Introduction
Chronic rhinosinusitis (CRS) is subdivided into two
different phenotypes: Chronic rhinosinusitis with nasal polyps
(CRSwNP) and without nasal polyps (CRSsNP). The morbidity rate of
CRS is relatively high, with 5-15% of the Caucasian population
affected (1). However, the
etiopathology has remained unclear to date. Recently, study has
further investigated the influence of lymphocytes, particularly T
cells, and their contribution to maintaining the chronic
inflammation of the mucosa. The findings led to a different
classification of inflammatory endotypes (2). CRSwNP is characterized by a T helper
(TH)2, and CRSsNP by a mixed TH1 and TH17
inflammatory response, though previous data shows heterogeneous
cell types in both endotypes with a predominant inflammation type
(3). In nasal polyps, the
inflammatory process is primarily modulated by the expression of
interleukin (IL)-4, -5 and -13(4).
Peters et al (5) additionally
detected significantly increased levels of IL-6 in CRSwNP, as a
further potential pathogenic signaling pathway in this disease.
Furthermore, a switch from mainly cluster of differentiation
(CD)4+ T cells in peripheral blood to CD8+ T
cells in nasal polyps in patients with CRSwNP has been demonstrated
(6). Both subpopulations exhibited a
significant increase in effector T cells (6). According to the classification of Miyara
et al (7), a significant
increase in activated regulatory T cells (aTregs) and
conventional Forkhead box P3 (Foxp3low) memory T cells
(Tconv) was identified in nasal polyps compared with in
peripheral blood in patients with CRSwNP (6).
Human barrier models are already well established
and frequently used for immunological in vitro studies
(8-11). Due to their effective illustration of the in vivo
situation they are useful for avoiding animal testing (8). Mechanistic papers have depicted the
generation of an air-liquid interface model (9), confirmed the similarity of in
vitro air-liquid interface models to in vivo models
(10) and demonstrated a mucin
production in the air-liquid interface equal to normal sputum
(11). Certain authors have
investigated the influence of cell lines, for instance tumor cells
(12) or retinal pigment cells
(13), in a co-culture system to
determine their phenotype modulation by fibroblasts (12) or T cells (13). Steelant et al (14) used a co-culture system to measure the
epithelial dysfunction in allergic rhinitis. All these studies
demonstrate the effectiveness of co-culture systems to assess the
influence of one cell type on an other cell type.
The question arises if nasal polyp tissue itself is
responsible for the consistency of the inflammatory reaction in
CRSwNP. The purpose of the current study was to detect the direct
influence of the nasal polyp tissue on the differentiation or
activation of lymphocytes via the secretion of cytokines. The
current study has hypothesized that a major difficulty of T cell
investigation in multicolor flow cytometry analysis is the low
quantity of these cells within polyp tissue. To circumvent this
problem, a co-culture system was developed with nasal polyp tissue
and peripheral lymphocytes. Additionally, the cytokine expression
of the polyp tissue was measured, since this is responsible for the
T cell responses in this system.
Materials and methods
Preparation of human lymphocytes
Heparinized blood samples (10 ml) were obtained
intraoperatively by venous puncture from 16 patients (mean age,
51.80±18.12; sex ratio, 6:10 female:male) undergoing paranasal
sinus surgery between March 2016 and March 2017. Patients were
recruited from the Department of Otorhinolaryngology, Plastic,
Aesthetic and Reconstructive Head and Neck Surgery at the
University of Würzburg, Würzburg, Germany. Diagnosis of nasal
polyposis and indication for surgery was determined according to
the European Position Paper on Rhinosinusitus and Nasal Polyps 2012
guidelines (1). Patients with
Churg-Strauss syndrome, primary ciliary dyskinesia or cystic
fibrosis were excluded. The study was approved by the Ethics Board
of the Medical Faculty of Julius-Maximilian-University, Würzburg,
Germany (approval no. 16/06), and written informed consent was
obtained from all patients.
Peripheral lymphocytes were separated by
density-gradient centrifugation for 10 min at 1,000 x g at room
temperature with 3 ml of Ficoll (Biochrom GmbH, Berlin, Germany),
using a membrane-containing 10 ml cell tube (Greiner Bio-One,
Kremsmünster, Austria). Tubes were washed twice with
phosphate-buffered saline and cell number and viability were
determined using a Cell Counter+ Analyzer System (CASY TT;
Innovatis Technologies, Inc., Fairfax, VA, USA) according to the
manufacturer's protocol. Following centrifugation at 500 x g at
20˚C for 5 min, cells were transferred into 1 ml freezing medium,
which contained 10 parts fetal bovine serum (Linaris GmbH,
Dossenheim, Germany) and one part dimethylsulfoxide. The cell
suspension was then stored at -80˚C.
Isolation and cultivation of human
nasal polypoid tissue cells
All polypoid tissue samples were collected
intraoperatively from the same 16 patients undergoing standard
paranasal sinus surgery due to CRSwNP as described above. Isolation
and cultivation of the cells were performed as previously reported
(15,16). Following isolation, the polypoid
tissue cells were cultured on porous membrane inserts (Corning
Transwell polycarbonate membrane inserts, 0.4 µm pore size,
12 mm diameter; Corning Incorporated, Corning, NY, USA) and covered
with 150 µl collagen I (66 ng/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Culture medium was refreshed every second day.
After reaching 70-80% confluence on day 7, the medium apical to the
membrane was removed, and nutrition was provided to the cells by
adding 1.3 ml Airway Epithelial Cell Medium (PromoCell GmbH,
Heidelberg, Germany) per insert under the membrane. At this point,
the cultures reached an air-liquid interface condition, which was
maintained from day 7 to 14 to stabilize the culture conditions.
Subsequently, basal cell medium was removed and a cytokine-free
medium was added, which contained 7.5 ml Dulbecco's modified
Eagle's medium (DMEM)/F12 and 7.5 ml DMEM-low glucose supplemented
with bovine serum albumin (0.4 g albumin/100 ml medium; all from
PAA Laboratories, Inc.; GE Healthcare Life Sciences, Little
Chalfont, UK), as previously described (12). Peripheral lymphocytes from the same
patients were activated with Dynabeads™ Human T-Activator CD3/CD28
(111161D; Gibco™; Thermo Fisher Scientific Inc., Waltham, MA, USA)
according to the manufacturer's protocol, and 1x106 cells were
inserted into the basal compartment. After 3 days in co-culture at
37˚C, lymphocytes within the basal compartment were removed and
flow cytometry analysis was performed. As a control, peripheral
lymphocytes from the same patient were cultured without the
Transwell insert in a mono-culture system.
Measurement of cytokine secretion from
polypoid tissue under air-liquid interface conditions
Polypoid tissue of 4 patients was cultured according
to the method described above, without the peripheral lymphocytes
in the basal layers. After 3 days, supernatants were collected and
a TH1/TH2/TH17 human cytokine
array (AAH-TH17-1-2; RayBiotech, Inc., Norcross, GA, USA) with 34
possible targets was performed according to the manufacturer's
protocol. Densities of the dot plots were measured by ImageJ v.1.50
(National Institutes of Health, Bethesda, MD, USA).
Fluorescence-activated cell sorting
(FACS)
The following antibodies were used: Anti-CD45
Pacific Orange (1:300; MHCD4530; Thermo Fisher Scientific, Inc.),
anti-CD3 phycoerythrin (PE)-Cyanine (Cy)7 (1:300; 300420) anti-CD4
Pacific Blue (1:50; 300521), anti-CD8a Alexa 700 (1:50; 301028),
anti-CD45RA peridinin chlorophyll protein complex-Cy5.5 (1:50;
304122), anti-CCR7 Alexa488 (1:80; 353206), anti-CD4 fluorescein
isothiocyanate (1:40; 300506), anti-FoxP3 Pacific Blue (1:25;
320216), anti-human leukocyte antigen (HLA)-DR isotype (AlexaFluor
700; 1:25; 307626), anti-CD52 [also known as cytotoxic T-lymphocyte
associated protein 4 (CTLA-4)] PE (1:400; 349906; all from
BioLegend, Inc., San Diego, CA, USA) and anti-Ki-67 (1:200; 556027;
BD Biosciences, San Jose, CA, USA). Isotype control staining was
performed using mouse-immunoglobulin G (IgG) antigen-presenting
cell (APC; 1:80; 137214; BioLegend, Inc.) and mouse-IgG PE (1:25;
556027; BD Biosciences). Viability Dye 780 (1:10; 65-0865-14;
eBioscience; Thermo Fisher Scientific Inc.) was used to detect
apoptotic and dead cells. Following blocking with 25 µg/ml
normal mouse IgG (1:50; I5381; Sigma-Aldrich; Merck KGaA) for 15
min on ice, all cells underwent cell surface staining on ice for 30
min, followed by intracellular staining. For intracellular staining
of Ki-67, Foxp3 and CTLA-4, cells were treated with 100 µl
fixation buffer (eBioscience; Thermo Fisher Scientific, Inc.) per
well for 30 min at room temperature. Permeabilization buffer (200
µl) was subsequently applied (eBioscience; Thermo Fisher
Scientific, Inc.) followed by staining with anti-Foxp3, anti-CTLA-4
and anti-Ki-67 for 45 min at room temperature. All antibodies were
used according to the manufacturer's protocol. FACS analysis was
performed using an LSR II flow cytometer and the data were analyzed
using FlowJo 10.2 software (FlowJo LLC, Ashland, OR, USA).
Statistics
Data are presented as the mean ± standard deviation.
The statistical significance of data was determined by two-tailed
paired t-tests using GraphPad Prism software 6.0c (GraphPad
Software, Inc., La Jolla, CA, USA). For data with non-parametric
distribution, the Wilcoxon test was applied. Values with P<0.05
were considered statistically significant in all comparisons.
Results
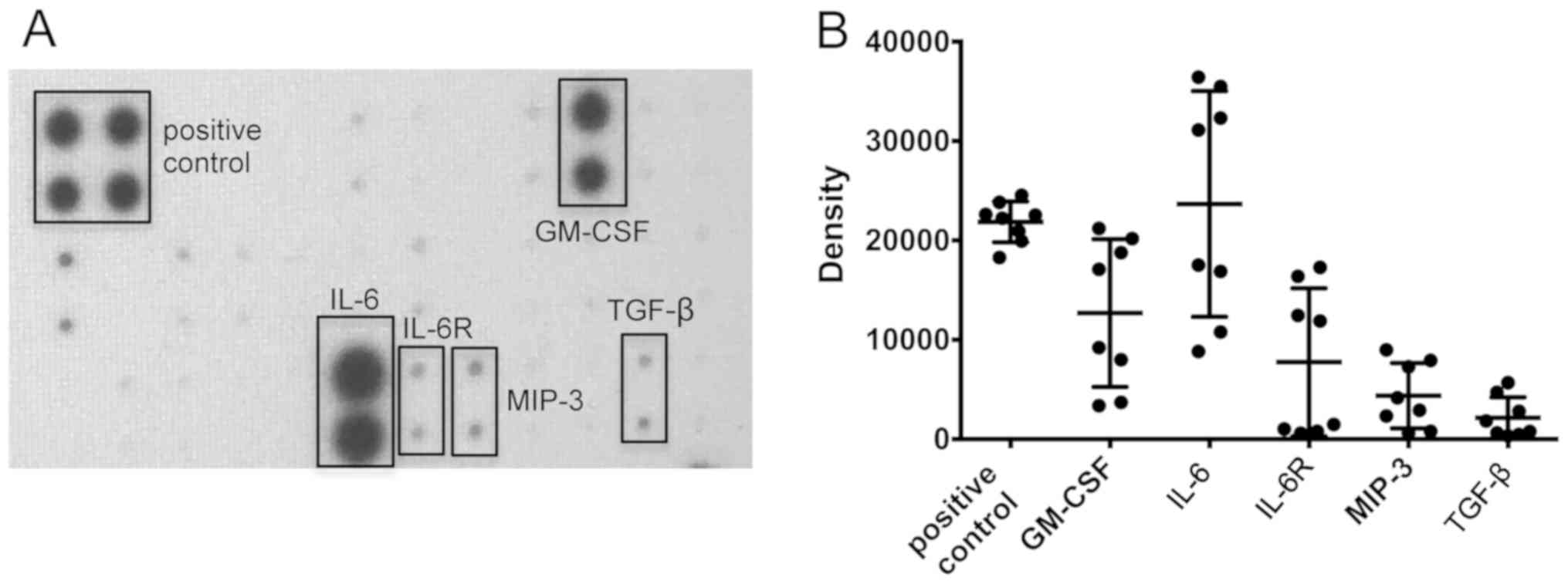
Cytokine secretion from polypoid
tissue under air-liquid interface conditions
Cytokine secretion of air-liquid interface polypoid
tissue cultures was semi-quantitatively measured after 3 days with
a TH1/TH2/TH17 antibody array. A
secretion of granulocyte-macrophage colony-stimulating factor
(GM-CSF), IL-6, IL-6 receptor (IL-6R), macrophage inflammatory
protein-3 (MIP-3) and transforming growth factor-β (TGF-β) was
identified (Fig. 1).
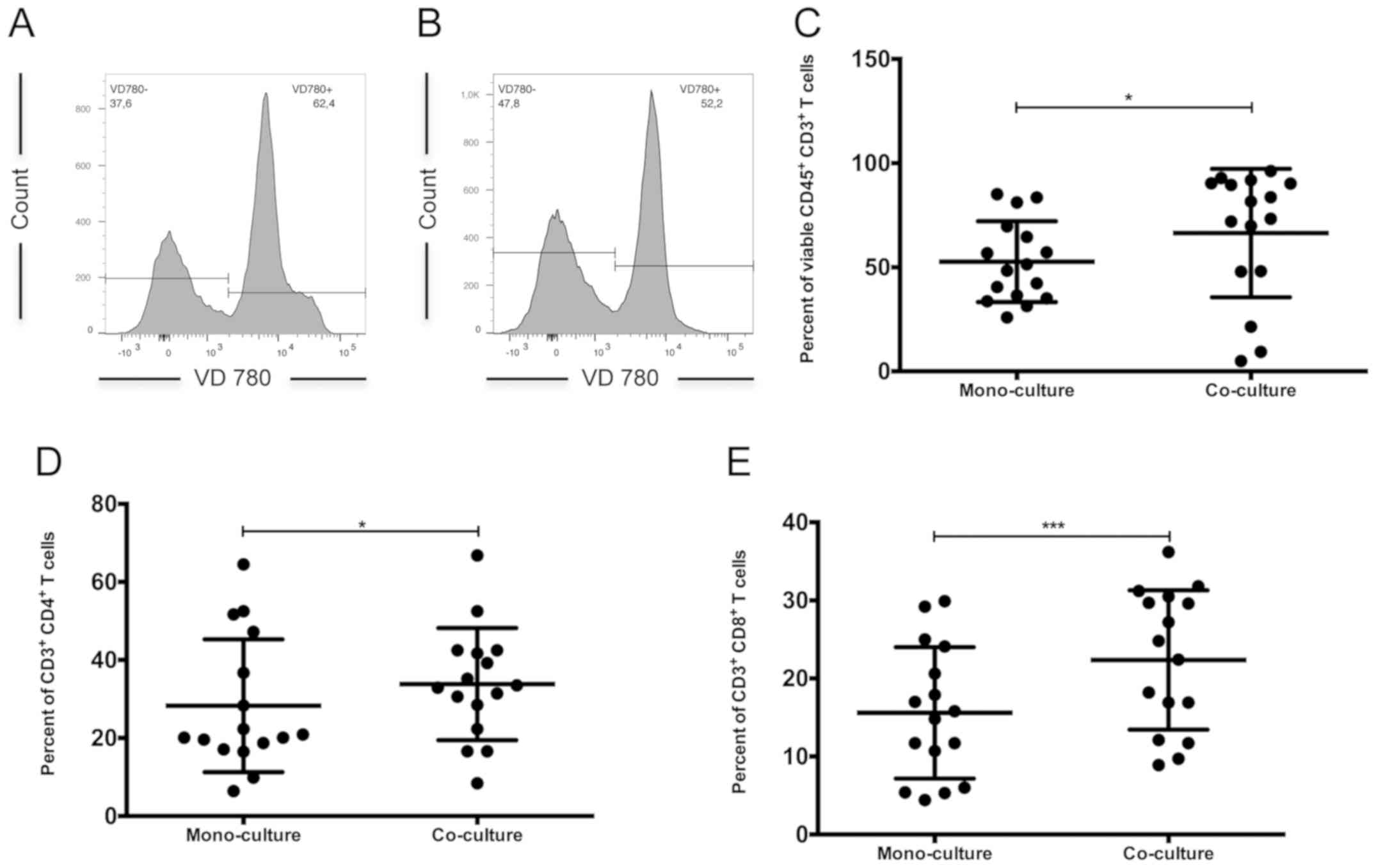
Viability of lymphocytes under
co-culture conditions
Overall, the viability of CD45+ and
CD3+ lymphocytes was significantly decreased under
mono-culture conditions, compared with that of the co-cultured
control group (viable cell rate, 52.70±19.42 vs. 66.43±30.83%,
respectively; P<0.05; Fig. 2A-C).
Furthermore, the percentage of viable
CD3+CD4+ (Fig.
2D) and CD3+CD8+ T cells (Fig. 2E) was significantly higher in the
co-culture (P=0.02 and P=0.008, respectively; Table I). Between these two subsets,
CD4+ T cells were the dominant subpopulation in both the
co-culture and mono-culture (Table
I).
 | Table I.CD3+ T cell
subpopulations. |
Table I.
CD3+ T cell
subpopulations.
| T cell
subpopulation | Co-culture | Mono-culture |
P-valuea |
|---|
|
CD4+ | 33.83±14.39 | 28.28±17.03 | 0.02 |
|
CD45RA-CCR7-
effector memory | 52.26±21.38 | 54.19±21.58 | 0.59 |
|
CD45RA+CCR7-
terminally differentiated | 18.72±17.11 | 23.02±19.07 | 0.18 |
|
CD45RA-CCR7+
central memory | 12.02±9.72 | 9.48±10.00 | 0.12 |
|
CD45RA+CCR7+
naïve | 17.17±14.50 | 13.71±15.62 | 0.23 |
|
CD8+ | 22.36±8.94 | 15.60±8.42 | 0.0008 |
|
CD45RA-CCR7-
effector memory | 44.98±20.43 | 44.28±22.95 | 0.83 |
|
CD45RA+CCR7-
terminally differentiated | 33.46±20.45 | 32.72±21.77 | 0.56 |
|
CD45RA-CCR7+
central memory | 7.74±11.97 | 9.60±12.35 | 0.25 |
|
CD45RA+CCR7+
naïve | 13.36±15.73 | 12.78±12.19 | 0.78 |
No changes in the differentiation of
CD3+CD4+ and CD3+CD8+ T
cell subpopulations
CD3+CD4+ and
CD3+CD8+ T cell subpopulations were analyzed
via staining of CD45RA and CCR7. The analysis of
CD3+CD4+ T cells identified no significant
difference in the frequency of CCR7+CD45RA+
naïve, CCR7+CD45- central memory,
CCR7-CD45RA- effector memory or
CCR7-CD45RA+ terminally differentiated T
cells (6) between mono- and
co-culture (Table I). Effector memory
T cells were the major subpopulation under co- and mono-culture.
Also among CD3+CD8+ T cell subpopulations, no
differences were detected, and effector memory T cells were again
the dominant subpopulation in both groups (Table I).
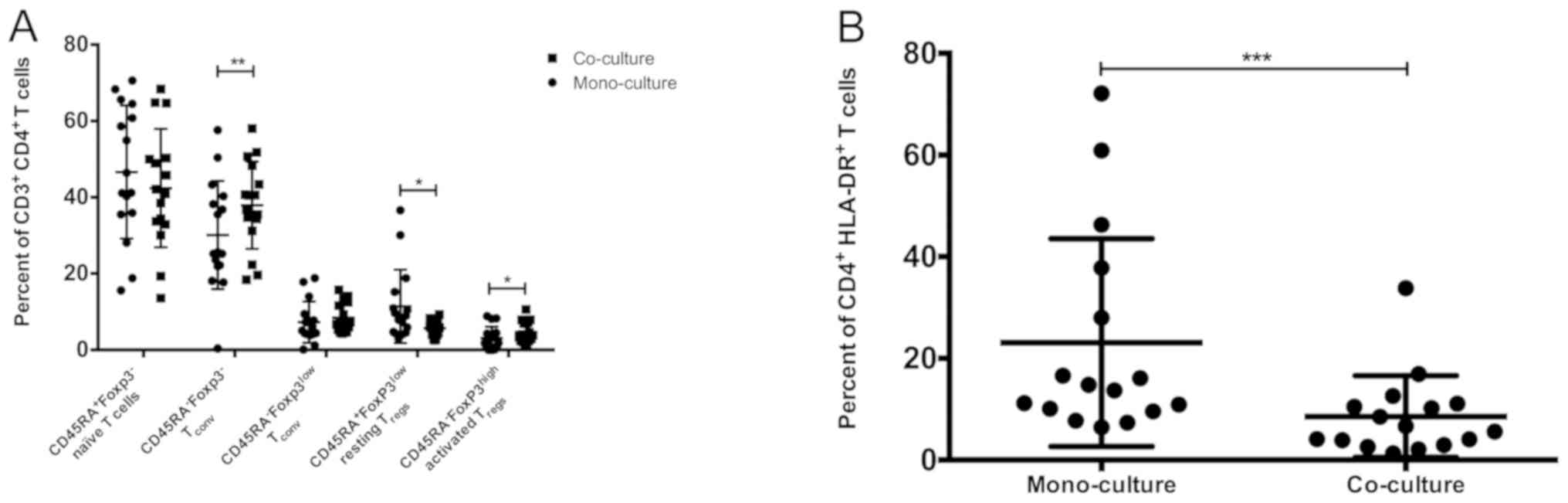
Significant increases in
aTreg and Tconv and decrease in resting
(r)Treg cells under co-culture conditions
Further determination of
CD3+CD4+ T cell subpopulations by staining
for Foxp3 and CD45RA revealed differences between mono- and
co-culture (Fig. 3A). The frequency
of Foxp3-CD45RA- T cells (Tconv)
(7) was significantly higher in
co-culture than among mono-cultured lymphocytes (P=0.01; Table II). The co-cultured lymphocytes also
exhibited significantly increased aTregs
(CD45RA-FoxP3highCTLA-4high; P=0.04) and
significantly decreased rTregs
(CD45RA+Foxp3lowCTLA-4low; P=0.01)
(7) than under mono-cultured
conditions. No differences in the frequencies of naïve
CD45RA+ FoxP3- and nonsuppressive
CD45RA- Foxp3low Tconv (7) were determined. In mono- and co-culture,
the majority of the CD3+CD4+ T cells were
naïve CD45RA+FoxP3- T cells (Table II).
 | Table II.Treg subpopulations analyzed by
staining for CD4, CD45RA and FoxP3. |
Table II.
Treg subpopulations analyzed by
staining for CD4, CD45RA and FoxP3.
|
CD3+CD4+ cell
subpopulation | Co-culture | Mono-culture |
P-valuea |
|---|
|
CD45RA+FoxP3lowCTLA-4low
resting Tregs | 5.70±2.05 | 11.40±9.63 | 0.01 |
|
CD45RA-FoxP3highCTLA-4high
activated Tregs | 4.75±2.78 | 3.07±3.05 | 0.04 |
|
CD45RA-Foxp3low
memory cells (Tconv) | 8.42±3.82 | 7.25±5.40 | 0.50 |
|
CD45RA-Foxp3- memory
cells (Tconv) | 37.93±11.41 | 30.13±14.17 | 0.01 |
|
CD45RA+Foxp3- naïve
T cells | 42.40±15.52 | 46.64±17.43 | 0.23 |
Significant downregulation of HLA-DR
on CD3+ and CD3+CD4+ T cells under
co-culture conditions
The activation marker HLA-DR (17) was significantly downregulated on
CD3+ lymphocytes (P=0.01) and
CD3+CD4+ T cells (Fig. 3B; P=0.0002) on co-cultured compared
with mono-cultured lymphocytes (Table
III). HLA-DR expression detection was not performed on
CD8+ T cells, due to the multicolor flow cytometry panel
utilized. Meanwhile, the expression of the intracellular
proliferation marker Ki-67(18) was
also measured. No differences in expression were observed among
CD3+, CD4+ and CD8+ T cells
between mono- and co-culture (Table
III).
 | Table III.Detection of T cells expressing the
activation markers HLA-DR and Ki-67. |
Table III.
Detection of T cells expressing the
activation markers HLA-DR and Ki-67.
| T cell | Co-culture | Mono-culture |
P-valuea |
|---|
| CD3+ T
cells |
|
HLA-DR+ | 7.74±5.06 | 17.42±14.86 | 0.01 |
|
Ki-67+ | 47.30±25.62 | 38.46±26.51 | 0.22 |
| CD4+ T
cells |
|
HLA-DR+ | 8.55±8.05 | 23.10±20.43 | <0.0001 |
|
Ki-67+ | 53.99±25.66 | 55.03±36.25 | 0.88 |
| CD8+ T
cells |
|
Ki-67+ | 62.17±30.52 | 55.93±35.16 | 0.71 |
Discussion
The current study determined differences in the
activation and differentiation of peripheral T cell subpopulations
following co-culture with nasal polyp tissue cells. Epithelial
cells from nasal polyps were cultured under air-liquid interface
conditions and a expression of cytokines, particularly IL-6, IL-6R,
GM-CSF, MIP-3 and TGF-β, was measured. This respiratory epithelial
cell culture under air-liquid interface conditions is widely used
in airway research due to a high functional and morphological in
vivo-in vitro correlation (19-21).
Mucociliary differentiation of nasal epithelial
cells has been described for regular nasal mucosa (19) and nasal polyps (20). De Borja Callejas et al
(21) described a 3D in vitro
model for nasal polyposis, which is similar to the air-liquid
interface model used in the present study. They also showed a
time-dependent mucociliary differentiation of epithelial cells
derived from nasal polyps which were cultivated under air-liquid
interface conditions. Furthermore, they described increased levels
of pro-inflammatory cytokines such as IL-8 and GM-CSF compared with
control nasal mucosa under monolayer culture conditions. Increased
GM-CSF levels have been described in vivo in patients with
an acute CRSwNP exacerbation (22).
Besides GM-CSF, high levels of IL-6 and IL-6R were also detected in
the present study. Several studies have highlighted the
pro-inflammatory role of IL-6 in patients with CRSwNP and reported
on significantly increased tissue levels of IL-6 (5,22) and
IL-6R (5). IL-6 and its specific
receptor IL-6R appear to serve a crucial role in CRSwNP (5), and its signaling pathway is important
for T cell recruitment and survival, particularly in preventing
apoptosis, preventing the differentiation of Tregs, and
in retaining T cells in the local tissue (5,23).
Consistent with the presented in vitro study, increased
levels of MIP-3 (24,25) and TGF-β (26) have been identified in vivo in
patients with CRSwNP. In summary, these findings underscore the
reliability of the air-liquid interface model with epithelial cells
from nasal polyps and its validity for further immunological
studies.
Co-cultured peripheral lymphocytes were
significantly more viable and higher frequencies of CD4+
and CD8+ T cells were measured. The secretion of growth
factors by the epithelial cells, particularly IL-6, as described
above is likely to mediate these findings. This supports the
hypothesis that polypoid tissue itself is responsible for
maintaining the inflammatory reaction. However, further analysis of
T cell subpopulations determined no differences between naïve,
central memory, effector memory and terminally differentiated
CD4+ and CD8+ T cells compared with
mono-cultured peripheral lymphocytes. In mono- and co-culture,
effector memory T cells were the major subpopulation among
CD4+ and CD8+ T cells, followed by terminally
differentiated and naïve cells. The subpopulation with the lowest
frequency was central memory T cells in both culture conditions.
Previous studies have demonstrated differences in CD4+
and CD8+ T cell subpopulations with differentiation from
mostly naïve T cells in peripheral blood lymphocytes to T cells
with an effector memory phenotype in lymphocytes from peripheral
blood (6,27). Due to the absence of APCs in the in
vitro model, cells had to be stimulated by CD3/CD28 prior to
adding them to the co-culture. This compromises the direct
comparison to in vivo findings, but it appears that polypoid
tissue has no immediate influence on the differentiation of T cell
subpopulations. Perhaps long-term co-culturing would induce such
differences, but in this case, an additional substitution of IL-2
to the basal medium is necessary to support survival of the
lymphocytes beyond the period of 3 days.
In a study by Miyara et al (7), further analysis of CD4+ T
cell differentiation into naïve FoxP3- and
Foxp3low Tconv, aTregs and
rTregs was performed. Interestingly, aTregs
and FoxP3- Tconv significantly increased and
rTregs significantly decreased in the co-culture
compared with a mono-culture system. These finding are similar to a
previous in vivo study by our group, where a significant
increase in aTregs and FoxP3-
Tconv was also identified (6). The current study hypothesizes that the
differentiation of rTregs to aTregs may be
classified as a feedback mechanism in response to inflammatory
stimulation of the nasal polyp epithelial cells. The role of memory
T cells such as FoxP3- Tconv is of particular
interest (28,29), and seems to be responsible for an
immediate defense against viruses or bacterial infection (30). Taken together, the data suggests that
nasal polyp epithelium mediates the immediate memory T cell
recruitment.
The intensity of T cell activation was measured by
evaluating the expression of HLA-DR and Ki-67. A significant
decrease in HLA-DR on CD3+ and CD4+
lymphocytes in the co-culture compared with in the mono-culture was
detected. There were no differences in the expression of the
intracellular proliferation marker Ki-67. A lack of HLA-DR
upregulation on CD3+ lymphocytes was similarly measured
previously in vivo (6), and
thus it appears that the nasal polyp epithelium is not responsible
for reactivation of T cells in the tissue. Interestingly, the
expression of HLA-DR significantly decreased in the presence of
nasal polyp epithelium cells.
CRS is a heterogeneous group of varying diseases,
and subclassifications attempt to structure the variety of
manifestations according to pathophysiological parameters. T cells
appear to serve a major role in the development of polyps (3). However, the communication between
epithelium and the T cell infiltrate is yet to be fully understood.
Results of the current study suggest that there exists such an
intercellular communication. The epithelium may promote T cell
survival within the tissue by the secretion of various cytokines.
One of the most notable findings of the current study was that
nasal epithelial cells were able to modify T cell subsets similarly
to the situation observed in vivo (6). Hence, it hypothesized that the
air-liquid interface co-culture model imitates the functional
aspects of the above-mentioned cell-cell communication, and is thus
a suitable tool for research on airway immunology. However, there
are several limitations to the present study. The use of peripheral
blood lymphocytes does not match the in vivo situation
exactly. It would be more appropriate to use local tissue-resident
T cells from unaffected nasal mucosa and submucosa. However, the
number of T cells available under healthy conditions is markedly
low, so that a large quantity of mucosal tissues would need to be
collected. Furthermore, previous study has also compared
polyp-associated T cells with peripheral blood T cells (6), and there is notable variability between
the individual results. The present study was declared as a pilot
project with a limited amount of samples. Regarding research using
primary cells, high interindividual variations have to be
tolerated, and, in addition to this, cells are not immortalized or
transformed (31,32). A previous activation of peripheral
lymphocytes was necessary in the current study due to the absence
of APCs in the co-culture system. This may also influence the
results; however, we believe that this fact is likely to be
insignificant. The air-liquid interface is able to imitate the
effects of cytokines derived from epithelial cells on T
lymphocytes, which is considered to be the most important mode of
communication (15). However, various
settings involving direct contact of lymphocytes with epithelial
cells should also be measured. Finally, little is known about the
opposite effect, and future studies should focus on the possibility
of inducing epithelial cell transformation following co-cultivation
with T cells derived from CRSwNP patients.
In conclusion, the present study underscores the
influence of nasal polyp epithelial cells in maintaining the
inflammatory reaction by expressing pro-inflammatory cytokines and
preventing T cells from apoptosis. Furthermore, the study results
support that the air-liquid interface co-culture is an appropriate
standardized and reliable model for analyzing the contribution of
nasal polyp epithelium cells to the etiopathology of CRSwNP.
Acknowledgements
The authors would like to thank Niklas Beyersdorf
for providing advice on the methods used. This study was presented
in an abstract at the 11th Symposium on Experimental Rhinology and
Immunology of the Nose (SERIN) Mar 30 - Apr 01, 2017 in Düsseldorf,
Germany and published as abstract no. S330 in Laryngo-Rhino-Otol 97
(S 02): 2018.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PI was principally responsible for drafting the
manuscript and performed, analyzed and interpreted all experiments.
AS, NK, RH and CG aided to analyze the data and were major
contributors to the writing of the manuscript. SH was involved in
the selection, analysis and interpretation of the experiments and
was a major contributor to the writing of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Board of the
Medical Faculty of Julius-Maximilian-University, Würzburg, Germany
(approval no. 16/06). Written informed consent was obtained from
each participating patient.
Patient consent for publication
Written informed consent was obtained from all
patients for the publication of their associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: European Position Paper on Rhinosinusitis and Nasal Polyps
2012. Rhinol Suppl. 23(3)2012.PubMed/NCBI
|
|
2
|
Tomassen P, Vandeplas G, Van Zele T,
Cardell LO, Arebro J, Olze H, Förster-Ruhrmann U, Kowalski ML,
Olszewska-Ziąber A, Holtappels G, et al: Inflammatory endotypes of
chronic rhinosinusitis based on cluster analysis of biomarkers. J
Allergy Clin Immunol. 137:1449–56, e4. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Derycke L, Eyerich S, Van Crombruggen K,
Pérez-Novo C, Holtappels G, Deruyck N, Gevaert P and Bachert C:
Mixed T helper cell signatures in chronic rhinosinusitis with and
without polyps. PLoS One. 9(e97581)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Van Zele T, Claeys S, Gevaert P, Van Maele
G, Holtappels G, Van Cauwenberge P and Bachert C: Differentiation
of chronic sinus diseases by measurement of inflammatory mediators.
Allergy. 61:1280–1289. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Peters AT, Kato A, Zhang N, Conley DB, Suh
L, Tancowny B, Carter D, Carr T, Radtke M, Hulse KE, et al:
Evidence for altered activity of the IL-6 pathway in chronic
rhinosinusitis with nasal polyps. J Allergy Clin Immunol.
125:397–403, e10. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ickrath P, Kleinsasser N, Ding X, Ginzkey
C, Beyersdorf N, Hagen R, Kerkau T and Hackenberg S:
Characterization of T-cell subpopulations in patients with chronic
rhinosinusitis with nasal polyposis. Allergy Rhinol (Providence).
8:139–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Miyara M, Yoshioka Y, Kitoh A, Shima T,
Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al:
Functional delineation and differentiation dynamics of human CD4+ T
cells expressing the FoxP3 transcription factor. Immunity.
30:899–911. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schweinlin M, Rossi A, Lodes N, Lotz C,
Hackenberg S, Steinke M, Walles H and Groeber F: Human barrier
models for the in vitro assessment of drug delivery. Drug Deliv
Transl Res. 7:217–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Everman JL, Rios C and Seibold MA:
Utilization of air-liquid interface cultures as an in vitro model
to assess primary airway epithelial cell responses to the type 2
cytokine interleukin-13. Methods Mol Biol. 1799:419–432.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pezzulo AA, Starner TD, Scheetz TE, Traver
GL, Tilley AE, Harvey BG, Crystal RG, McCray PB Jr and Zabner J:
The air-liquid interface and use of primary cell cultures are
important to recapitulate the transcriptional profile of in vivo
airway epithelia. Am J Physiol Lung Cell Mol Physiol. 300:L25–L31.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kesimer M, Kirkham S, Pickles RJ,
Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ and
Sheehan JK: Tracheobronchial air-liquid interface cell culture: A
model for innate mucosal defense of the upper airways? Am J Physiol
Lung Cell Mol Physiol. 296:L92–L100. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Steinbichler TB, Metzler V, Pritz C,
Riechelmann H and Dudas J: Tumor-associated fibroblast-conditioned
medium induces CDDP resistance in HNSCC cells. Oncotarget.
7:2508–2518. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Juel HB, Faber C, Udsen MS, Folkersen L
and Nissen MH: Chemokine expression in retinal pigment epithelial
ARPE-19 cells in response to coculture with activated T cells.
Invest Ophthalmol Vis Sci. 53:8472–8480. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Steelant B, Seys SF, Van Gerven L, Van
Woensel M, Farre R, Wawrzyniak P, Kortekaas Krohn I, Bullens DM,
Talavera K, Raap U, et al: Histamine and T helper cytokine-driven
epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin
Immunol. 141:951–63, e8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Koehler C, Paulus M, Ginzkey C, Hackenberg
S, Scherzad A, Ickrath P, Hagen R and Kleinsasser N: The
Proinflammatory potential of nitrogen dioxide and its influence on
the house dust mite allergen der p 1. Int Arch Allergy Immunol.
171:27–35. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Koehler C, Ginzkey C, Friehs G, Hackenberg
S, Froelich K, Scherzed A, Burghartz M, Kessler M and Kleinsasser
N: Aspects of nitrogen dioxide toxicity in environmental urban
concentrations in human nasal epithelium. Toxicol Appl Pharmacol.
245:219–225. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Revenfeld AL, Steffensen R, Pugholm LH,
Jørgensen MM, Stensballe A and Varming K: Presence of HLA-DR
Molecules and HLA-DRB1 mRNA in Circulating CD4(+) T Cells. Scand J
Immunol. 84:211–221. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Motamedi M, Xu L and Elahi S: Correlation
of transferrin receptor (CD71) with Ki67 expression on stimulated
human and mouse T cells: The kinetics of expression of T cell
activation markers. J Immunol Methods. 437:43–52. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yoon JH, Moon HJ, Seong JK, Kim CH, Lee
JJ, Choi JY, Song MS and Kim SH: Mucociliary differentiation
according to time in human nasal epithelial cell culture.
Differentiation. 70:77–83. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bleier BS, Mulligan RM and Schlosser RJ:
Primary human sinonasal epithelial cell culture model for topical
drug delivery in patients with chronic rhinosinusitis with nasal
polyposis. J Pharm Pharmacol. 64:449–456. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
de Borja Callejas F, Martínez-Antón A,
Alobid I, Fuentes M, Cortijo J, Picado C, Roca-Ferrer J and Mullol
J: Reconstituted human upper airway epithelium as 3-d in vitro
model for nasal polyposis. PLoS One. 9(e100537)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Divekar RD, Samant S, Rank MA, Hagan J,
Lal D, O'Brien EK and Kita H: Immunological profiling in chronic
rhinosinusitis with nasal polyps reveals distinct VEGF and GM-CSF
signatures during symptomatic exacerbations. Clin Exp Allergy.
45:767–778. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Romano M, Sironi M, Toniatti C,
Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van
Hinsbergh V, Sozzani S, et al: Role of IL-6 and its soluble
receptor in induction of chemokines and leukocyte recruitment.
Immunity. 6:315–325. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Poposki JA, Uzzaman A, Nagarkar DR, Chustz
RT, Peters AT, Suh LA, Carter R, Norton J, Harris KE, Grammer LC,
et al: Increased expression of the chemokine CCL23 in eosinophilic
chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol.
128:73–81, e4. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Poposki JA, Keswani A, Kim JK, Klingler
AI, Suh LA, Norton J, Carter RG, Peters AT, Hulse KE, Grammer LC,
et al: Tissue proteases convert CCL23 into potent monocyte
chemoattractants in patients with chronic rhinosinusitis. J Allergy
Clin Immunol. 137:1274–1277, e9. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Van Bruaene N, Derycke L, Perez-Novo CA,
Gevaert P, Holtappels G, De Ruyck N, Cuvelier C, Van Cauwenberge P
and Bachert C: TGF-beta signaling and collagen deposition in
chronic rhinosinusitis. J Allergy Clin Immunol. 124:253–9,
259.e1-2. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pant H, Hughes A, Miljkovic D, Schembri M,
Wormald P, Macardle P, Grose R, Zola H and Krumbiegel D:
Accumulation of effector memory CD8+ T cells in nasal polyps. Am J
Rhinol Allergy. 27:e117–e126. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pham OH and McSorley SJ: Divergent
behavior of mucosal memory T cells. Mucosal Immunol. 8:731–734.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Carbone FR: Tissue-resident memory T cells
and fixed immune surveillance in nonlymphoid organs. J Immunol.
195:17–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ely KH, Cauley LS, Roberts AD, Brennan JW,
Cookenham T and Woodland DL: Nonspecific recruitment of memory CD8+
T cells to the lung airways during respiratory virus infections. J
Immunol. 170:1423–1429. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schmitz S: Der Experimentator: Zellkultur.
Spektrum Akademischer Verlag, Heidelberg. 2011.
|
|
32
|
Gstraunthaler G and Lindl T: Zell- und
Gewebekultur: Allgemeine Grundlagen und spezielle Anwendungen.
Springer Spektrum Akademischer Verlag, Heidelberg. 2013.
|