Introduction
According to recent data, ~40% of the U.S.
population over the age of 20 years, and 18% of children aged 2-19
years, are obese (1). In addition to
metabolic disorders, obesity is also associated with reproductive
complications, such as menstrual irregularities, subfertility,
endometrial hyperplasia and gynecological cancers (2-4). The
adipose tissue is an endocrine organ that produces active protein
hormones, referred to as adipokines, which are involved in the
regulation of energy metabolism, appetite, insulin sensitivity,
inflammation, diabetes and metabolic syndrome (5-9).
One of the most extensively studied biologically relevant
adipokines is adiponectin, which is abundantly produced and
secreted by adipose tissue. Furthermore, it has a low expression in
obese individuals. Antidiabetic, anti-inflammatory, antiatherogenic
and cardioprotective properties of adiponectin are widely known
(5-11).
Adiponectin is a 30-kDa glycoprotein hormone
produced by mature adipocytes (12).
Its mechanism of action occurs mainly in the periphery through
binding to two receptors, AdipoR1 and AdipoR2(12). These receptors are found in
reproductive tissues, such as the ovaries, oviduct, endometrium and
testis (12). Furthermore,
adiponectin modulates gonadotropin release, normal pregnancy, and
affects assisted reproduction outcomes (13). Higher adiponectin levels are
associated with improved menstrual regularities and better in
vitro fertilization (IVF) outcomes (13). In the ovaries, adiponectin plays a key
role in oocyte maturation, granulosa cell proliferation and steroid
secretion (11,14-16).
In animal studies, adiponectin knockout (KO) mice have fewer
oocytes, more atretic follicles and prolonged diestrus cycles,
indicating that adiponectin plays an important role in
folliculogenesis (17). In a
retrospective case-controlled study, adiponectin levels were found
to be higher among women who conceived after IVF, and were
positively correlated with the number of oocytes retrieved,
regardless of the woman's body mass index (BMI) (18). Similarly, while adiponectin expression
is low in human and mouse granulosa cells, its presence is
associated with better fertilization rates and better embryonic
development (19,20).
Adiponectin is associated with two important genes
involved in folliculogenesis: Anti-Müllerian hormone (AMH) and
kisspeptin. Interestingly, adiponectin is a regulator of AMH
production, possibly via its effects on insulin sensitivity
(21). In addition to having low
serum and follicular fluid adiponectin levels, obese women also
have low AMH levels, which is one of the most reliable markers of
ovarian reserve (22). In a previous
study, obese women in their late reproductive years were found to
have ~65% lower serum AMH levels compared with normoweight women of
the same age (23,24). Through its effects on downstream
targets, such as AMP-activated protein kinase (AMPK), adiponectin
can mediate the phosphorylation of the transcription factor
specificity protein-1 (SP1), which is a regulator of the kisspeptin
gene (25). In addition to its
regulation of female reproduction at the level of the hypothalamus
(26,27), the neuropeptide kisspeptin is
expressed in the rat ovary, suggesting a role for kisspeptin in
ovarian function (28,29). Obese individuals were found to have
lower serum kisspeptin levels compared with normoweight individuals
(30). Furthermore, serum kisspeptin
levels were found to be negatively correlated with body mass index
(BMI) (30), but positively
correlated with serum adiponectin (30).
Given this association of adiponectin with AMH and
kisspeptin in non-ovarian tissues, the aim of the present study was
to assess whether there is any such association in the ovaries.
Materials and methods
Experimental animals
All protocols were conducted in accordance with the
National Institutes of Health guidelines for the care and use of
laboratory animals and were approved by the Institutional Animal
Care and Use Committee of the University of Vermont College of
Medicine. Adiponectin-KO mice, B6.129-Adipoqtm1Chan/J,
were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). A
targeting vector was designed to replace exon 2 of the targeted
gene with a PGKneo cassette. This construct was electroporated into
(129X1/SvJ x 129S1/Sv) F1-derived R1 embryonic stem (ES) cells.
Correctly targeted ES cells were injected into blastocysts and
chimeric mice were bred with C57BL/6J to generate mutant mice.
Mutant mice were backcrossed to C57BL/6J for at least eight
generations. The mice were bred with C57BL/6J inbred mice for at
least one generation to establish the colony. A 32
single-nucleotide polymorphism (SNP) panel analysis, with 27
markers covering all 19 chromosomes and the X chromosome, as well
as 5 markers that distinguish between the C57BL/6J and C57BL/6N
substrains, was performed on the re-derived living colony. This
analysis revealed two markers on chromosome 15 and one marker on
chromosome 19 that are segregating for 129, suggesting an
incomplete backcross. Homozygous mice are viable and fertile, with
absence of targeted allele expression confirmed in adipose tissue
(mRNA) and plasma (adiponectin protein). While homozygous mice have
normal glucose tolerance and insulin resistance, beta-oxidation
activity is significantly increased in the muscle and liver.
Homozygotes also exhibit endothelial dysfunction (increased
leukocyte rolling and leukocyte adhesion), and are more susceptible
to myocardial ischemia/reperfusion. According to The Jackson
Laboratory, these mice may be useful for studying obesity,
diabetes, insulin resistance, metabolism, inflammation,
leukocyte-endothelium interactions and colitis.
Adiponectin-KO mice (5 months old; n=6) and C57BL/6J
wild-type control female mice (5 months old; n=6), were also
obtained from The Jackson Laboratory, were housed five to a cage
and bred in-house under specific pathogen-free conditions and
normal light/dark cycles (14 h light and 10 h dark cycle), with
ad libitum access to standard mouse chow (ProLab®
Isopro®; chemical composition: 5% fat, 22% protein, 59%
carbohydrate, 3.46 kcal/g; PMI Nutrition International, Brentwood,
MO, USA) and water. All the mice were sacrificed at 5 months of age
by cervical dislocation. At the time of sacrifice, oophorectomy was
performed and the ovaries were snap-frozen in liquid nitrogen and
stored at -80˚C to be used for gene expression analysis.
RNA extraction from mouse ovaries and
reverse transcription-quantitative polymerase chain reaction
(RT-PCR) analysis
RT-PCR analysis was performed to quantify the mRNA
levels of specific genes that are known to be important in
folliculogenesis and their receptors: amh and its receptor
(Amhr2), as well as kisspeptin (Kiss1) and its
receptor (Kiss1r). RNA extraction and RT-qPCR were performed
on mouse ovaries that were lysed and homogenized using a
homogenizer. RNA was isolated using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) and chloroform
extraction using RNeasy mini kit (Qiagen Sciences, Inc.,
Germantown, MD, USA) according to the manufacturer's instructions.
RNA analysis quality was assessed by a Nanodrop spectrophotometer
and Agilent Bioanalyzer (Agilent Technologies, Inc., Santa Clara,
CA, USA). Only samples with a minimum concentration of 10 ng/µl,
optical density 260:280, and ratio of 1.8-2.0 were used for
quantification. The RNA quality was additionally confirmed using
RNA electrophoresis. The mRNA expression levels were measured by
RT-PCR kinetics using SYBR Green I Chemistry (Roche Diagnostics,
Indianapolis, IN, USA), as described elsewhere (31). The primers used were synthesized by
Thermo Fisher Scientific (Pittsburgh, PA, USA; Table I). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) primers were used as a loading control and
the levels of mRNA for each gene relative to GAPDH were calculated
using the 2-ΔΔCq method (32).
 | Table IPrimers used for reverse
transcription-polymerase chain reaction analysis. |
Table I
Primers used for reverse
transcription-polymerase chain reaction analysis.
| Gene | Sequence primers
(5'-3') |
|---|
| Kiss1 | Forward:
ATGATCTCAATGGCTTCTTGG |
| | Reverse:
CCAGGCATTAACGAGTTCCT |
| Kiss1r | Forward:
GCACATGCAGACAGTTACCAA |
| | Reverse:
CACGCAGCACAGTAGGAAAGT |
| Amh | Forward:
CGTCACCGCAGCCAGCACA |
| | Reverse:
CCCGCAGAGCACGAACCAAG |
| Amhr2 | Forward:
CCACAGACCACCACCTTTCC |
| | Reverse:
GTCTGCGTCCCAGCAATCTT |
Subjects and follicular fluid
adiponectin levels
A total of 25 women of reproductive age who
underwent controlled ovarian hyperstimulation for IVF at Albert
Einstein College of Medicine/Montefiore Medical Center (Bronx, NY,
USA) between August 2008 and April 2011 were enrolled. Standard IVF
procedures were used. Briefly, the participants underwent
controlled ovarian hyperstimulation with a combination of
gonadotropins (Follistim, Merck & Co., Inc., Whitehouse
Station, NJ, USA; Gonal-F, EMD-Serono, Rockland, MA, USA; Menopur
and Bravelle, Ferring, Parsippany, NJ, USA) using either a long
agonist (Lupron, AbbVie, North Chicago, IL, USA) or antagonist
(Ganirelix acetate, Merck & Co., Inc., or cetrorelix acetate,
EMD-Serono) protocol. When ≥2 follicles had reached a diameter of
≥17 mm, human chorionic gonadotropin (hCG; Ovidrel, EMD-SeronoMA;
or Novarel, Ferring) was administered for oocyte maturation,
followed by transvaginal ultrasound-guided oocyte retrieval 34-36 h
later. Women who had known endometriosis or anovulatory
infertility, such as polycystic ovary syndrome (PCOS) or
hypothalamic amenorrhea, were excluded to minimize the effect of
these confounding variables on the results. Women with tubal or
male factor infertility were included. BMI was calculated using the
following formula: Weight (kg)/height (m2). For each
woman, follicular phase serum AMH was determined by ELISA according
to the manufacturer's recommendations; the intra- and inter-assay
coefficients of variation were <15%. Following controlled
ovarian hyperstimulation and at the time of oocyte retrieval,
follicular fluid was collected for the measurement of adiponectin
levels from the first aspirate of the large follicle (>14 mm) to
prevent any blood contamination. Adiponectin protein levels were
measured by human ELISA kits according to the manufacturer's
protocol (Quantakine kit; R&D Systems, Inc., Minneapolis, MN,
USA); the intra- and inter-assay coefficients of variation were
<15%. Each participant reviewed and signed an informed consent
document. The study was approved by the Institutional Review Board
of Albert Einstein College of Medicine of Yeshiva University and
Montefiore Medical Center (approval no. 04-08-199E).
Statistical analysis
Data are expressed as mean ± standard error of the
mean (SEM). The RT-PCR results are expressed as relative number of
copies ± SEM. As the data were not normally distributed, the
Mann-Whitney U test was used for comparison of genes between
wild-type mice and adiponectin-KO mice. Spearman's correlation
analysis was used to evaluate the association between serum AMH and
follicular fluid adiponectin levels among the participants. All
statistical procedures were run on STATA software (StataCorp LP,
College Station, TX, USA) and GraphPad Prism 7 (GraphPad Software,
Inc., La Jolla, CA, USA). A P-value ≤0.05 was considered to
indicate statistically significant differences.
Results
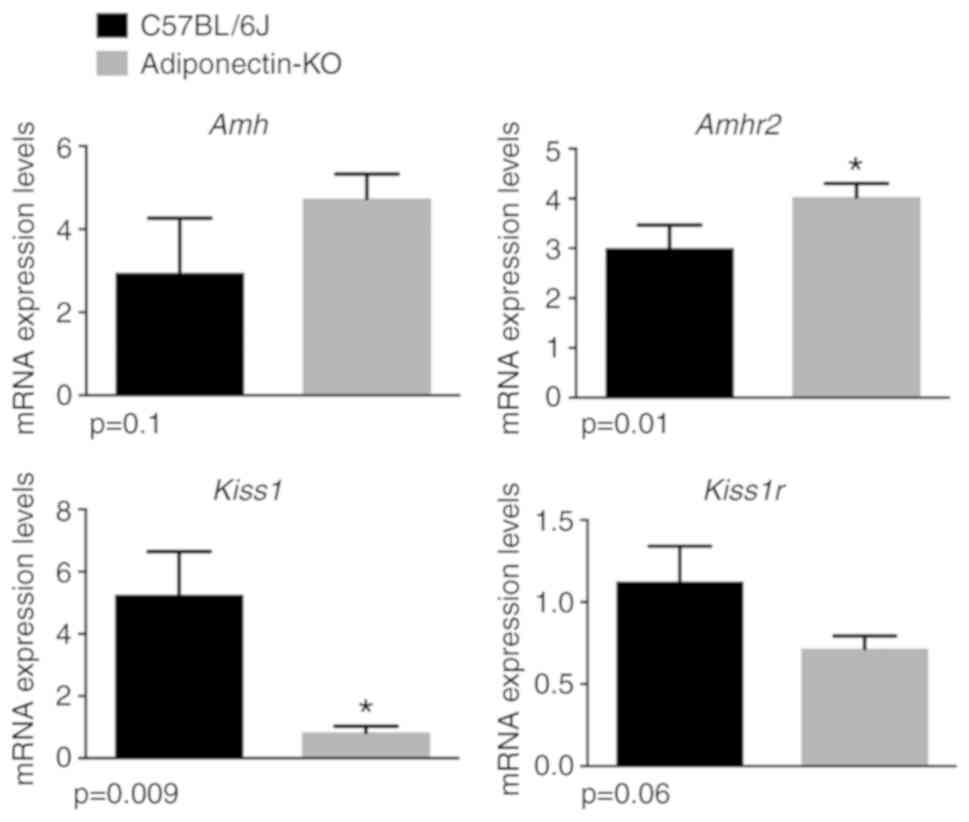
Effect of adiponectin knockout on
Kiss1, Kiss1r, amh and Amhr2 mRNA in mouse ovaries
Adiponectin-KO mice (n=6) on normal chow diet had
similar weights compared with wild-type control mice (n=6) on
normal chow diet (P>0.05). Compared with control mice,
adiponectin-KO mice had 6.5 times lower ovarian Kiss1 mRNA
levels (P=0.009) and the tendency for lower ovarian Kiss1r
mRNA expression levels (P=0.06; Fig.
1). By contrast, adiponectin-KO mice had significantly higher
Amhr2 mRNA expression levels (P=0.01) compared with control
mice (Fig. 1). Both adiponectin-KO
and control mice had similar amh mRNA expression levels
(P=0.1).
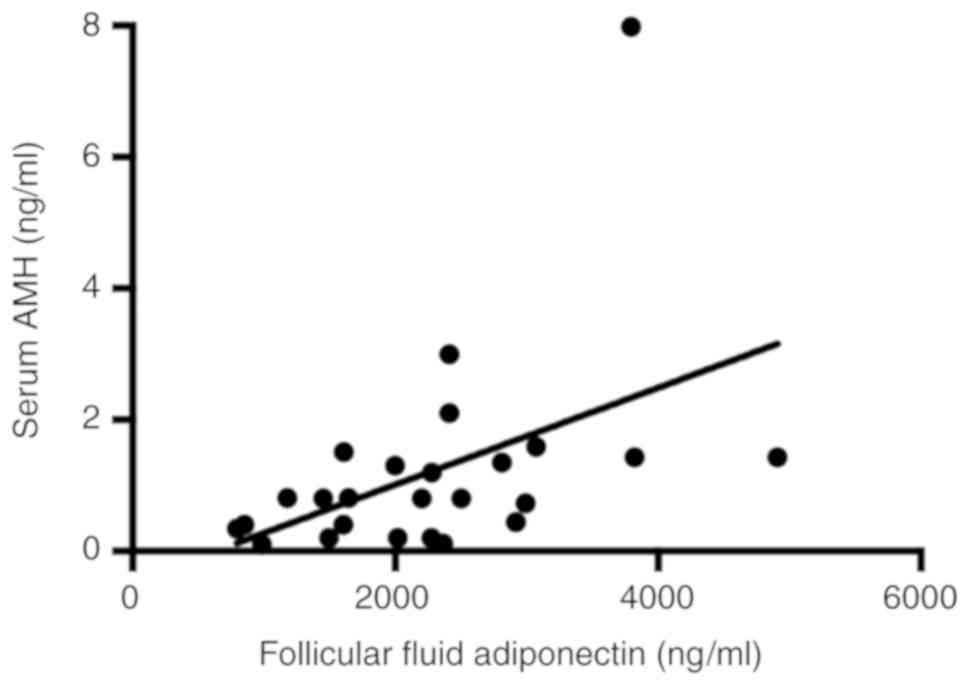
Correlation between follicular fluid
adiponectin and serum AMH
The demographics and IVF cycle characteristics of
the study participants are summarized in Table II. The age of the participants ranged
from 19 to 42 years. In all participants, the mean serum AMH level
was 1.2±0.3 ng/ml and the mean follicular fluid adiponectin level
was 2,256±197 ng/ml. There was a positive correlation between serum
AMH levels and follicular fluid adiponectin concentration (r=0.54,
P=0.006; Fig. 2). BMI was negatively
correlated with follicular fluid adiponectin levels (r=-0.4,
P=0.04, Table II).
 | Table IIDemographics and clinical
characteristics of the participants. |
Table II
Demographics and clinical
characteristics of the participants.
|
Characteristics | Mean ± SEM | Correlation with
follicular fluid adiponectin levels |
|---|
| Age (years) | 38.9±0.9 | r=-0.3, P=0.1 |
| Body mass index
(kg/m2) | 25.6±1.1 | r=-0.4, P=0.04 |
| Baseline day 3
follicle-stimulating hormone (mIU/ml) | 9.3±0.7 | r=-0.3, P=0.2 |
| Baseline day 3
estradiol (pg/ml) | 48.3±3.6 | r=-0.1, P=0.5 |
| Dose of
gonadotropin per cycle (USP) | 4,491.0±339.6 | r=-0.4, P=0.09 |
| Peak estradiol
following ovarian stimulation (pg/ml) | 1,916.5±217.5 | r=0.1, P=0.5 |
| Number of oocytes
retrieved | 10.0±1.2 | r=0.2, P=0.4 |
| Number of mature
oocytes | 7.7±1.2 | r=0.08, P=0.7 |
Discussion
To the best of our knowledge, this descriptive study
was the first to evaluate the effect of adiponectin gene knockout
on Kiss1, Kiss1r, amh and Amhr2 genes in mouse
ovaries. It also assessed the association between serum AMH levels
and follicular fluid adiponectin levels in women who underwent IVF.
First, the results indicated that adiponectin-KO mice had
significantly lower Kiss1 mRNA, but higher Amhr2 mRNA
expression levels. Adiponectin-KO mice also exhibited a tendency to
have lower ovarian Kiss1r mRNA expression levels and similar
amh mRNA levels. The clinical part of this study revealed a
positive correlation between serum AMH and follicular fluid
adiponectin levels. Furthermore, BMI was found to be negatively
correlated with follicular fluid adiponectin levels.
The association between adiponectin and AMH in the
serum was previously investigated (33). Nelson et al (33) demonstrated that serum AMH levels in
the first trimester of pregnancy were negatively correlated with
maternal adiposity, and that AMH levels decline in the 2nd and 3rd
trimester of pregnancy as maternal adiposity increases; they also
observed that adiponectin was positively correlated with serum AMH
(33). Park et al explored the
association of insulin resistance and adipokines with AMH levels in
women without PCOS (34). Their
findings revealed a negative correlation between insulin resistance
and AMH levels (34). Furthermore,
they also found a positive correlation between serum AMH and
adiponectin levels (34). Although
their study focused on serum adiponectin levels, their findings are
consistent with those of our study, indicating that follicular
fluid adiponectin levels are positively correlated with serum AMH
levels. In our assessment, adiponectin-KO mice were found to have
higher Amhr2 mRNA expression levels compared with control
mice, but similar amh mRNA expression levels. This may be
due to adiponectin deficiency-mediated upregulation of Amhr2
that could affect the sensitivity to the action of the AMH protein
in the ovary. It is well known that adiponectin is abundantly
produced and secreted by adipose tissue, and that its expression
and serum levels are lower in obese women (12). In a previous study, obese women in
their late reproductive years were found to have ~65% lower serum
AMH levels compared with normoweight women of the same age
(23). Thus, a possible explanation
for the negative association of AMH and obesity may be
adiponectin.
The association between adiponectin and kisspeptin
levels has been previously investigated (35,36). Two
hormones secreted by adipocytes, leptin and adiponectin, mediate
food intake, energy hemostasis and insulin sensitivity (35). Their action involves regulation of
gonadotropin-releasing hormone secretion from the hypothalamus, and
this is mediated by kisspeptin (35).
Specifically, these two hormones modulate Kiss1 gene
expression in GT1-7 neurons in the hypothalamus (25). Furthermore, while leptin decreases
insulin sensitivity, adiponectin increases insulin sensitivity
(36). In addition to its modulatory
effects on the hypothalamus, adiponectin was found to inhibit the
transcription of Kiss1 and Kiss1r in islet cells of
the pancreas (35). These findings
indicate that certain related metabolic disorders, such as obesity
and diabetes mellitus, are involved in the regulation of kisspeptin
through hormones such as adiponectin. Obese individuals were found
to have lower serum kisspeptin levels compared with normoweight
individuals (30). Furthermore, there
is an inverse association between serum kisspeptin levels and BMI,
but a positive correlation between kisspeptin and serum adiponectin
levels (30). This correlation was
further supported by Zhou et al (37), who demonstrated that high-fat
diet-induced obesity caused a marked suppression of ovarian
Kiss1 mRNA levels in mice at postnatal days 42 and 70
compared with normoweight mice on normal chow diet. The association
between adiponectin and kisspeptin may be better understood at the
biochemical level: Wen et al (25) reported that, through its effects on
downstream targets such as AMPK, adiponectin can mediate the
phosphorylation of the transcription factor SP1, which is a
regulator of the kisspeptin gene. Our study further confirmed this
association between adiponectin and kisspeptin, as it was
demonstrated that adiponectin-KO mice had significantly lower
Kiss1 mRNA levels compared with control mice (Fig. 1). This is opposite to the findings
reported by Latif et al (38),
who concluded that there was no significant association between
kisspeptin and adiponectin in any of the menstrual phases, despite
the proven presence of kisspeptin receptors on adipocytes. That
study had several limitations, including a small sample size,
making it difficult to draw a definitive conclusion (38). Furthermore, due to the minimal
fluctuations of kisspeptin during the menstrual cycle, the levels
of kisspeptin during this time period may not correlate to the
secretion of adiponectin and, thus, may not provide an accurate
assessment of the association between kisspeptin and adiponectin
(38). Our findings have potential
implications in reproduction and may help us to better understand
and overcome barriers to reproductive success. Kisspeptin has been
shown to affect ovarian follicular development and ovarian reserve.
We have demonstrated that older mice had significantly higher
Kiss1 and Kiss1r levels in their ovaries compared
with younger mice (39). The
sensitivity of kisspeptin may be altered by age-related
upregulation of Kiss1r (39).
Therefore, a better understanding of the kisspeptinergic system and
its mediators, such as adiponectin, may reveal new treatment
strategies to overcome reproductive barriers, particularly in obese
women, who usually have low levels of adiponectin.
This study had several limitations. When quantifying
mRNA expression levels, we used the whole mouse ovary, which
includes a number of different components, such as granulosa, theca
and stromal cells (39). Previous
studies have demonstrated that theca and stromal cells are the
major sites of Kiss1 expression, but another study reported
that the granulosa cells are the main site of Kiss1
expression (37). Therefore, future
studies should focus on identifying the specific type of cell where
the hormone being studied is mainly expressed. Another limitation
of this study is that we did not measure serum AMH, a measure of
ovarian reserve, in our adiponectin-KO animal model. In this
experiment, mRNA expression was used as a determinant of gene
expression, but mRNA may not necessarily translate into protein;
thus, future mechanistic studies should involve western blotting
and immunofluorescence experiments. In the present study, human
participants who underwent controlled ovarian hyperstimulation with
gonadotropins for IVF treatment were exposed to a combination of
hormones that may affect adiponectin levels. Future studies should
evaluate ovarian histology and follicular dynamics (e.g., number of
primordial follicles) in adiponectin-KO mice, whereas in humans,
they should focus on controlling for the number and type(s) of
gonadotropins used per cycle for controlled ovarian
hyperstimulation. Furthermore, AMH levels were measured in the
serum in order to determine a baseline ovarian reserve measure,
while adiponectin levels were measured in the follicular fluid in
order to assess its ovarian levels.
In conclusion, the present study suggests that
adiponectin may play a key role in ovarian physiology through its
impact on genes important for ovarian follicular development and
ovarian reserve, such as kisspeptin and AMH. Our results support
the presence of a possible common denominator and modulator, such
as adiponectin, for genes important for reproduction. Further
elucidating this association and the underlying biochemical
pathways that affect its expression may lead to targeted and
specific therapies to improve ovarian health, particularly in obese
women who have low systemic adiponectin levels.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
American Society for Reproductive Medicine (ASRM) and by a grant
from Ferring Pharmaceuticals.
Availability of data and materials
All the data are available from our clinical patient
software upon reasonable request.
Ethics approval and consent to
participate
All protocols were conducted in accordance with the
National Institutes of Health guidelines for the care and use of
laboratory animals and were approved by the Institutional Animal
Care and Use Committee of the University of Vermont College of
Medicine. Studies on human participants were approved by the
Institutional Review Board of Albert Einstein College of Medicine
of Yeshiva University and Montefiore Medical Center (approval no.
04-08-199E). All participants reviewed and signed an informed
consent document.
Patient consent for publication
Not applicable.
Authors' contributions
ZM, EB and EAB participated in the design of the
animal and human studies, collected data from participants, and
performed the statistical analysis; AB participated in writing the
manuscript and performed the literature search. All the authors
have read and approved the final version of this manuscript for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hales CM, Carroll MD, Fryar CD and Ogden
CL: Prevalence of obesity among adults and youth: United States,
2015-2016. NCHS Data Brief. 1–8. 2017.PubMed/NCBI
|
|
2
|
Wang YC, McPherson K, Marsh T, Gortmaker
SL and Brown M: Health and economic burden of the projected obesity
trends in the USA and the UK. Lancet. 378:815–825. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tzeng CR, Chang YC, Chang YC, Wang CW,
Chen CH and Hsu MI: Cluster analysis of cardiovascular and
metabolic risk factors in women of reproductive age. Fertil Steril.
101:1404–1410. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wei S, Schmidt MD, Dwyer T, Norman RJ and
Venn AJ: Obesity and menstrual irregularity: Associations with
SHBG, testosterone, and insulin. Obesity (Silver Spring).
17:1070–1076. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wise MR, Jordan V, Lagas A, Showell M,
Wong N, Lensen S and Farquhar CM: Obesity and endometrial
hyperplasia and cancer in premenopausal women: A systematic review.
Am J Obstet Gynecol. 214(689): e1–-689.e17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Massetti GM, Dietz WH and Richardson LC:
Excessive weight gain, obesity, and cancer: Opportunities for
clinical intervention. JAMA. 318:1975–1976. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee B and Shao J: Adiponectin and energy
homeostasis. Rev Endocr Metab Disord. 15:149–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Arita Y, Kihara S, Ouchi N, Takahashi M,
Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K,
et al: Paradoxical decrease of an adipose-specific protein,
adiponectin, in obesity. Biochem Biophys Res Commun. 257:79–83.
1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kondo H, Shimomura I, Matsukawa Y, Kumada
M, Takahashi M, Matsuda M, Ouchi N, Kihara S, Kawamoto T, Sumitsuji
S, et al: Association of adiponectin mutation with type 2 diabetes:
A candidate gene for the insulin resistance syndrome. Diabetes.
51:2325–2328. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ohashi K, Ouchi N, Kihara S, Funahashi T,
Nakamura T, Sumitsuji S, Kawamoto T, Matsumoto S, Nagaretani H,
Kumada M, et al: Adiponectin I164T mutation is associated with the
metabolic syndrome and coronary artery disease. J Am Coll Cardiol.
43:1195–1200. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kadowaki T, Yamauchi T, Kubota N, Hara K,
Ueki K and Tobe K: Adiponectin and adiponectin receptors in insulin
resistance, diabetes, and the metabolic syndrome. J Clin Invest.
116:1784–1792. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Nigro E, Scudiero O, Ludovica MM, Palmieri
A, Mazzarella G, Costagliola C, Bianco A and Daniele A: New insight
into adiponectin role in obesity and obesity-related diseases.
Biomed Res Int Article. 2014(658913)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Michalakis KG and Segars JF: The role of
adiponectin in reproduction: From polycystic ovary syndrome to
assisted reproduction. Fertil Steril. 94:1949–1957. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chabrolle C, Tosca L and Dupont J:
Regulation of adiponectin and its receptors in rat ovary by human
chorionic gonadotrophin treatment and potential involvement of
adiponectin in granulosa cell steroidogenesis. Reproduction.
133:719–731. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ledoux S, Campos DB, Lopes FL, Dobias-Goff
M, Palin MF and Murphy BD: Adiponectin induces periovulatory
changes in ovarian follicular cells. Endocrinology. 147:5178–5186.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chabrolle C, Tosca L, Crochet S, Tesseraud
S and Dupont J: Expression of adiponectin and its receptors
(AdipoR1 and AdipoR2) in chicken ovary: Potential role in ovarian
steroidogenesis. Domest Anim Endocrinol. 33:480–487.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cheng L, Shi H, Jin Y, Li X, Pan J, Lai Y,
Lin Y, Jin Y, Roy G, Zhao A and Li F: Adiponectin deficiency leads
to female subfertility and ovarian dysfunctions in mice.
Endocrinology. 157:4875–4887. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu YH, Tsai EM, Wu LC, Chen SY, Chang YH,
Jong SB and Chan TF: Higher basal adiponectin levels are associated
with better ovarian response to gonadotropin stimulation during in
vitro fertilization. Gynecol Obstet Invest. 60:167–170.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Richards JS, Liu Z, Kawai T, Tabata K,
Watanabe H, Suresh D, Kuo FT, Pisarska MD and Shimada M:
Adiponectin and its receptors modulate granulosa cell and cumulus
cell functions, fertility, and early embryo development in the
mouse and human. Fertil Steril. 98:471–479.e1. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chang HJ, Lee JH, Lee JR, Jee BC, Suh CS
and Kim SH: Relationship between follicular fluid adipocytokines
and the quality of the oocyte and corresponding embryo development
from a single dominant follicle in in vitro
fertilization/intracytoplasmic sperm injection cycles. Clin Exp
Reprod Med. 41:21–28. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seifer DB and Maclaughlin DT: Mullerian
Inhibiting Substance is an ovarian growth factor of emerging
clinical significance. Fertil Steril. 88:539–546. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
van Rooij IA, Broekmans FJ, te Velde ER,
Fauser BC, Bancsi LF, de Jong FH and Themmen AP: Serum
anti-Müllerian hormone levels: A novel measure of ovarian reserve.
Hum Reprod. 17:3065–3071. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Freeman EW, Gracia CR, Sammel MD, Lin H,
Lim LC and Strauss JF III: Association of anti-mullerian hormone
levels with obesity in late reproductive-age women. Fertil Steril.
87:101–106. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Buyuk E, Seifer DB, Illions E, Grazi RV
and Lieman H: Elevated body mass index is associated with lower
serum anti-mullerian hormone levels in infertile women with
diminished ovarian reserve but not with normal ovarian reserve.
Fertil Steril. 95:2364–2368. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wen JP, Liu C, Bi WK, Hu YT, Chen Q, Huang
H, Liang JX, Li LT, Lin LX and Chen G: Adiponectin inhibits KISS1
gene transcription through AMPK and specificity protein-1 in the
hypothalamic GT1-7 neurons. J Endocrinol. 214:177–189.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hameed S, Jayasena CN and Dhillo WS:
Kisspeptin and fertility. J Endocrinol. 208:97–105. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
de Roux N, Genin E, Carel JC, Matsuda F,
Chaussain JL and Milgrom E: Hypogonadotropic hypogonadism due to
loss of function of the KiSS1-derived peptide receptor GPR54. Proc
Natl Acad Sci USA. 100:10972–10976. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Seminara SB, Messager S, Chatzidaki EE,
Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W,
Schwinof KM, Hendrick AG, et al: The GPR54 gene as a regulator of
puberty. N Engl J Med. 349:1614–1627. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Terao Y, Kumano S, Takatsu Y, Hattori M,
Nishimura A, Ohtaki T and Shintani Y: Expression of KiSS-1, a
metastasis suppressor gene, in trophoblast giant cells of the rat
placenta. Biochim Biophys Acta. 1678:102–110. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kołodziejski PA, Pruszyńska-Oszmałek E,
Korek E, Sassek M, Szczepankiewicz D, Kaczmarek P, Nogowski L,
Maćkowiak P, Nowak KW, Krauss H and Strowski MZ: Serum levels of
spexin and kisspeptin negatively correlate with obesity and insulin
resistance in women. Physiol Res. 67:45–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Merhi Z, Buyuk E, Berger DS, Zapantis A,
Israel DD, Chua S Jr and Jindal S: Leptin suppresses anti-Mullerian
hormone gene expression through the JAK2/STAT3 pathway in
luteinized granulosa cells of women undergoing IVF. Hum Reprod.
28:1661–1669. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nelson SM, Stewart F, Fleming R and
Freeman DJ: Longitudinal assessment of antimüllerian hormone during
pregnancy-relationship with maternal adiposity, insulin, and
adiponectin. Fertil Steril. 93:1356–1358. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Park HT, Cho GJ, Ahn KH, Shin JH, Kim YT,
Hur JY, Kim SH, Lee KW and Kim T: Association of insulin resistance
with anti-Mullerian hormone levels in women without polycystic
ovary syndrome (PCOS). Clin Endocrinol (Oxf). 72:26–31.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mahmoodzadeh Sagheb M, Azarpira N and
Yaghobi R: The effect of leptin and adiponectin on KiSS-1 and KissR
mRNA expression in rat islets of langerhans and CRI-D2 cell line.
Int J Endocrinol Metab. 12(e15297)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ahima RS and Lazar MA: Adipokines and the
peripheral and neural control of energy balance. Mol Endocrinol.
22:1023–1031. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhou Q, Chen H, Yang S, Li Y, Wang B, Chen
Y and Wu X: High-fat diet decreases the expression of Kiss1 mRNA
and kisspeptin in the ovary, and increases ovulatory dysfunction in
postpubertal female rats. Reprod Biol Endocrinol.
12(127)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Latif R, Rafique N, Salem AM, AlSheikh MH
and Chathoth S: Correlation between circulatory Kisspeptin and
Adipokines in normal and over-weight Saudi females during menstrual
cycle. Biol Rhythm Res. 49:169–174. 2018. View Article : Google Scholar
|
|
39
|
Merhi Z, Thornton K, Bonney E, Cipolla MJ,
Charron MJ and Buyuk E: Ovarian kisspeptin expression is related to
age and to monocyte chemoattractant protein-1. J Assist Reprod
Genet. 33:535–543. 2016.PubMed/NCBI View Article : Google Scholar
|