Introduction
Kidney transplantation is the most effective
treatment for end-stage renal disease. The widespread use of
crossmatch assays and improved immunosuppressive regiments to
prevent acute antibody-mediated rejection and acute cellular
rejection respectively, have increased early graft survival
significantly; however, late graft outcome remains fairly poor in
part due to chronic antibody-mediated rejection, which is the
result of de novo production of antibodies against the graft
(1,2).
Thus, further investigation into humoral alloimmunity is imperative
in order to reduce late graft loss.
Indoleamine 2,3-dioxygenase (IDO) is an
immunomodulatory enzyme. Its immunosuppressive properties were
originally identified by the detection of its role in preventing
semi-allogenic fetal rejection (3).
Subsequently, the immunosuppressive effects of IDO have been
extensively studied and confirmed in various models of
transplantation; autoimmunity and tumor escape from
immunosurveillance have also been reported (4,5). Upon
inflammation, IDO expression is upregulated in antigen presenting
cells resulting in L-tryptophan degradation via the kynurenine
pathway (4,5). L-tryptophan depletion suppresses T cells
by activating general control nonderepressible-2 (GCN2) kinase
(6); products of the kynurenine
pathway favor naïve CD4+ T cell differentiation towards
a regulatory state instead of an effector phenotype by activating
the aryl-hydrocarbon receptor (AhR) (7). The effects of IDO on T cells are
mediated by alterations in the expression level of numerous
transcription factors and are partial associated with alterations
in cell metabolism (8-12).
The role of IDO in preventing acute cellular
rejection of allografts has been reported in various models of
solid organ transplantation (13-22);
however, the effect of this enzyme on humoral alloimmunity requires
further investigation. In the present study, we developed a method
for assessing de novo antibody production during an in
vitro alloimmune response. In order to evaluate the role of IDO
in humoral alloimmunity, the specific inhibitor
1-methyl-DL-tryptophan (1-MT) was employed. 1-MT is a competitive,
non-toxic IDO inhibitor (23), which
has been successfully used for suppressing immune tolerance in
models of semi-allogenic pregnancy (3), transplantation (24), autoimmunity (25) and cancer (26). To further understand the molecular
mechanisms by which IDO may affect humoral alloimmunity, the GCN2
kinase activator tryptophanol (TRP) was used. TRP is a competitive
inhibitor of tryptophanyl-tRNA synthetase and by raising the pool
of uncharged tRNA, it acts as a pharmacological activator of GCN-2
kinase (6,27). Thus, the AhR inhibitor CH223191 was
used. CH223191 does not have detectable AhR agonist-like activity
and protects mice from dioxin toxicity (28).
Materials and methods
Subjects
Blood was collected from 4 unrelated healthy
subjects (aged 32.5±7.05-year-old) from a blood vessel in the arm,
inside of the elbow or wrist, at the laboratory of the Nephrology
Department, University of Thessaly. In order to exclude any
pre-sensitization event, all subjects were males without a history
of blood transfusion. Written informed consent was obtained from
each individual enrolled; the present study was approved by the
Ethics Committee of the Faculty of Medicine, University of Thessaly
(Larissa, Greece) (approval no. 558/10-2-2017).
Peripheral blood mononuclear cell
(PBMC) isolation and culture
PBMCs were isolated from whole blood samples by
Ficoll-Hypaque density gradient centrifugation at 600 x g for 25
min at 18-20˚C using Histopaque-1077 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). The interface was collected and washed with
RPMI-1640 medium (Sigma Aldrich; Merck KGaA). To count the isolated
PBMCs, a Neubauer chamber (Paul Marienfeld GmbH & Co. KG,
Lauda-Königshofen, Germany) and an optical microscope at x40 (x4
objective) were used. Cell viability was assessed using the trypan
blue exclusion assay (Sigma-Aldrich; Merck KGaA) and for each PBMC
sample cells were counted in the four fields of the Neubauer
chamber. All cell cultures were performed using RPMI-1640 medium,
supplemented with 2 mM L-glutamine, 10 mM HEPES, 10% fetal bovine
serum (Sigma-Aldrich; Merck KGaA) and 1% antibiotic-antimycotic
solution containing penicillin, streptomycin and amphotericin B
(Sigma-Aldrich; Merck KGaA). Cultures were incubated at 37˚C in an
atmosphere of 95% relative humidity and 5% CO2.
Assessment of 1-MT or TRP effect on
lactate dehydrogenase (LDH) release following treatment with
CH223191
The concentrations of 1-MT (100 µM), TRP (0.25 mM)
and CH223191 (3 µM) were selected according to previous studies
(8,29,30). In
particular, the concentration of 100 µM for 1-MT was selected
according to the results that were repeatedly obtained from several
of our previous studies, which revealed efficacy in inhibiting IDO
and reduced L-tryptophan consumption in mixed lymphocyte reactions
(MLRs) without toxicity (9-12,29).
In addition, an LDH release assay for the selected concentrations
of the aforementioned substances was performed in resting PBMCs
seeded in 96-well plates (1x105 cells/well) and cultured
at 37˚C for a 7-day period. The LDH release assay was performed
using the Cytotox Non-Radioactive Cytotoxic Assay kit (cat no.
G1780; Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocols. LDH release was calculated by the
following equation: LDH release (%) = (LDH in the supernatant /
total LDH) x 100. All experiments were performed in triplicate and
the results were presented as the mean of the three
measurements.
Of note, our previous study originally planned to
apply the sodium
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-2H-tetrazolium
(XTT) assay to analyze cell proliferation. This assay comprises a
colorimetric technique that assesses actual cytosolic NADH content,
a key compound in the mitochondria, in which the tricarboxylic acid
cycle takes place (31). The results
revealed that IDO inhibition by 1-MT significantly decreased
mitochondrial function in PBMCs stimulated with lymphocyte-specific
stimulus tetanus toxoid (TT), which was unexpected. In addition,
compared with untreated cells, TT stimulation notably increased the
optical density (OD) value from the XTT assay, indicating enhanced
mitochondrial function. On the contrary, in cells treated with TT
and 1-MT, the XTT assay presented a significantly lower OD value
compared with TT-treated cells. This decrease suggested that IDO
enhances mitochondrial function in stimulated PBMCs, and its
inhibition by 1-MT may markedly abrogate this effect. Additionally,
as 1-MT did not alter the proliferation of TT-stimulated
lymphocytes, the use of tetrazolium dyes for assessing cell
proliferation may not be reliable (32).
Assessment of cellular alloimmunity in
two-way MLRs
MLRs are ex vivo cellular immunoassays that
occur between two genetically distinct allogeneic lymphocyte
populations of the same species. In a one-way MLR, only one
lymphocyte population can respond or proliferate. In a two-way MLR,
the two populations can proliferate. MLRs are performed to assess
the response of T cells to external stimuli. The assay comprises
the purification of responder lymphocytes from peripheral blood
followed by co-culturing with stimulator cells. Stimulator cell
populations that also contain T cells (two-way MLR) can replicate
in the presence of the responder cells. Therefore, for a one-way
MLR, stimulator cells are prevented from replicating by mitomycin
C, a DNA crosslinker to restricT cell replication. The maximal
measurable cellular proliferation occurs ~5-7 days of the MLR
(33).
In the present study, two-way MLRs were performed in
96-well plates for 7 days in the presence or absence of 100 µΜ
1-MT, 0.25 mM TRP or 3 µM CH223191. A total of 6 different pairs of
PBMC samples of the 4 different healthy aforementioned individuals.
For each pair, lymphocytes from a subject were mixed with
lymphocytes from a different subject. In particular, PBMCs from
subject #1 were co-cultured with PBMCs from subject #2, 3 or 4;
thus three different MLRs could be obtained. PBMCs from subject #2
were cultured with PBMCs from subject #3 or 4, which provided two
more MLRs. Additionally, PBMCs from subject #3 were cultured with
PBMCs from subject #4 yielding only one MLR that differed from the
others. The number of PBMCs from each member of the MLR pairs was
5x104, which comprised 1x105 PBMCs in each
well. Cultures of 1x105 resting PMBCs per well were used
as controls. At the end of the 7-day period, cell proliferation was
assessed by chemiluminescence with Cell Proliferation ELISA (Roche
Diagnostics, Indianapolis, IN, USA) using bromodeoxyuridine (BrdU)
labeling overnight at 37˚C and immunoenzymatic detection according
to the manufacturer's protocols. For determining the results of the
BrdU Cell Proliferation Assay, the fluorescence intensity was
measured and analyzed using an EnSpire® Multimode Plate
Reader (PerkinElmer, Inc., Waltham, MA, USA). The proliferation
index was calculated by the following equation: Proliferation index
(%) = (OD derived from each MLR / the mean OD derived from the
control resting PBMCs cultures of the two subjects that constituted
the specific MLR) x 100. All aforementioned MLRs were performed in
triplicate and the results were representative of the mean of three
measurements.
Assessment of humoral
alloimmunity
In order to evaluate humoral alloimmunity, the
following method was developed. One-way MLRs were performed in
24-well plates. Mitomycin-C-treated PBMCs (0.5x106
cells) from one subject were used as stimulator cells. For the
mitomycin-C treatment, PBMCs were incubated for 30 min at 37˚C with
50 µg/ml mitomycin C (Sigma Aldrich; Merck Merck KGaA) and then
washed three times with complete RPMI-1640 medium supplemented with
2 mM L-glutamine and 10% FBS. As responder cells,
0.5x106 PBMCs from another individual were used. A total
of 12 different pairs of PBMC samples of the 4 healthy
aforementioned subjects using one-way MLR cultures. For each pair,
lymphocytes from a responder subject were mixed with inactivated
lymphocytes from a stimulator subject and cultured as follows: Pair
1, responder subject #1 + stimulator subject #2; pair 2, stimulator
subject #1 + responder subject #2; pair 3, responder subject #1 +
stimulator subject #3; pair 4, stimulator subject #1 + responder
subject #3; pair 5, responder subject #1 + stimulator subject #4;
pair 6, stimulator subject #1 + responder subject #4; pair 7,
responder subject #2 + stimulator subject #3, pair 8, stimulator
subject #2 + responder subject #3; pair 9, responder subject #2 +
stimulator subject #4; pair 10, stimulator subject #2 + responder
subject #4; pair 11, responder subject #3 + stimulator subject #4
and pair 12, stimulator subject #3 + responder subject #4. MLRs
lasted for 7 days in culture at 37˚C in the presence or absence of
100 µΜ 1-MT, 0.25 mM TRP or 3 µM CH223191. Of note, a 7-day period
was reported as adequate for the production of IgM and IgG
alloantibodies in human mixed lymphocyte cultures (34). Following this period, the supernatants
from each one-way MLR were harvested and expected to contain
antibodies produced against the stimulator PBMCs.
In parallel to the aforementioned one-way MLRs,
resting untreated PBMCs were cultured in 6-well plates. At the end
of the 7-day one-way MLRs, resting PBMCs similar to those used as
stimulator cells in MLRs but untreated, were counted using a
Neubauer chamber (Paul Marienfeld GmbH & Co. KG) and an optical
microscope at x40 (x4 objective). Cell viability was assessed using
the trypan blue exclusion assay and for each PBMC sample, cells
were counted in the four different fields of the Neubauer chamber.
Then, cells were placed in 96-well plates at a number of
0.5x105 in a volume of 50 µl of RPMI-1640 medium,
supplemented with L-glutamine, 10 mM HEPES, 10% fetal bovine serum
and 1% antibiotic-antimycotic solution containing penicillin,
streptomycin and amphotericin B. Cultures were incubated at 37˚C in
an atmosphere of 95% relative humidity and 5% CO2. For
assessing antibody-mediated complement-dependent cytotoxicity (CDC)
a modified protocol, which was initially developed for the
assessment of antigen specific antibodies in serum samples was
conducted (35). In brief, 50 µl of
each supernatant collected from each one-way MLR, undiluted or
diluted 1:2 with complete RPMI-1640, was added into 96-well plates
that were pre-seeded with the resting target PBMCs. The plates were
incubated on ice for 30 min. Subsequently, 11 µl of rabbit
complement (Low-Tox-H rabbit complement, Cedarlane Corporation,
Burlington, Canada) was added to each well at a final concentration
of 10%. The 96-well plates were incubated for another 2 h at 37˚C.
As a control, 50 µl of complete RMPI-1640 was added instead of the
one-way MLR supernatant, along with 11 µl of rabbit complement.
As cell-mediated cytotoxicity occurs in MLRs
(36) and the used compounds (1-MT,
TRP and CH223191) affected the intensity of MLRs leading to the
release of various quantities of LDH in the supernatants,
antibody-mediated CDC induced by these supernatants in cells was
not investigated directly. Instead, the present study analyzed cell
survival. As the exact time-points of cell death for each of the
differenT cell types involved in MLRs have not been clearly defined
yet, Annexin V staining for the detection of apoptosis was not
selected in the present study; however, it is considered to be the
gold standard for the analysis of cell survival/death. Therefore,
cell survival was assessed colorimetrically by measuring the
reduction of XTT, a yellow tetrazolium salt, to orange formazan via
the metabolic targeting of cells. Target cells were incubated with
XTT reagent for 1 h at 37˚C. For this purpose, the TACS XTT assay
kit (Trevigen, Inc., Gaithersburg, MD, USA) was used according to
manufacturer's protocols. Cell survival was calculated by the
following equation: Cell survival (%) = (XTT assay OD of the
control / XTT assay OD of the evaluated condition) x 100. A total
of twelve experiments were performed, each in triplicates and the
results were representative of the mean of the three
measurements.
Statistical analysis
Statistical analyses were performed using SPSS
Statistics, Version 20 (IBM Corp., Armonk, NY, USA). The normality
of the evaluated variables was assessed and confirmed by the
one-sample Kolmogorov-Smirnov test. For the comparison of means, a
paired t-test or one-way repeated measures analysis of variance
followed by Bonferroni's correction test were used. The results
were expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
In some cases, to avoid the violation of the
prerequisite for normal distribution of the compared variables when
applying parametric statistical tests, we compared the OD values as
derived from the aforementioned assays prior to normalization to
the control; however, the results were expressed and depicted
following the normalization of values to the control group.
Results
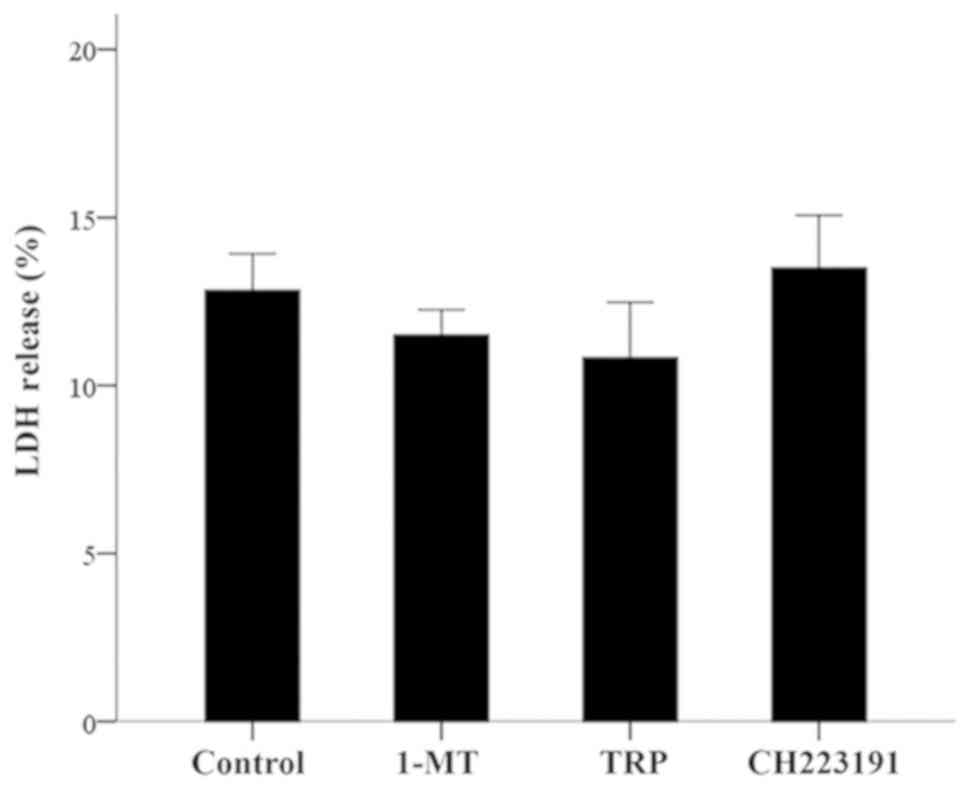
1-MT, TRP and CH223191 does not affect
LDH release in resting PBMCs
At the concentrations of 100 µM for 1-MT, 0.25 mM
for TRP or 3 µM for CH223191, none of the evaluated compounds
induced a statistically significant change in the LDH release of
resting PBMCs. The LDH release assay revealed a value of
12.83±1.01% for the control, 11.50±0.75% for 1-MT (P>0.05),
10.83±1.64% for TRP (P>0.05) and 13.50±1.56% for CH223191
(P>0.05; Fig. 1).
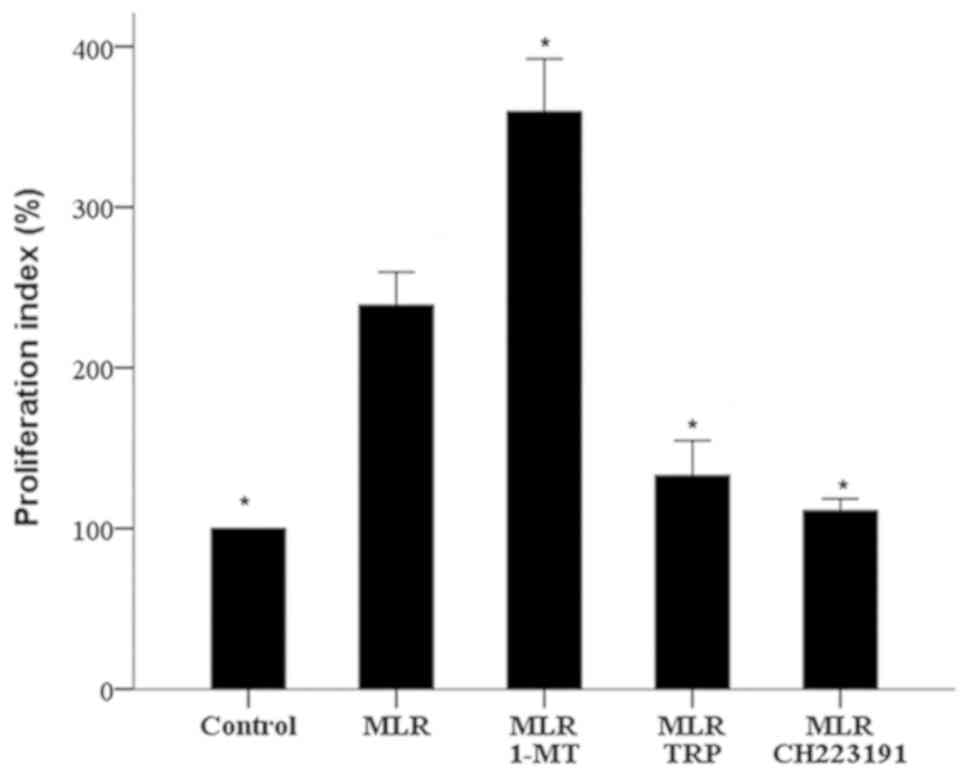
1-MT increases cellular alloimmunity,
but is decreased by TRP and CH223191
The IDO inhibitor 1-MT increased cellular
alloimmunity, as assessed by cell proliferation in two-way MLRs.
The proliferation index in 1-MT-treated MLRs was significantly
higher than that of untreated MLRs (359.37±32.93 vs. 238.99±20.55%
respectively, P<0.001; Fig.
2).
On the contrary, compared with MLRs of untreated
cells, treatment with the GCN2 kinase activator TRP significantly
decreased the proliferation index to 132.89±21.65% (P<0.001;
Fig. 2). Similarly, the AhR inhibitor
CH223191 significantly reduced the proliferation index to
111.07±7.36% (P<0.001; Fig.
2).
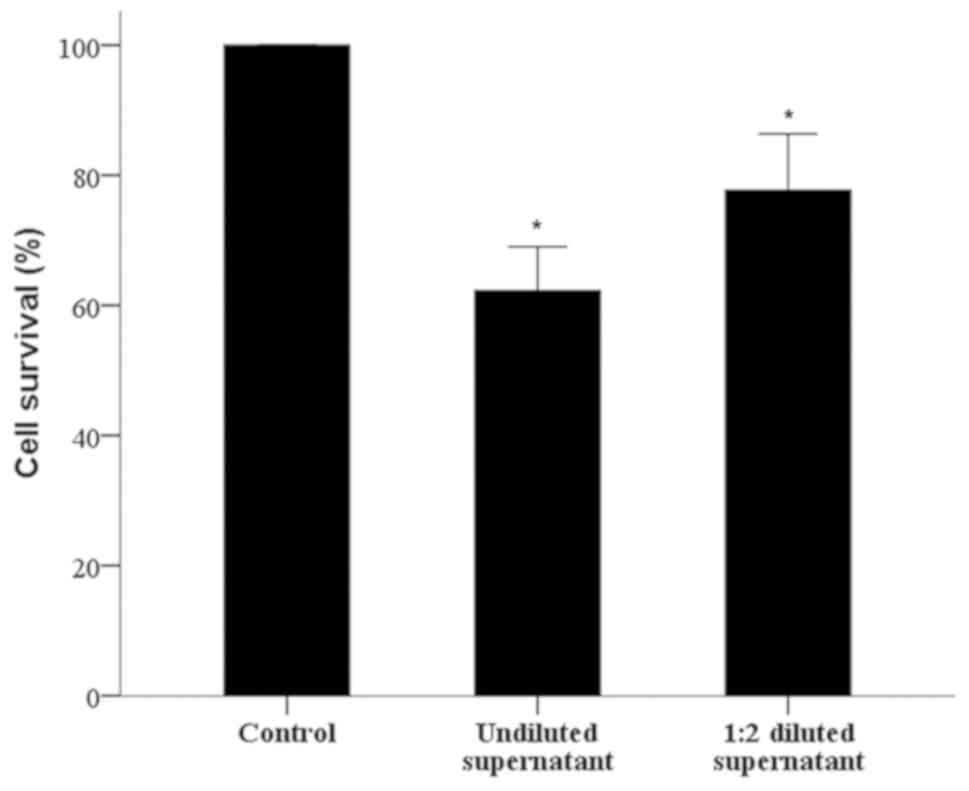
Supernatants from one-way MLRs contain
specific antibodies against the stimulator PBMCs
The antibody-mediated CDC assay revealed that
one-way MLR-antibodies specific for the stimulator PBMCs were
produced by the responder PBMCs. The cell survival of untreated
PBMCs, similar to stimulator cells, decreased to 62.25±6.76%
compared with the control when supernatants from the respective
MLRs were undiluted (P<0.001), and to 77.71±8.62% when diluted
1:2 (P<0.001). Interestingly, the diluted supernatants exhibited
less antibody-mediated CDC against the untreated PBMCs, similar to
stimulator cells, target PBMCs (P<0.001; Fig. 3).
1-MT increases humoral alloimmunity,
but is not affected by TRP and CH22319
The antibody-mediated CDC assay revealed that the
treatment of one-way MLRs with 1-MT increased the production of
specific for the stimulator PBMCs antibodies. When undiluted
supernatants from untreated MLRs were used, the survival of target
PBMCs was 62.25±6.76%, which was significantly decreased to
35.39±7.75% following treatment with 1-MT (P<0.001; Fig. 4A). Similar results were obtained from
the 1:2 diluted supernatants compared with the control; however,
decreased antibody-mediated CDC was observed, (cell survival of
diluted supernatant, 77.71±8.62% versus diluted supernatant with
1-MT, 47.48±10.72%, P<0.001; Fig.
4B).
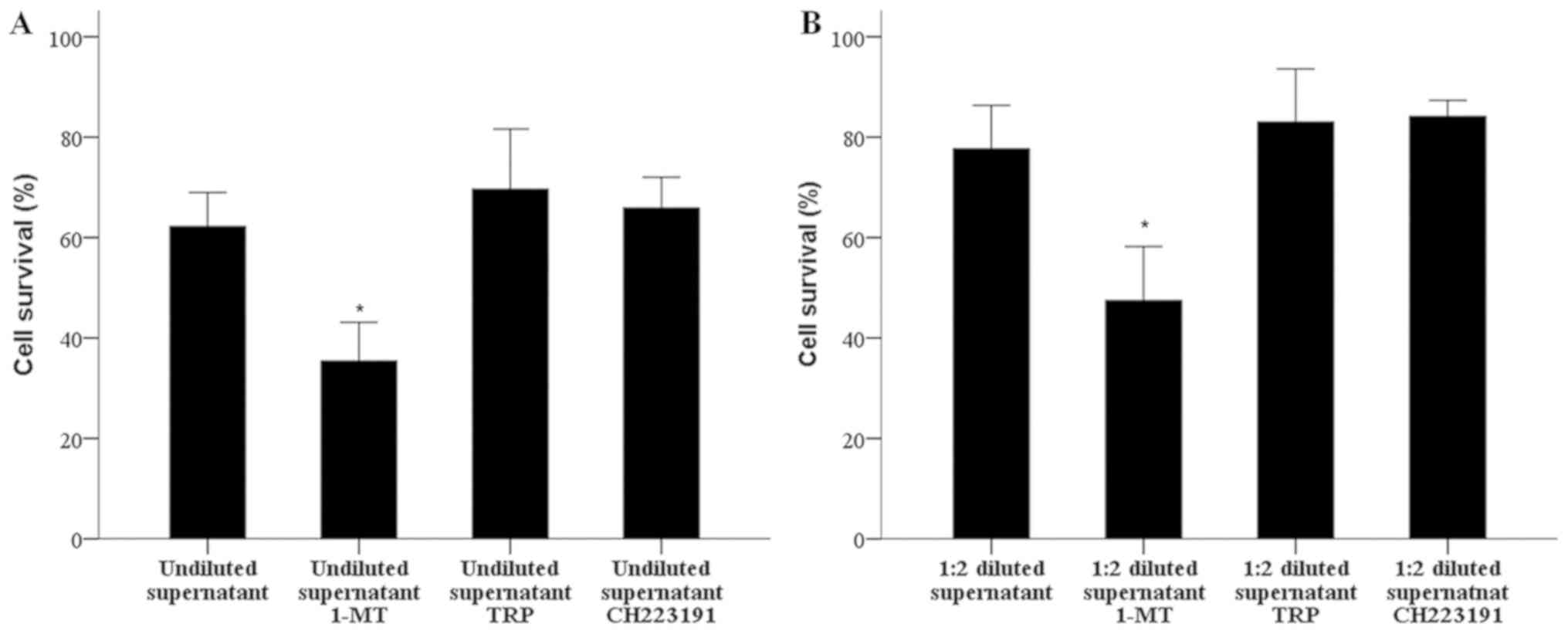 | Figure 4.1-MT increases humoral alloimmunity,
but is notably affected by TRP and CH223191. One-way MLRs were
performed in the presence or absence of indoleamine 2,3-dioxygenase
inhibitor 1-MT, the GCN-2 kinase activator TRP or the aryl
hydrocarbon receptor inhibitor CH223191. (A) Then the supernatants
were collected and used in an antibody-mediated CDC assay against
resting target PBMCs similar to those used as stimulator cells in
the respective MLRs; (B) supernatants were diluted 1:2. When
undiluted supernatants were employed, those derived from
1-MT-treated MLRs exhibited greater antibody-mediated CDC than
those derived from untreated MLRs. On the contrary, supernatants
from TRP- or CH223191-treated MLRs did not exhibit notably
different antibody-mediated CDC, than those from untreated MLRs.
*P<0.05 vs. untreated supernatant. Error bars
represent standard deviation. 1-MT, 1-methyl-DL-tryptophan; CDC,
complement-dependent cytotoxicity; MLR, mixed lymphocyte reaction;
TRP, tryptophanol. |
In one-way MLRs, the GCN-2 kinase activator TRP did
not markedly affect the production of specific antibodies against
the stimulator PBMCs. The antibody-mediated CDC assay revealed that
when undiluted supernatants from untreated MLRs were used, the
survival of untreated, target PMBCs, which are similar to
stimulatory cells, was 62.25±6.76%, while in the case of undiluted
supernatants from TRP-treated MLRs cell survival was 69.63±12.01%
(P>0.05; Fig. 4A). In the case of
1:2 diluted supernatants, cell survival was 77.71±8.62 and
83.04±10.58% in the untreated as TRP-treated groups, respectively
(P>0.05; Fig. 4B).
Similarly, in one-way MLRs, the AhR inhibitor
CH223191 did not notably alter the production of specific
antibodies against the stimulatory PBMCs. The antibody-mediated CDC
assay demonstrated that the cell survival of untreated target
PMBCs, which are similar to stimulatory cells, was 62.25±6.76%,
while that of undiluted supernatants from CH223191-treated MLRs,
cell survival was 65.87±6.17% (P>0.05; Fig. 4A). In the case of 1:2 diluted
supernatants, cell survival was 77.71±8.62 and 84.13±5.21%, in the
untreated and CH223191-treated groups, respectively (P>0.05;
Fig. 4B).
Discussion
Developments in immunological assays and
immunosuppressive medications have reduced the incidence of acute
antibody-mediated rejection and acute cellular rejection; however,
little is known of the pathophysiology and treatment of chronic
antibody-mediated rejection, which notably contributes to late
graft loss (1,2). The aim of the present study was to
evaluate the effects of IDO, an immunomodulatory enzyme (4,5), on
humoral alloimmunity. In addition, cellular alloimmunity was also
assessed.
Cellular alloimmunity was investigated by measuring
cell proliferation in two-way MLRs. As expected, inhibition of IDO
by 1-MT at a non-toxic concentration, increased cell proliferation.
The immunosuppressive effects of IDO on cellular adaptive immunity
has been extensively studied (4,5), and
numerous experimental models of transplantation have revealed the
protective role of IDO in acute cellular rejection (13-22).
IDO may affect T cell function via L-tryptophan depletion and GCN-2
kinase activation (6,29). In accordance with this, MLR treatment
with non-toxic concentrations of the GCN-2 kinase activator TRP
inhibited cell proliferation in the present study.
In addition, IDO affects T cell function and favors
naïve CD4+ T cell differentiation toward a regulatory
phenotype via the activation of AhR by L-tryptophan degradation
products (7). On the contrary, the
AhR inhibitor CH223191 decreased cell proliferation. Generally,
there are contradicting results regarding the role of AhR in
cellular alloimmunity. For instance, a previous study revealed that
the prototype AhR activator 2,3,7,8-tetrachlorodibenzo-p-dioxin
(TCDD) decreased cell proliferation in MLRs (37), while the same activator enhanced T
cell proliferation by increasing the expression of MHCII and
cluster of differentiation 86 in allogenic bone marrow-derived
dendritic cells (38). It has been
also been proposed, that under different conditions, the activation
of AhR may affect the differentiation of naïve CD4+ T
cells into the Th17 or Treg cell lineages (39). The effects of AhR activation on
cellular alloimmunity requires further investigation, taking into
consideration that AhR regulation differs between human and murine
cells (40). Thus, primary human
PBMCs were employed for analysis in the present study.
To assess humoral alloimmunity, we developed a
simple experimental model using one-way MLRs and an
antibody-mediated CDC assay. The results confirmed that in the
7-day period of MLR culture, specific antibodies against the
stimulator cells were produced and secreted into the supernatant by
the responder cells. These antibodies were identified and
quantified via an antibody-mediated CDC assay of resting PBMCs
derived from an individual conferring stimulator PBMCs for the
one-way MLR.
In our model, the IDO inhibitor 1-MT increased
humoral alloimmunity, which suggests that IDO inhibits humoral
alloimmunity. Providing this may be simply attributed to
1-MT-induced increased cellular autoimmunity, it would be expected
that humoral alloimmunity would be reduced in the presence of TRP
or CH223191, as these substances decreased cellular immunity.
Additionally, neither the GCN-2 kinase activator TRP, nor the AhR
inhibitor CH223191 exerted any significant effects on humoral
alloimmunity. These pathways have been evaluated mainly in T cells
and in models where IDO1 is upregulated in antigen presenting cells
due to inflammation (4-7).
The effects of IDO and the involved molecular pathways on humoral
immunity require further investigation. For instance, IDO is
generally considered to possess immunosuppressive properties;
however, IDO1 expression in B cells increased antibody production
against T cell-independent (TI) antigens (41). In addition, in vitro activation
with Toll-like receptor ligands or B cell receptor crosslinking
rapidly induced IDO1 expression and activity in purified B cells,
whereas IDO1-/- B cells exhibited enhanced proliferation
and survival, associated with increased immunoglobulin and cytokine
production compared with wild type B cells. Murine B cells purified
from the spleen of knockout or wild type mice activated with TI
type I antigens LPS and CpG, or anti-IgM B cell receptor exhibited
crosslinking to mimic TI-2 antigen responses (41). On the contrary, to the best of our
knowledge, we are the first to examine the role of the IDO pathway
in B cell responses in a more physiologically relevant setting of
MLRs using human cells and 1-MT as a treatment in order to inhibit
IDO activity. Furthermore, IDO inhibition with 1-MT has been
reported to exacerbate autoimmune diseases (25,42),
whereas other studies revealed opposing findings (43,44). This
could be due to the inhibitory effects of 1-MT on IDO1 and IDO2.
IDO2 is a low-efficiency L-tryptophan catabolizing enzyme, which
was associated with immunomodulation; its molecular mode of action
remains unknown (45). A recent study
demonstrated that IDO2 expression in B cells enhances humoral
autoimmunity by supporting cross talk between autoreactive T and B
cells; however, humoral immune responses to T cell-dependent
antigens were unaffected (46). Our
study revealed that IDO inhibition via 1-MT increased humoral
alloimmunity; this may provide insight into the prevention of
chronic antibody-mediated rejection. The lack of effects from TRP
and CH223191 may indicate that other molecular pathways are
responsible for the inhibitory effects of IDO in humoral
alloimmunity, which may differ to the mechanisms reported in T
cells. Thus, IDO2 may serve as a promising candidate for further
research.
Another possible explanation for the lack of effects
of the AhR inhibitor CH223191 on humoral alloimmunity in the
present study may be due to the activation of AhR that directly
affects B cells. An endogenous tryptophan catabolism-derived AhR
agonist has been shown to suppress the differentiation of B cells
into immunoglobulin-secreting plasma cells (47). A recent study revealed that the
prototypical AhR activator TCDD decreases antibody production by
promoting an interaction between AhR and an essential
transcriptional element in the gene of the immunoglobulin heavy
chain (48). Thus, inhibition of AhR
may be proposed to increase humoral alloimmunity. This may explain
our finding of steady humoral alloimmunity in CH223191-treated
one-way MLRs despite the decreased T cell response detected in
two-way MLRs treated with the same inhibitor. In MLRs with human
cells, 1-MT-induced decreases in L-tryptophan degradation reduced
AhR activation (12), supporting the
finding of the present study in which that 1-MT increased humoral
alloimmunity.
Of note, inter-species variation should be taken
into consideration when interpreting the results of the
aforementioned studies. Additionally, AhR regulation differs
between human and murine cells (40).
For instance, in humans, the antibody response to a vaccine against
the surface antigen of hepatitis B is decreased in association with
increased IDO levels (49), whereas
administration of the same antigen in mice along with IDO inhibitor
1-MT resulted in a suppressed humoral immune response (50). Nevertheless, the use of primary human
PBMCs in the present study due to certain inter-species differences
in AhR regulation yields several limitations. In particular,
primary human PBMCs have a short lifespan in culture (51); thus, experiments with overexpression
of IDO, which may provide further insight into underlying
mechanisms, would be rather ineffective in this particular
study.
In conclusion, IDO decreased humoral alloimmunity in
a GCN-2 kinase-dependent manner, which may occur independently of
AhR in humans. As humoral alloimmunity may induce chronic
antibody-mediated rejection, understanding the major cause of late
kidney allograft dysfunction and the underlying mechanisms may
contribute to developments in prolonging kidney allograft
survival.
Acknowledgements
The present study was based on a previously
published abstract by Eleftheriadis et al in The Journal of
Immunology, volume 146, pages 292-300 (2015) entitled as:
Indoleamine 2,3-dioxygenase depletes tryptophan, activates general
control non-derepressible 2 kinase and down-regulates key enzymes
involved in fatty acid synthesis in primary human CD4+ T
cells.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All of the authors contributed to the preparation of
the manuscript and have read and agree to the manuscript as
written. MS and GP performed the experiments; TE made substantial
contributions to the conception of the present study; MS, GP, TE,
GA, SG, and VL analyzed the data; MS, GP, and TE wrote the
manuscript; IS supported all stages.
Ethics approval and consent to
participate
Written informed consent for the use of blood
samples was obtained from all participants. The present study was
approved by the Ethics Committee of the Faculty of Medicine,
University of Thessaly (Larissa, Greece; approval no.
558/10-2-2017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Solez K, Colvin RB, Racusen LC, Sis B,
Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris
AJ, et al: Banff '05 Meeting Report: Differential diagnosis of
chronic allograft injury and elimination of chronic allograft
nephropathy (‘CAN’). Am J Transplant. 7:518–526. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Solez K, Colvin RB, Racusen LC, Haas M,
Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, et
al: Banff 07 classification of renal allograft pathology: Updates
and future directions. Am J Transplant. 8:753–760. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Munn DH, Zhou M, Attwood JT, Bondarev I,
Conway SJ, Marshall B, Brown C and Mellor AL: Prevention of
allogeneic fetal rejection by tryptophan catabolism. Science.
281:1191–1193. 1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
King NJC and Thomas SR: Molecules in
focus: Indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol.
39:2167–2172. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Curti A, Trabanelli S, Salvestrini V,
Baccarani M and Lemoli RM: The role of indoleamine 2,3-dioxygenase
in the induction of immune tolerance: Focus on hematology. Blood.
113:2394–2401. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Munn DH, Sharma MD, Baban B, Harding HP,
Zhang Y, Ron D and Mellor AL: GCN2 kinase in T cells mediates
proliferative arrest and anergy induction in response to
indoleamine 2,3-dioxygenase. Immunity. 22:633–642. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mezrich JD, Fechner JH, Zhang X, Johnson
BP, Burlingham WJ and Bradfield CA: An interaction between
kynurenine and the aryl hydrocarbon receptor can generate
regulatory T cells. J Immunol. 185:3190–3198. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Eleftheriadis T, Pissas G, Yiannaki E,
Markala D, Arampatzis S, Antoniadi G, Liakopoulos V and Stefanidis
I: Inhibition of indoleamine 2,3-dioxygenase in mixed lymphocyte
reaction affects glucose influx and enzymes involved in aerobic
glycolysis and glutaminolysis in alloreactive T-cells. Hum Immunol.
74:1501–1509. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Eleftheriadis T, Pissas G, Antoniadi G,
Spanoulis A, Liakopoulos V and Stefanidis I: Indoleamine
2,3-dioxygenase increases p53 levels in alloreactive human T cells,
and both indoleamine 2,3-dioxygenase and p53 suppress glucose
uptake, glycolysis and proliferation. Int Immunol. 26:673–684.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eleftheriadis T, Pissas G, Antoniadi G,
Liakopoulos V and Stefanidis I: Indoleamine 2,3-dioxygenase
depletes tryptophan, activates general control non-derepressible 2
kinase and down-regulates key enzymes involved in fatty acid
synthesis in primary human CD4+ T cells. Immunology. 146:292–300.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eleftheriadis T, Pissas G, Antoniadi G,
Tsogka K, Sounidaki M, Liakopoulos V and Stefanidis I: Indoleamine
2,3 dioxygenase downregulates T cell receptor complex ζ chain and c
Myc, and reduces proliferation, lactate dehydrogenase levels and
mitochondrial glutaminase in human T cells. Mol Med Rep.
13:925–932. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Eleftheriadis T, Pissas G, Sounidaki M,
Tsogka K, Antoniadis N, Antoniadi G, Liakopoulos V and Stefanidis
I: Indoleamine 2,3-dioxygenase, by degrading L-tryptophan, enhances
carnitine palmitoyltransferase I activity and fatty acid oxidation,
and exerts fatty acid-dependent effects in human alloreactive
CD4+ T-cells. Int J Mol Med. 38:1605–1613.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vavrincova-Yaghi D, Deelman LE, Goor H,
Seelen M, Kema IP, Smit-van Oosten A, Zeeuw D, Henning RH and
Sandovici M: Gene therapy with adenovirus-delivered indoleamine
2,3-dioxygenase improves renal function and morphology following
allogeneic kidney transplantation in rat. J Gene Med. 13:373–381.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Sun X, Gong ZJ, Wang ZW, Li T, Zhang JY,
Sun HC, Liu S, Huang L, Huang C and Peng ZH: IDO-competent-DCs
induced by IFN-γ attenuate acute rejection in rat liver
transplantation. J Clin Immunol. 32:837–847. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Iken K, Liu K, Liu H, Bizargity P, Wang L,
Hancock WW and Visner GA: Indoleamine 2,3-dioxygenase and
metabolites protect murine lung allografts and impair the calcium
mobilization of T cells. Am J Respir Cell Mol Biol. 47:405–416.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hosseini-Tabatabaei A, Jalili RB,
Khosravi-Maharlooei M, Hartwell R, Kilani RT, Zhang Y and Ghahary
A: Immunoprotection and Functional Improvement of Allogeneic Islets
in Diabetic Mice, Using a Stable Indoleamine 2,3-Dioxygenase
Producing Scaffold. Transplantation. 99:1341–1348. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xie FT, Cao JS, Zhao J, Yu Y, Qi F and Dai
XC: IDO expressing dendritic cells suppress allograft rejection of
small bowel transplantation in mice by expansion of Foxp3+
regulatory T cells. Transpl Immunol. 33:69–77. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
He Y, Zhou S, Liu H, Shen B, Zhao H, Peng
K and Wu X: Indoleamine 2,3-Dioxgenase Transfected Mesenchymal Stem
Cells Induce Kidney Allograft Tolerance by Increasing the
Production and Function of Regulatory T cells. Transplantation.
99:1829–1838. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ebrahimi A, Kardar GA, Teimoori-Toolabi L,
Ghanbari H and Sadroddiny E and Sadroddiny E: Inducible expression
of indoleamine 2,3-dioxygenase attenuates acute rejection of
tissue-engineered lung allografts in rats. Gene. 576:412–420.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li C, Liu T, Zhao N, Zhu L, Wang P and Dai
X: Dendritic cells transfected with indoleamine 2,3-dioxygenase
gene suppressed acute rejection of cardiac allograft. Int
Immunopharmacol. 36:31–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Na N, Luo Y, Zhao D, Yang S, Hong L, Li H,
Miao B and Qiu J: Prolongation of kidney allograft survival
regulated by indoleamine 2, 3-dioxygenase in immature dendritic
cells generated from recipient type bone marrow progenitors. Mol
Immunol. 79:22–31. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Khosravi-Maharlooei M, Pakyari M, Jalili
RB, Kilani RT and Ghahary A: Intraperitoneal injection of
IDO-expressing dermal fibroblasts improves the allograft survival.
Clin Immunol. 174:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jia L, Schweikart K, Tomaszewski J, Page
JG, Noker PE, Buhrow SA, Reid JM, Ames MM and Munn DH: Toxicology
and pharmacokinetics of 1-methyl-d-tryptophan: Absence of toxicity
due to saturating absorption. Food Chem Toxicol. 46:203–211.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alexander AM, Crawford M, Bertera S,
Rudert WA, Takikawa O, Robbins PD and Trucco M: Indoleamine
2,3-dioxygenase expression in transplanted NOD Islets prolongs
graft survival after adoptive transfer of diabetogenic splenocytes.
Diabetes. 51:356–365. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sakurai K, Zou J-P, Tschetter JR, Ward JM
and Shearer GM: Effect of indoleamine 2,3-dioxygenase on induction
of experimental autoimmune encephalomyelitis. J Neuroimmunol.
129:186–196. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Uyttenhove C, Pilotte L, Théate I,
Stroobant V, Colau D, Parmentier N, Boon T and Van den Eynde BJ:
Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med.
9:1269–1274. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Lowe G and Tansley G: An investigation of
the mechanism of activation of tryptophan by tryptophanyl-tRNA
synthetase from beef pancreas. Eur J Biochem. 138:597–602.
1984.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ,
Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, et al: Novel
compound 2-methyl-2H-pyrazole-3-carboxylic acid
(2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents
2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon
receptor. Mol Pharmacol. 69:1871–1878. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Eleftheriadis T, Pissas G, Antoniadi G,
Liakopoulos V, Tsogka K, Sounidaki M and Stefanidis I: Differential
effects of the two amino acid sensing systems, the GCN2 kinase and
the mTOR complex 1, on primary human alloreactive CD4+
T-cells. Int J Mol Med. 37:1412–1420. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Eleftheriadis T, Pissas G, Antoniadi G,
Liakopoulos V and Stefanidis I: Kynurenine, by activating aryl
hydrocarbon receptor, decreases erythropoietin and increases
hepcidin production in HepG2 cells: A new mechanism for anemia of
inflammation. Exp Hematol. 44:60–7, e1. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Berridge MV, Herst PM and Tan AS:
Tetrazolium dyes as tools in cell biology: New insights into their
cellular reduction. Biotechnol Annu Rev. 11:127–152.
2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eleftheriadis T, Pissas G, Karioti A,
Antoniadi G, Liakopoulos V, Dafopoulou K, Pournaras S, Koukoulis G
and Stefanidis I: The indoleamine 2,3-dioxygenase inhibitor
1-methyl-tryptophan suppresses mitochondrial function, induces
aerobic glycolysis and decreases interleukin-10 production in human
lymphocytes. Immunol Invest. 41:507–520. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Meo T: The MLR test in the mouse. In:
Immunological methods. Lefkovits I and Pernis B (eds). Academic
Press, New York, NY. pp227–239. 1979.
|
|
34
|
Rümke HC, Terpstra FG, Huis B, Out TA and
Zeijlemaker WP: Immunoglobulin production in human mixed lymphocyte
cultures: Implications for co-cultures of cells from patients and
healthy donors. J Immunol. 128:696–701. 1982.PubMed/NCBI
|
|
35
|
Konishi E, Kitai Y and Kondo T:
Utilization of complement-dependent cytotoxicity to measure low
levels of antibodies: Application to nonstructural protein 1 in a
model of Japanese encephalitis virus. Clin Vaccine Immunol.
15:88–94. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sato T, Deiwick A, Raddatz G, Koyama K and
Schlitt HJ: Interactions of allogeneic human mononuclear cells in
the two-way mixed leucocyte culture (MLC): Influence of cell
numbers, subpopulations and cyclosporin. Clin Exp Immunol.
115:301–308. 1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cai LJ, Yu DW, Gao Y, Yang C, Zhou HM and
Chen ZK: Activation of aryl hydrocarbon receptor prolongs survival
of fully mismatched cardiac allografts. J Huazhong Univ Sci
Technolog Med Sci. 33:199–204. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee JA, Hwang JA, Sung HN, Jeon CH, Gill
BC, Youn HJ and Park JH: 2,3,7,8-Tetrachlorodibenzo-p-dioxin
modulates functional differentiation of mouse bone marrow-derived
dendritic cells Downregulation of RelB by
2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 173:31–40.
2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Julliard W, Fechner JH and Mezrich JD: The
aryl hydrocarbon receptor meets immunology: Friend or foe? A little
of both. Front Immunol. 5(458)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Panchanathan R, Liu H and Choubey D:
Activation of p53 in Human and Murine Cells by DNA-Damaging Agents
Differentially Regulates Aryl Hydrocarbon Receptor Levels. Int J
Toxicol. 34:242–249. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shinde R, Shimoda M, Chaudhary K, Liu H,
Mohamed E, Bradley J, Kandala S, Li X, Liu K and McGaha TL: B
cell-Intrinsic IDO1 Regulates Humoral Immunity to T
cell-Independent Antigens. J Immunol. 195:2374–2382.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Criado G, Simelyte E, Inglis JJ, Essex D
and Williams RO: Indoleamine 2,3 dioxygenase-mediated tryptophan
catabolism regulates accumulation of Th1/Th17 cells in the joint in
collagen-induced arthritis. Arthritis Rheum. 60:1342–1351.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Scott GN, DuHadaway J, Pigott E, Ridge N,
Prendergast GC, Muller AJ and Mandik-Nayak L: The immunoregulatory
enzyme IDO paradoxically drives B cell-mediated autoimmunity. J
Immunol. 182:7509–7517. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xu H, Oriss TB, Fei M, Henry AC, Melgert
BN, Chen L, Mellor AL, Munn DH, Irvin CG, Ray P, et al: Indoleamine
2,3-dioxygenase in lung dendritic cells promotes Th2 responses and
allergic inflammation. Proc Natl Acad Sci USA. 105:6690–6695.
2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Merlo LMF, Pigott E, DuHadaway JB, Grabler
S, Metz R, Prendergast GC and Mandik-Nayak L: IDO2 is a critical
mediator of autoantibody production and inflammatory pathogenesis
in a mouse model of autoimmune arthritis. J Immunol. 192:2082–2090.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Merlo LMF, DuHadaway JB, Grabler S,
Prendergast GC, Muller AJ and Mandik-Nayak L: IDO2 Modulates T
cell-Dependent Autoimmune Responses through a B Cell-Intrinsic
Mechanism. J Immunol. 196:4487–4497. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yoshida T, Katsuya K, Oka T, Koizumi S,
Wakita D, Kitamura H and Nishimura T: Effects of AhR ligands on the
production of immunoglobulins in purified mouse B cells. Biomed
Res. 33:67–74. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wourms MJ and Sulentic CEW: The aryl
hydrocarbon receptor regulates an essential transcriptional element
in the immunoglobulin heavy chain gene. Cell Immunol. 295:60–66.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Eleftheriadis T, Liakopoulos V, Antoniadi
G, Stefanidis I and Galaktidou G: Indoleamine 2,3-dioxygenase is
increased in hemodialysis patients and affects immune response to
hepatitis B vaccination. Vaccine. 29:2242–2247. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Eleftheriadis T, Sparopoulou T, Antoniadi
G, Liakopoulos V, Stefanidis I and Galaktidou G: Suppression of
humoral immune response to hepatitis B surface antigen vaccine in
BALB/c mice by 1-methyl-tryptophan co-administration. Daru.
19:236–239. 2011.PubMed/NCBI
|
|
51
|
Patel AA, Zhang Y, Fullerton JN, Boelen L,
Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B,
et al: The fate and lifespan of human monocyte subsets in steady
state and systemic inflammation. J Exp Med. 214:1913–1923.
2017.PubMed/NCBI View Article : Google Scholar
|