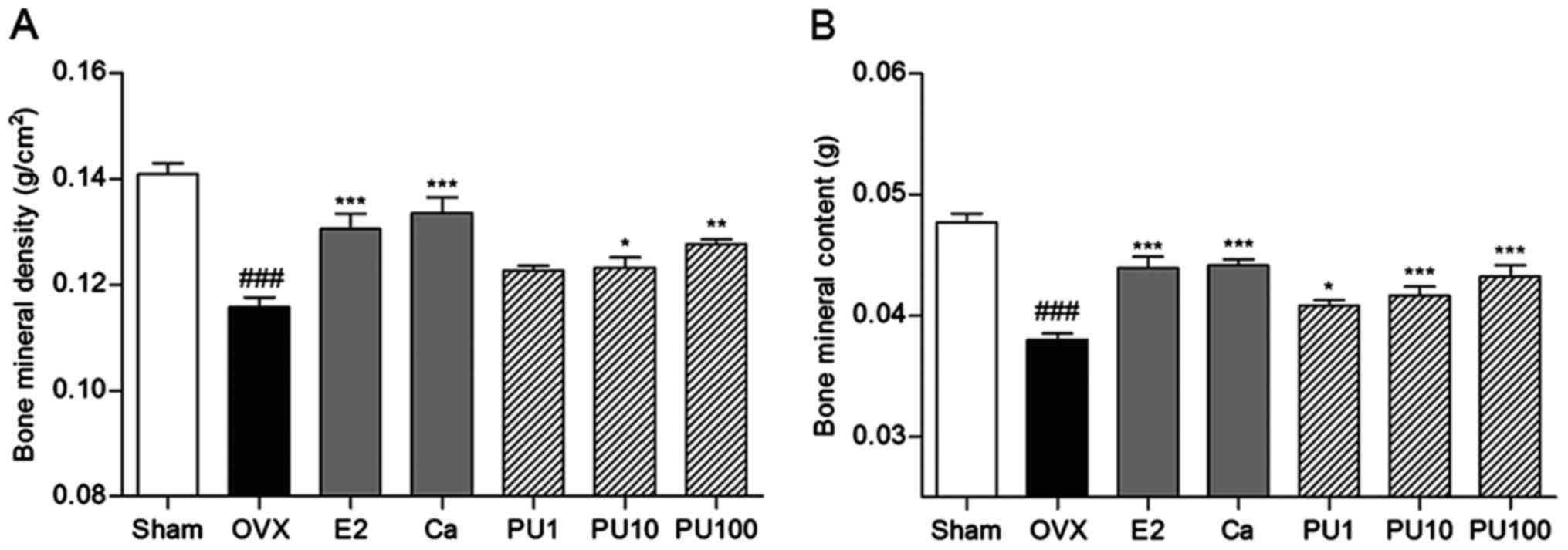
Effects of PU on BMD and BMC
The BMD level of the OVX group (0.115±0.004
g/cm2) was significantly decreased by 0.025
g/cm2 compared with the Sham group (0.140±0.006
g/cm2). E2 injection and Ca administration as positive
controls significantly increased the level of BMD (0.130±0.005 and
0.133±0.006 g/cm2, respectively). Similar to the results
from the positive controls, there were significant increases in BMC
level in 10 and 100 mg/kg PU-treated femurs (0.123±0.005 and
0.127±0.002 g/cm2, respectively; Fig. 2A) compared with in the OVX group.
The BMC level of the OVX mice (0.038±0.001 g) was
19.15% decreased compared with the Sham group (0.047±0.002 g). E2
injection and Ca administration as positive controls significantly
increased BMC (0.043±0.001 and 0.044±0.001 g, respectively). PU
treatment with 1, 10 and 100 mg/kg induced significant increases in
BMC levels at all concentrations to 0.040±0.001; 0.041±0.002 and
0.043±0.002 g, respectively, compared with in OVX mice (Fig. 2B).
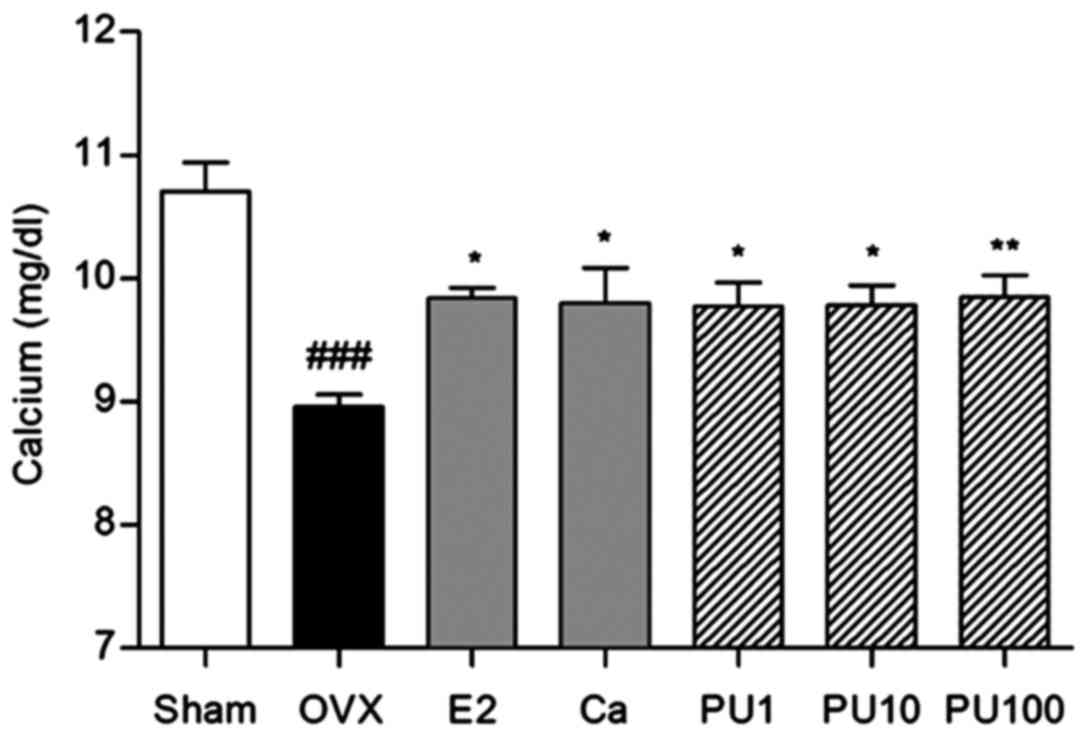
Effect of PU on serum calcium
levels
The serum calcium level was significantly decreased
in OVX group (8.96±0.24 mg/dl) compared with the Sham group
(10.70±0.52 mg/dl). E2 and Ca treatment significantly increased the
calcium level compared with the OVX group (9.83±0.14 and 9.80±0.63
mg/dl, respectively). Similarly, administration of 1, 10 and 100
mg/kg PU markedly increased the calcium levels to 9.77±0.49,
9.78±0.50 and 9.84±0.55 mg/dl, respectively (Fig. 3).
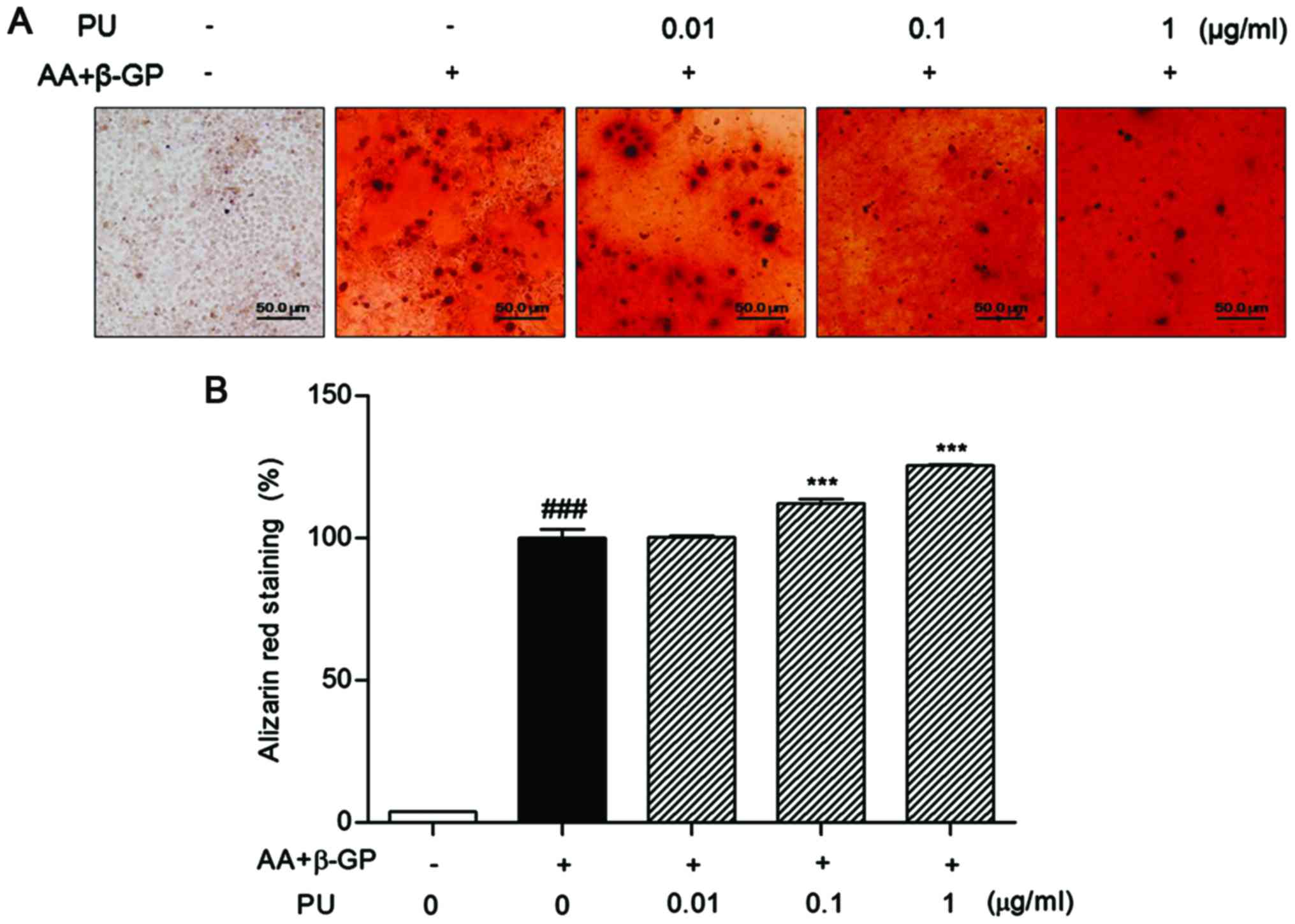
Effect of PU on matrix mineralization
of Saos-2 osteoblast cells
The cells in the presence of AA and β-GP exhibited
intense red coloring. Addition of 0.1 and 1 µg/ml PU markedly
increased the intensities of Alizarin Red S staining compared with
control cells in the absence of PU. The percent of calcification
was 12.32±3.80 and 25.62±0.68%, respectively (Fig. 4). No cytotoxic effect was observed in
Saos-2 cells at any concentration of PU.
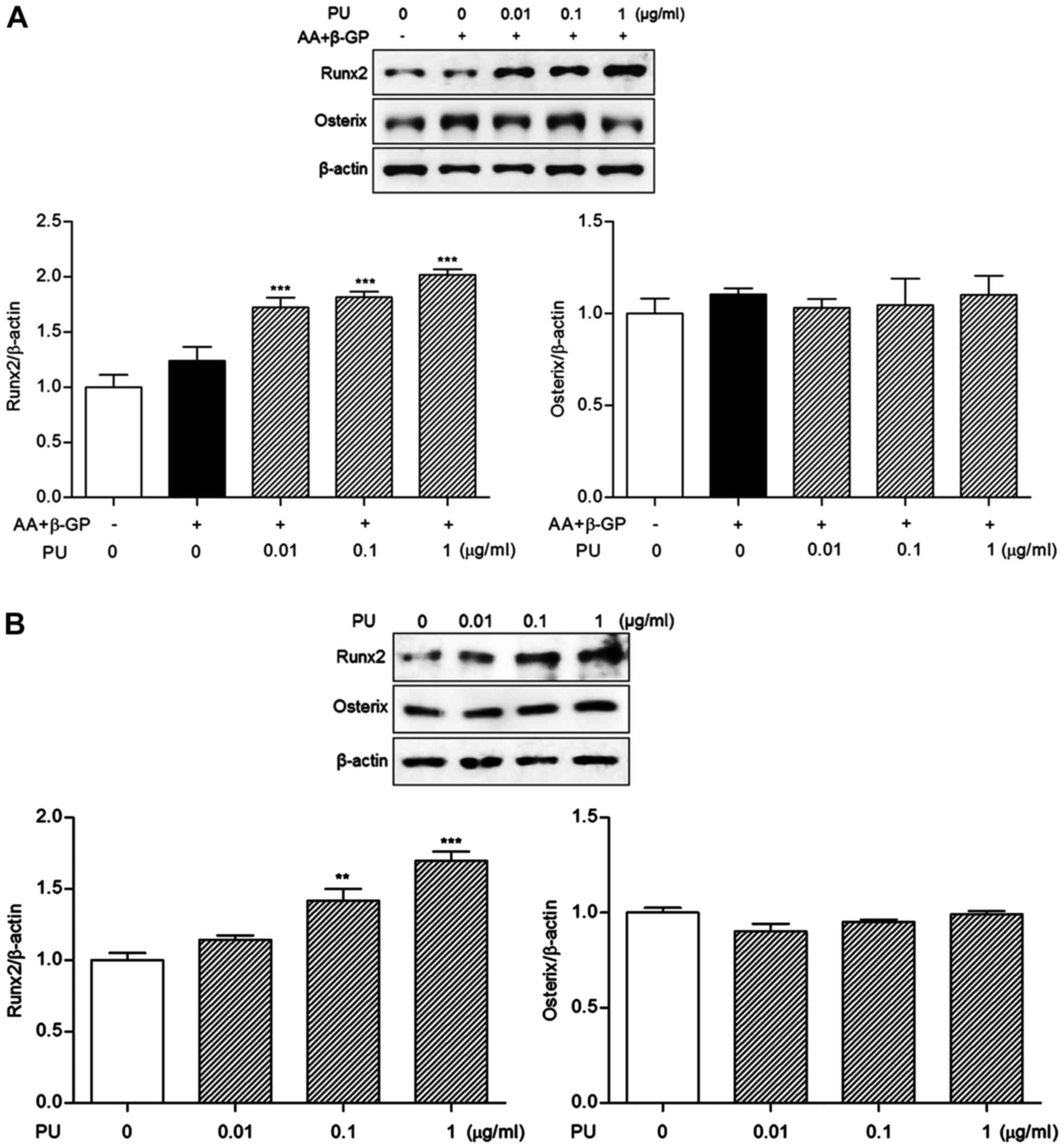
Effects of PU on Runx2 and osterix
expression levels
Runx2 expression levels were significantly increased
by 0.01, 0.1 and 1 µg/ml PU treatment as compared with
differentiated Saos-2 osteoblast cells (39.04±13.03, 46.73±7.32 and
63.06±6.92%, respectively; Fig. 5A).
However, osterix expression was not significantly altered compared
with the differentiated cells. To confirm the osteogenic effects of
PU on Runx2 expression, cells were treated with PU in the absence
of differentiation medium for 10 days. Consistent with the result
from differentiated osteoblasts, Runx2 expression was significantly
increased by 0.1 and 1 μg/ml PU treatment (41.62±14.31 and
69.81±11.00%, respectively; Fig. 5B)
in undifferentiated Saos-2 osteoblast cells. By contrast, osterix
expression was not increased in PU-treated cells.
Discussion
Osteoporotic bone exhibits high incidence rates of
fracture risk factors, including loss of bone mass, deterioration
of bone structure and hyperplasia of epiphyseal growth plate
(12,13). BMD and BMC measurements are the
primary parameters used for the diagnosis of osteoporosis (14). In the present study, the thickness of
epiphyseal growth plate was markedly increased in OVX mice. Also,
the bone fragility parameters BMD and BMC were decreased in OVX
mice, as expected, and administration of PU recovered the
hyperplasia of growth plate and the loss of bone mass. In addition,
serum calcium level is positively associated with activity of bone
formation and maintenance of bone integrity (15). The results of the present study
demonstrated that treatment with PU significantly increased serum
calcium levels. Therefore, it appears that PU treatment ameliorates
the destruction of bone structure and bone minerals in
osteoporosis.
Imbalance between bone resorption and bone
deposition is a crucial pathogenic event in osteoporosis (16), as development and maintenance of bone
tissue requires a continuous process of bone resorption and bone
deposition (3). Osteoblasts,
differentiated from bone marrow mesenchymal stem cells, are
responsible for bone formation (17).
As calcium deposition is accompanied by bone mineralization in the
process of bone formation, calcium content in mature osteoblasts
differentiated from osteoblast like Saos-2 cells was observed in
the present study. PU treatment notably increased the calcium
content of the mineralized matrix. Therefore, these results
demonstrate that PU treatments have the capability to promote bone
matrix mineralization in osteoblasts.
To clarify this osteogenic effect of PU, the
expression of bone differentiation-associated markers including
Runx2 and osterix were analyzed in Saos-2 cells. The mineralization
of osteoblasts is regulated by several osteogenic factors including
Runx2, osterix, bone morphogenic protein (BMP), mothers against
decapentaplegic homolog 1, insulin-like growth factor (IGF)-1,
β-catenin and transforming growth factor-β (18,19). In
particular, Runx2 and osterix serve key roles in the
differentiation and proliferation of the osteoblast lineage
(20,21). Osteoblast-specific transcription
factors are also involved in the process of newly-formed matrix
mineralization, which leads to osteogenesis (22). In the present study, the expression of
Runx2 during osteogenic differentiation was improved by PU
treatment in Saos-2 cells, while osterix expression was not
increased by PU. These data suggest that PU may induce osteoblast
differentiation and mineralization by stimulating Runx2.
Lee et al (11)
identified that the longitudinal bone growth rate of adolescent
rats was increased by P. umbrosa administration via
upregulation of IGF-1 and BMP-2. Also, a herbal-based formula
including P. umbrosa was demonstrated to exhibit
ameliorative effects on pre-, peri and post-menopausal symptoms in
a randomized, double-blind, placebo-controlled trial involving 72
subjects (ISRCTN 959534) (23). In
the present study, PU exhibited osteogenic effects in OVX-induced
osteoporosis mice, which was consistent with data from previous
studies (11,23). Considering the data from previous
studies and the experimental results of the present study, P.
umbrosa may possess the potential to be used in post-menopausal
osteoporosis.
P. umbrosa ameliorates osteoporosis through
its osteogenic effects. P. umbrosa recovered bone mineral
loss and the structure of osteoporotic bone. P. umbrosa
promoted matrix formation in osteoblasts by regulating Runx2.
Accordingly, P. umbrosa may represent a novel
anti-osteoporotic herbal candidate for the treatment of
osteoporosis as a bone-forming agent.
Acknowledgements
Not applicable.
Funding
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea
Grant funded by the Korean Government (grant no.
NRF-2016R1D1A2B03935368).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
All authors participated in the study design,
interpretation and analysis of the data and review of the
manuscript; JEL, MHK and WMY contributed to the analysis design,
JEL, HL and MHK analyzed the data; JEL and WMY drafted the
manuscript; and WMY provided supervision of the study.
Ethics approval and consent to
participate
Experimental protocols involving animals were
approved by the Institutional Animal Ethics Committee of Kyung Hee
University, Seoul, Korea [approval no. KHUASP (SE)-15-079].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang W, Yang GJ, Wu SX, Li DQ, Xu YB, Ma
CH, Wang JL and Chen WW: The guiding role of bone metabolism test
in osteoporosis treatment. Am J Clin Exp Immunol. 7:40–49.
2018.PubMed/NCBI
|
|
2
|
Das S and Crockett JC: Osteoporosis - a
current view of pharmacological prevention and treatment. Drug Des
Devel Ther. 7:435–448. 2013.PubMed/NCBI
|
|
3
|
Sims NA and Martin TJ: Coupling the
activities of bone formation and resorption: A multitude of signals
within the basic multicellular unit. Bonekey Rep. 3:4812014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanis JA and Reginster JY: European
guidance for the diagnosis and management of osteoporosis in
postmenopausal women - what is the current message for clinical
practice? Pol Arch Med Wewn. 118:538–540. 2008.PubMed/NCBI
|
|
5
|
Fujiwara S, Hamaya E, Sato M,
Graham-Clarke P, Flynn JA and Burge R: Systematic review of
raloxifene in postmenopausal Japanese women with osteoporosis or
low bone mass (osteopenia). Clin Interv Aging. 9:1879–1893.
2014.PubMed/NCBI
|
|
6
|
Sunyecz JA: The use of calcium and vitamin
D in the management of osteoporosis. Ther Clin Risk Manag.
4:827–836. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Canderelli R, Leccesse LA, Miller NL and
Davidson Unruh J: Benefits of hormone replacement therapy in
postmenopausal women. J Am Acad Nurse Pract. 19:635–641. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Ma X, An J, Ding J, Dai G, Liu Z,
Song Z and Lin N: Treatment with QiBaoMeiRan, a Chinese herbal
formula, prevents bone loss in ovariectomized rat. Climacteric.
19:98–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shang X, Wang J, Li M, Miao X, Pan H, Yang
Y and Wang Y: Antinociceptive and anti-inflammatory activities of
Phlomis umbrosa Turcz extract. Fitoterapia. 82:716–721.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
López V, Jäger AK, Akerreta S, Cavero RY
and Calvo MI: Antioxidant activity and phenylpropanoids of
Phlomis lychnitis L.: A traditional herbal tea. Plant Foods
Hum Nutr. 65:179–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee D, Kim YS, Song J, Kim HS, Lee HJ, Guo
H and Kim H: Effects of Phlomis umbrosa Root on Longitudinal
Bone Growth Rate in Adolescent Female Rats. Molecules. 21:4612016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sandhu SK and Hampson G: The pathogenesis,
diagnosis, investigation and management of osteoporosis. J Clin
Pathol. 64:1042–1050. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MH, Choi YY, Han JM, Lee HS, Hong SB,
Lee SG and Yang WM: Ameliorative effects of Schizandra chinensis on
osteoporosis via activation of estrogen receptor (ER)-α/-β. Food
Funct. 5:1594–1601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soen S, Fukunaga M, Sugimoto T, Sone T,
Fujiwara S, Endo N, Gorai I, Shiraki M, Hagino H, Hosoi T, et al:
Japanese Society for Bone and Mineral Research and Japan
Osteoporosis Society Joint Review Committee for the Revision of the
Diagnostic Criteria for Primary Osteoporosis: Diagnostic criteria
for primary osteoporosis: Year 2012 revision. J Bone Miner Metab.
31:247–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takayanagi H: Inflammatory bone
destruction and osteoimmunology. J Periodontal Res. 40:287–293.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosen CJ and Bouxsein ML: Mechanisms of
disease: Is osteoporosis the obesity of bone? Nat Clin Pract
Rheumatol. 2:35–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Q, Shou P, Zheng C, Jiang M, Cao G,
Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al: Fate decision of
mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death
Differ. 23:1128–1139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang W, Yang S, Shao J and Li YP:
Signaling and transcriptional regulation in osteoblast commitment
and differentiation. Front Biosci. 12:3068–3092. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Ghori-Javed FY, Rashid H, Adhami
MD, Serra R, Gutierrez SE and Javed A: Runx2 regulates endochondral
ossification through control of chondrocyte proliferation and
differentiation. J Bone Miner Res. 29:2653–2665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koga T, Matsui Y, Asagiri M, Kodama T, de
Crombrugghe B, Nakashima K and Takayanagi H: NFAT and Osterix
cooperatively regulate bone formation. Nat Med. 11:880–885. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Byers BA and García AJ: Exogenous Runx2
expression enhances in vitro osteoblastic differentiation and
mineralization in primary bone marrow stromal cells. Tissue Eng.
10:1623–1632. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang A, Kwak BY, Yi K and Kim JS: The
effect of herbal extract (EstroG-100) on pre-, peri- and
post-menopausal women: A randomized double-blind,
placebo-controlled study. Phytother Res. 26:510–516. 2012.
View Article : Google Scholar : PubMed/NCBI
|