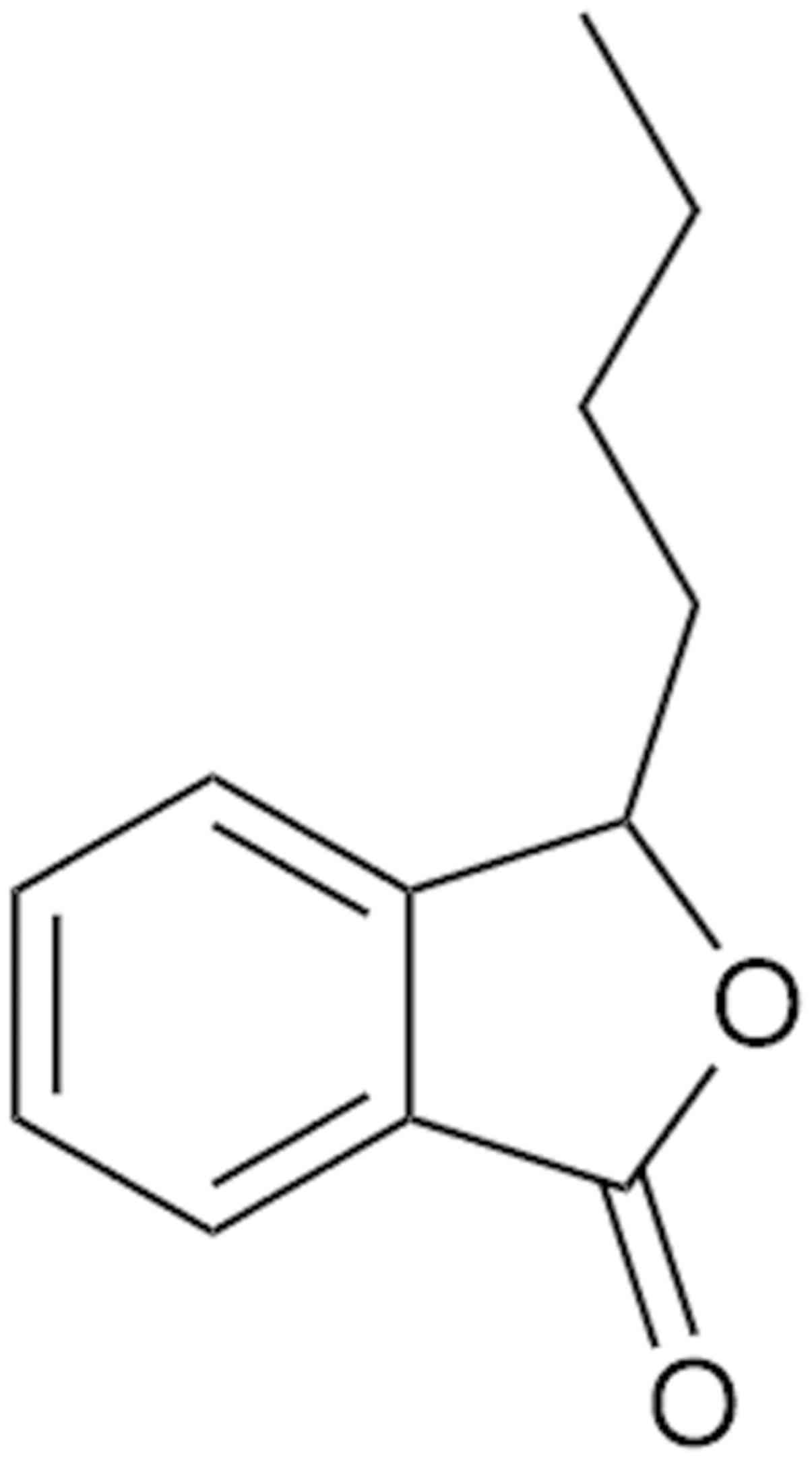
3-n-butylphthalide (NBP), approved by the China Food
and Drug Administration for the treatment of acute ischemic stroke,
is a type of compound isolated from the seeds of Chinese celery
(1). The molecular structure of NBP
is presented in Fig. 1. Therapy using
NBP has been recommended by Chinese guidelines for acute ischemic
stroke (2). A randomized double-blind
trial (clinical trial no. ChiCTR-TRC-09000483) reported that NBP
significantly improves clinical outcomes, including the modified
Rankin Scale (3) and National
institute of Health Stroke Scale scores (4), of patients who experienced ischemic
stroke (5). In addition, a study
demonstrated that NBP therapy persistently increases the level of
endothelial progenitor cells in peripheral blood, ameliorate
cerebral blood flow and improve neuronal functions (6). Furthermore, NBP has been reported to be
a safe treatment for cerebral ischemia stroke (5-7). A
study has indicated that NBP exhibits protective effects in several
neurodegenerative diseases (8).
However, to the best of our knowledge, the neuroprotective
mechanism of NBP remains unclear. Therefore, the present review
discusses the potential mechanism of neuroprotective effects of
NBP. The aim of the current review is to provide further
understanding regarding the advances of NBP.
Inflammation, a complex biological response to
injury, is associated with neurodegenerative diseases, including
Alzheimer's disease, Parkinson's disease (PD), multiple sclerosis,
amyotrophic lateral sclerosis, traumatic brain injury (TBI) and
more (9-11).
NBP has exhibited anti-inflammatory effects in various models of
these diseases and certain mechanisms have been identified. NBP has
been reported to reduce the inflammatory reaction by inhibiting
nucleotide binding oligomerization domain like receptor protein
3-inflammasome microglia activation and mitigating the
Alzheimer's-like pathology via the nuclear factor
erythroid-2-related factor 2-thioredoxin-interacting
protein-TXNIP-thioredoxin axis in an APP/PS1 mouse model (12,13).
Furthermore, NBP inhibited the inflammatory reaction in
lipopolysaccharide (LPS)-induced rats via inhibition of c-Jun
N-terminal kinase activation and the NF-κB pathway (14,15). NBP
was reported to improve dyskinesia in a LPS-induced PD mouse model
via a reduction in the loss of dopaminergic neurons, activation of
mouse microglia, an increase in TNF-α levels and α-synuclein
deposition in the black substantia of the mouse midbrain (16). Additionally, NBP-treatment reduces
NF-κB activation following TBI (17),
and NBP also inhibits the inflammatory reaction via the same
pathway in spontaneously hypertensive rats (18). Notably, a number of studies have
indicated that NBP inhibits the inflammatory reaction in other
neuroassociated experimental models, such as an experimental model
of autoimmune encephalomyelitis of microglia or autoimmune myositis
in guinea pigs (19,20). In addition, NBP-treatment has been
demonstrated to significantly ameliorate cerebral ischemia
reperfusion-induced brain injury of Sprague-Dawley (SD) rats by
inhibiting toll like receptor 4/NF-κB-associated inflammation
(21). NBP attenuates advanced
glycation end products-induced endothelial dysfunction by
ameliorating inflammatory responses (22). In summary, there is some understanding
regarding the mechanism of NBP in the inhibition of
inflammation.
Mitochondria, the site of oxidative metabolism in
eukaryotes, produce energy through the oxidation of carbohydrates,
fats and amino acids (23).
Therefore, mitochondrial dysfunction in the form of oxidative
stress may contribute to the pathogenesis of various
neurodegenerative diseases (24).
Oxidative stress is considered a condition that is caused by an
imbalance between pro- and antioxidant factors, which leads to
molecular and cellular damage (25).
Oxidative stress serves an essential role in the development of
age-related diseases (26). NBP
exhibits a cumulative beneficial effect on the process of
mitochondrial damage (27). This
section will discuss the mechanisms involved in mitochondrial
oxidative stress.
Recently, NBP exhibited a powerful effect on
antioxidant stress in some different models. NBP inhibited
oxidative stress in K141N-induced SH-SY5Y cells and in LPS-induced
rats through activation of the Kelch-like ECH-associating protein 1
Nrf2-related factor 2-antioxidant response element signaling
pathway (15,28). Similarly, NBP reduced oxidative damage
to provide neuroprotection in mice following TBI and in rats
following carbon monoxide poisoning (29,30). In
addition, NBP protects against cerebral ischemia-reperfusion injury
by decreasing antioxidant stress via the ERK signaling pathway
(31). NBP also protects against
H2O2-induced injury in neural stem cells by
activation of the PI3K/Akt and the Mash1 signaling pathways
(32). Furthermore, NBP has been
reported to increase superoxide dismutase and catalase activity,
and reduce malondialdehyde activity in the experimental autoimmune
myositis (EAM) model, NBP directly protects muscle mitochondria and
muscle cells from oxidative damage (33). However, the protective effect of NBP
on mitochondrial function is not only limited to neurodegeneration,
but also appears in cardiovascular diseases. A study suggested that
NBP exerts a cardioprotective effect on cardiac ischemic injury via
the regulation of mitochondrial function both using in vivo
and in vitro experiments (34). In summary, the antioxidant effect of
NBP has been widely recognized.
Apoptosis and autophagy are basic biological
phenomena of cells, which serve essential roles in removing
abnormal cells in multicellular organisms. Disorders in the
apoptosis and autophagy processes may cause the occurrence of
neuropathy (35). The neuroprotective
effect of NBP via the regulation of apoptosis and autophagy has
been demonstrated. Treatment with NBP has been reported to reduce
apoptotic cell death by increasing the levels of cleaved caspase-3
and caspase-9 following TBI (17).
Furthermore, NBP blocks neural apoptosis in areas surrounding
cortical contusions on the brain that are induced by TBI (29). The neuroprotective mechanism of NBP
involves the mitochondrial apoptotic pathway. NBP inhibits HSPB8
K141N mutation-induced neurotoxicity, attenuates β-amyloid-induced
toxicity in SH-SY5Y cells, and protects rat cardiomyocytes from
ischemia or reperfusion through regulating mitochondrion-mediated
apoptosis (28,36,37).
Furthermore, certain studies have demonstrated the inhibition of
apoptosis by NBP via the Akt pathway. One study reported that NBP
activates Akt/mTOR signaling to inhibit neuronal apoptosis and
autophagy in mice with repeated cerebral ischemia reperfusion
injury (38). Another study
demonstrated that NBP improves cognitive impairment of APP/PS1 mice
by inhibiting apoptosis via the PI3K/AKT pathway (39). Additionally, NBP reduces the number of
apoptotic cells by regulating Bcl-2 in HUVECs and an EAM model
(22,33).
ERS is characterized by incorrect folding and
aggregation of unfolded proteins in the endoplasmic reticulum lumen
and a disturbance of the calcium balance, which can activate the
unfolded protein response and lead to disturbance of the cell
function and cell death (40). In
recent years, certain studies have reported an anti-ERS effect of
NBP. One study demonstrated that NBP inhibits doxorubicin-induced
ERS in SD rats (41). In addition,
NBP alleviates vascular cognitive impairment by regulating ERS and
the Sonic hedgehog/Patched homolog 1 signaling pathway in SD rats
(42). Both of these studies agreed
that NBP attenuates ERS through regulating the expression of 78-kDa
glucose-regulated protein (GRP78), CCAAT-enhancer binding protein
homologous protein (CHOP) and caspase-12. Furthermore, NBP also
inhibits ERS by attenuating activating transcription factory
(ATF)-4, ATF-6, X-box binding protein 1, protein disulfide
isomerase, GRP78, CHOP and cleaved-caspase-12 in a spinal cord
injury (SCI) model, which may improve functional recovery and
prevent disruption of the blood-spinal cord barrier (43,44).
However, this mechanism has only recently been identified;
therefore, there is limited literature about it. Further research
on this mechanism may lead to new findings.
Abnormal protein deposition is closely associated
with numerous neurodegenerative diseases (45), such as Alzheimer's disease, which is
associated with amyloid-β (Aβ) and tau proteins; and PD, which is
associated with α-synuclein (46). A
study has demonstrated that NBP significantly reduces total
cerebral Aβ plaque deposition and lowers Aβ levels in brain
homogenates in a triple-transgenic mouse model of Alzheimer's
disease via directing amyloid precursor protein processing toward a
non-amyloidogenic pathway (47).
Furthermore, NBP treatment inhibited tau hyperphosphorylation in
AβPP/PS1 mice, which may improve cognitive impairment (48). NBP enhances a
1-methyl-4-phenylpyridiniumion-induced cellular model and a
LPS-induced mice model of PD via reducing the accumulation of
α-synuclein (16,49). However, the molecular mechanisms of
how NBP reduces the accumulation of α-synuclein and inhibits tau
hyperphosphorylation remain unclear. Furthermore, to the best of
our knowledge, there is no associated study that provides the
clinical evidence that NBP is effective in multiple sclerosis or
Lewy body dementia via attenuating abnormal protein deposition.
Potentially, new findings can be revealed in additional
neurodegenerative diseases.
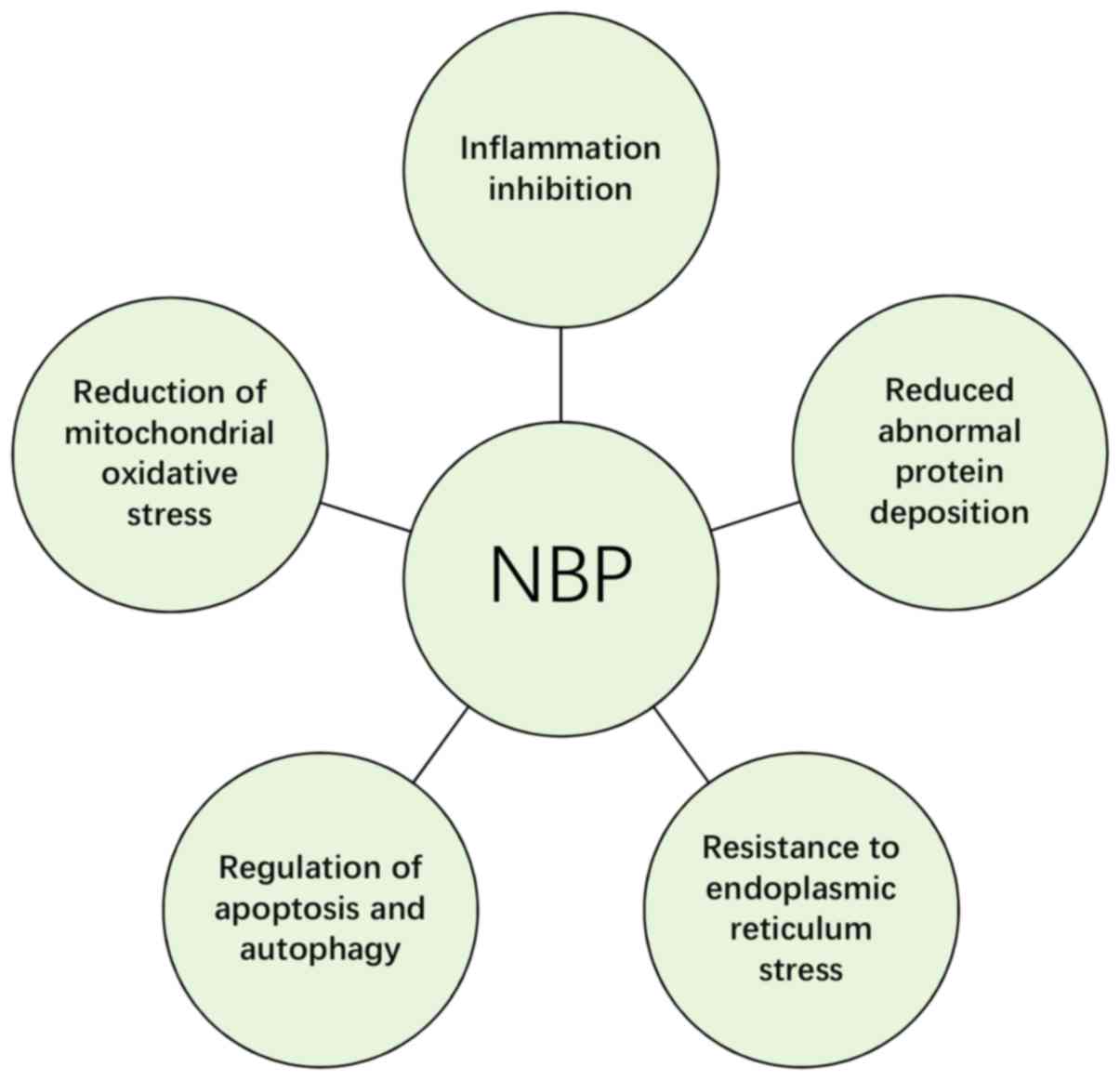
In summary, current studies suggest that NBP serves
a neuroprotective role through inhibiting inflammation, protecting
mitochondrial function, alleviating oxidative stress, regulating
apoptosis, resisting ERS and decreasing the abnormal protein
deposition (Fig. 2). Details on
specific molecular mechanisms are presented in Table I. Taken together, it is suggested that
NBP provides a promising therapeutic strategy for neurodegenerative
diseases. In further studies, the mechanism of action of NBP may be
further clarified, and the understanding regarding its potential
uses may be expanded.
Not applicable.
This study was supported by grants from the Natural
Science Foundation of China (grant no. 81371442), the Training
program for outstanding young teachers in higher education
institutions of Guangdong Province (grant no. YQ2015024) and the
Fundamental Research Funds for the Central Universities (grant no.
21617482).
All data generated or analyzed during this study are
included in this published article.
RL was a major contributor in writing the
manuscript. RL, RW, LZ and WB contributed to researching data,
discussing content and editing the manuscript. All authors read and
approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Xu ZQ, Zhou Y, Shao BZ, Zhang JJ and Liu
C: A Systematic Review of Neuroprotective Efficacy and Safety of
DL-3-N-Butylphthalide in Ischemic Stroke. Am J Chin Med.
47:507–525. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chinese Society of Cerebral Blood Flow and
Metabolism: The Chinese guidelines for the evaluation and
management of cerebral collateral circulation in ischemic stroke
(2017). Zhonghua Nei Ke Za Zhi 56: 460-471, 2017 (In Chinese).
|
|
3
|
Banks JL and Marotta CA: Outcomes validity
and reliability of the modified Rankin scale: implications for
stroke clinical trials: a literature review and synthesis. Stroke.
38:1091–1096. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Heldner MR, Zubler C, Mattle HP, Schroth
G, Weck A, Mono ML, Gralla J, Jung S, El-Koussy M, Lüdi R, et al:
National Institutes of Health stroke scale score and vessel
occlusion in 2152 patients with acute ischemic stroke. Stroke.
44:1153–1157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cui LY, Zhu YC, Gao S, Wang JM, Peng B, Ni
J, Zhou LX, He J and Ma XQ: Ninety-day administration of
dl-3-n-butylphthalide for acute ischemic stroke: A randomized,
double-blind trial. Chin Med J (Engl). 126:3405–3410.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao H, Yun W, Zhang Q, Cai X, Li X, Hui
G, Zhou X and Ni J: Mobilization of Circulating Endothelial
Progenitor Cells by dl-3-n-Butylphthalide in Acute Ischemic Stroke
Patients. J Stroke Cerebrovasc Dis. 25:752–760. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang C, Zhao S, Zang Y, Gu F, Mao S, Feng
S, Hu L and Zhang C: The efficacy and safety of
Dl-3n-butylphthalide on progressive cerebral infarction: A
randomized controlled STROBE study. Medicine (Baltimore).
96(e7257)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang L, Wang S, Ma F, Zhang Y and Peng Y,
Xing C, Feng Y, Wang X and Peng Y: From stroke to neurodegenerative
diseases: The multi-target neuroprotective effects of
3-n-butylphthalide and its derivatives. Pharmacol Res. 135:201–211.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Skaper SD, Facci L, Zusso M and Giusti P:
An Inflammation-Centric View of Neurological Disease: Beyond the
Neuron. Front Cell Neurosci. 12(72)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu J and Wang F: Role of
Neuroinflammation in Amyotrophic Lateral Sclerosis: Cellular
Mechanisms and Therapeutic Implications. Front Immunol.
8(1005)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Niu F, Sharma A, Feng L, Ozkizilcik A,
Muresanu DF, Lafuente JV, Tian ZR, Nozari A and Sharma HS:
Nanowired delivery of DL-3-n-butylphthalide induces superior
neuroprotection in concussive head injury. Prog Brain Res.
245:89–118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Y, Huang LJ, Shi S, Xu SF, Wang XL
and Peng Y: L-3-n-butylphthalide Rescues Hippocampal Synaptic
Failure and Attenuates Neuropathology in Aged APP/PS1 Mouse Model
of Alzheimer's Disease. CNS Neurosci Ther. 22:979–987.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang CY, Xu Y, Wang X, Guo C, Wang T and
Wang ZY: Dl-3-n-Butylphthalide Inhibits NLRP3 Inflammasome and
Mitigates Alzheimer's-Like Pathology via Nrf2-TXNIP-TrX Axis.
Antioxid Redox Signal. 30:1411–1431. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang M, Dang R, Xu P, Guo Y, Han W, Liao D
and Jiang P: Dl-3-n-Butylphthalide improves
lipopolysaccharide-induced depressive-like behavior in rats:
Involvement of Nrf2 and NF-κB pathways. Psychopharmacology (Berl).
235:2573–2585. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao CY, Lei H, Zhang Y, Li L, Xu SF, Cai
J, Li PP, Wang L, Wang XL and Peng Y: L-3-n-Butylphthalide
attenuates neuroinflammatory responses by downregulating JNK
activation and upregulating Heme oxygenase-1 in
lipopolysaccharide-treated mice. J Asian Nat Prod Res. 18:289–302.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen Y, Jiang M, Li L, Ye M, Yu M, Zhang
L, Ge B, Xu W and Wei D: DL-3-n-butylphthalide reduces microglial
activation in lipopolysaccharide-induced Parkinson's disease model
mice. Mol Med Rep. 17:3884–3890. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao Y, Lee JH, Chen D, Gu X, Caslin A, Li
J, Yu SP and Wei L: DL-3-n-butylphthalide induced neuroprotection,
regenerative repair, functional recovery and psychological benefits
following traumatic brain injury in mice. Neurochem Int. 111:82–92.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu J, Zhang Y and Yang C: Protective
effect of 3-n-butylphthalide against hypertensive nephropathy in
spontaneously hypertensive rats. Mol Med Rep. 11:1448–1454.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang Y, Bi Y, Xia Z, Shi W, Li B, Li B,
Chen L and Guo L: Butylphthalide ameliorates experimental
autoimmune encephalomyelitis by suppressing PGAM5-induced
necroptosis and inflammation in microglia. Biochem Biophys Res
Commun. 497:80–86. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen J, Wang J, Zhang J and Pu C: Effect
of butylphthalide intervention on experimental autoimmune myositis
in guinea pigs. Exp Ther Med. 15:152–158. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang P, Guo ZF, Xu YM, Li YS and Song JG:
N-Butylphthalide (NBP) ameliorated cerebral ischemia
reperfusion-induced brain injury via HGF-regulated TLR4/NF-κB
signaling pathway. Biomed Pharmacother. 83:658–666. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu CY, Zhao ZH, Chen ZT, Che CH, Zou ZY,
Wu XM, Chen SG, Li YX, Lin HB, Wei XF, et al: DL-3-n-butylphthalide
protects endothelial cells against advanced glycation end
product-induced injury by attenuating oxidative stress and
inflammation responses. Exp Ther Med. 14:2241–2248. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schapira AHV: Mitochondrial diseases.
Lancet. 379:1825–1834. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Arun S, Liu L and Donmez G: Mitochondrial
Biology and Neurological Diseases. Curr Neuropharmacol. 14:143–154.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hybertson BM, Gao B, Bose SK and McCord
JM: Oxidative stress in health and disease: The therapeutic
potential of Nrf2 activation. Mol Aspects Med. 32:234–246.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tan BL, Norhaizan ME, Liew WP and Sulaiman
Rahman H: Antioxidant and Oxidative Stress: A Mutual Interplay in
Age-Related Diseases. Front Pharmacol. 9(1162)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Abdoulaye IA and Guo YJ: A Review of
Recent Advances in Neuroprotective Potential of 3-N-Butylphthalide
and Its Derivatives. BioMed Res Int. 2016(5012341)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang XD, Cen ZD, Cheng HP, Shi K, Bai J,
Xie F, Wu HW, Li BB and Luo W: L-3-n-Butylphthalide Protects HSPB8
K141N Mutation-Induced Oxidative Stress by Modulating the
Mitochondrial Apoptotic and Nrf2 Pathways. Front Neurosci.
11(402)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu Z, Wang H, Shi X, Li L, Zhou M, Ding
H, Yang Y, Li X and Ding K: DL-3-n-Butylphthalide (NBP) Provides
Neuroprotection in the Mice Models After Traumatic Brain Injury via
Nrf2-ARE Signaling Pathway. Neurochem Res. 42:1375–1386.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Q, Cheng Y, Bi M, Lin H, Chen Y, Zou Y,
Liu Y, Kang H and Guo Y: Effects of N-butylphthalide on the
activation of Keap1/Nrf-2 signal pathway in rats after carbon
monoxide poisoning. Environ Toxicol Pharmacol. 40:22–29.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhu BL, Xie CL, Hu NN, Zhu XB and Liu CF:
Inhibiting of GRASP65 Phosphorylation by DL-3-N-Butylphthalide
Protects against Cerebral Ischemia-Reperfusion Injury via ERK
Signaling. Behav Neurol. 2018(5701719)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang S, Huang L, Zhang Y and Peng Y, Wang
X and Peng Y: Protective Effects of L-3-n-Butylphthalide Against
H2O2-Induced Injury in Neural Stem Cells by Activation of PI3K/Akt
and Mash1 Pathway. Neuroscience. 393:164–174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen J, Wang J, Zhang J and Pu C:
3-n-Butylphthalide reduces the oxidative damage of muscles in an
experimental autoimmune myositis animal model. Exp Ther Med.
14:2085–2093. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tian X, He W, Yang R and Liu Y:
Dl-3-n-butylphthalide protects the heart against ischemic injury
and H9c2 cardiomyoblasts against oxidative stress: Involvement of
mitochondrial function and biogenesis. J Biomed Sci.
24(38)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Booth LA, Tavallai S, Hamed HA,
Cruickshanks N and Dent P: The role of cell signalling in the
crosstalk between autophagy and apoptosis. Cell Signal. 26:549–555.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang YG, Li Y, Wang CY, Ai JW, Dong XY,
Huang HY, Feng ZY, Pan YM, Lin Y, Wang BX, et al:
L-3-n-Butylphthalide protects rats' cardiomyocytes from
ischaemia/reperfusion-induced apoptosis by affecting the
mitochondrial apoptosis pathway. Acta Physiol (Oxf). 210:524–533.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lei H, Zhao CY, Liu DM, Zhang Y, Li L,
Wang XL and Peng Y: l-3-n-Butylphthalide attenuates
β-amyloid-induced toxicity in neuroblastoma SH-SY5Y cells through
regulating mitochondrion-mediated apoptosis and MAPK signaling. J
Asian Nat Prod Res. 16:854–864. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xu J, Huai Y, Meng N, Dong Y, Liu Z, Qi Q,
Hu M, Fan M, Jin W and Lv P: L-3-n-Butylphthalide Activates
Akt/mTOR Signaling, Inhibits Neuronal Apoptosis and Autophagy and
Improves Cognitive Impairment in Mice with Repeated Cerebral
Ischemia-Reperfusion Injury. Neurochem Res. 42:2968–2981.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xiang J, Pan J, Chen F, Zheng L, Chen Y,
Zhang S and Feng W: L-3-n-butylphthalide improves cognitive
impairment of APP/PS1 mice by BDNF/TrkB/PI3K/AKT pathway. Int J
Clin Exp Med. 7:1706–1713. 2014.PubMed/NCBI
|
|
40
|
Iurlaro R and Muñoz-Pinedo C: Cell death
induced by endoplasmic reticulum stress. FEBS J. 283:2640–2652.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liao D, Xiang D, Dang R, Xu P, Wang J, Han
W, Fu Y, Yao D, Cao L and Jiang P: Neuroprotective Effects of
dl-3-n-Butylphthalide against Doxorubicin-Induced
Neuroinflammation, Oxidative Stress, Endoplasmic Reticulum Stress,
and Behavioral Changes. Oxid Med Cell Longev.
2018(9125601)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Niu XL, Jiang X, Xu GD, Zheng GM, Tang ZP,
Yin N, Li XQ, Yang YY and Lv PY: DL-3-n-butylphthalide alleviates
vascular cognitive impairment by regulating endoplasmic reticulum
stress and the Shh/Ptch1 signaling-pathway in rats. J Cell Physiol.
234:12604–12614. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zheng B, Zhou Y, Zhang H, Yang G, Hong Z,
Han D, Wang Q, He Z, Liu Y, Wu F, et al: Dl-3-n-butylphthalide
prevents the disruption of blood-spinal cord barrier via inhibiting
endoplasmic reticulum stress following spinal cord injury. Int J
Biol Sci. 13:1520–1531. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
He Z, Zhou Y, Huang Y, Wang Q, Zheng B,
Zhang H, Li J, Liu Y, Wu F, Zhang X, et al: Dl-3-n-butylphthalide
improves functional recovery in rats with spinal cord injury by
inhibiting endoplasmic reticulum stress-induced apoptosis. Am J
Transl Res. 9:1075–1087. 2017.PubMed/NCBI
|
|
45
|
Nonaka T, Masuda-Suzukake M and Hasegawa
M: Molecular mechanisms of the co-deposition of multiple
pathological proteins in neurodegenerative diseases.
Neuropathology. 38:64–71. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Goedert M: NEURODEGENERATION. Alzheimer's
and Parkinson's diseases: The prion concept in relation to
assembled Aβ, tau, and α-synuclein. Science.
349(1255555)2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Peng Y, Sun J, Hon S, Nylander AN, Xia W,
Feng Y, Wang X and Lemere CA: L-3-n-butylphthalide improves
cognitive impairment and reduces amyloid-beta in a transgenic model
of Alzheimer's disease. J Neurosci. 30:8180–8189. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Peng Y, Hu Y, Xu S, Li P, Li J, Lu L, Yang
H, Feng N, Wang L and Wang X: L-3-n-butylphthalide reduces tau
phosphorylation and improves cognitive deficits in
AβPP/PS1-Alzheimer's transgenic mice. J Alzheimers Dis. 29:379–391.
2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Huang JZ, Chen YZ, Su M, Zheng HF, Yang
YP, Chen J and Liu CF: dl-3-n-Butylphthalide prevents oxidative
damage and reduces mitochondrial dysfunction in an MPP(+)-induced
cellular model of Parkinson's disease. Neurosci Lett. 475:89–94.
2010.PubMed/NCBI View Article : Google Scholar
|