Introduction
Breast cancer is a serious life-threatening disease,
which with its different stages, ranging from benign to invasive
malignant tumors, has a debilitating effect on the health and
quality of life of women (1). Despite
considerable progress being made in the early detection and
diagnosis, the complex nature of breast cancer renders this
malignancy an incurable disease due to the acquisition of the
chemoresistant phenotype and the metastatic spread of cancer cells
to distant organs (2). Of note, it
has been demonstrated that women who are younger than 45 years are
at a higher risk of developing breast cancer in modern countries
(3,4).
Marked improvements in the laboratory and etiological
investigations have revolutionized the major understanding of the
relevant mechanisms involved in the pathogenesis of breast cancer,
providing new perspectives for the evolution of this type of
cancer. Recent studies have suggested that there is a tight
association between the incidence of breast cancer and numerous
risk factors, such as sex, age, smoking, alcohol addiction,
high-fat diet and salt consumption, infection diseases, pollution
and radiation (5-8).
Among the extensive list of risk factors associated
with breast cancer, the significant role of microRNAs (miRNAs or
miRs), which are crucial small molecules responsible for
regulation, has been noted (9). The
pivotal role of miRNAs in several types of human cancer has been
well-established in a mounting body of recent investigations.
Although some miRNAs serve as oncogenes and activate cancer-related
signaling pathways, others play the roles of tumor suppressors,
preventing numerous biomolecules that are important for the
formation of malignancies (10-13).
It has been suggested that miR-9, an important miRNA which
participates in cancer progression, can downregulate E-cadherin
expression in breast cancer and thereby increase the risk of cancer
metastasis in patients by reducing the ability of malignant cells
to adhere to the cancer microenvironment (10-12).
According to bioinformatics tools (miRWalk), there are several gene
targets for miR-9, such as claudin 14, cyclin G1 and leptin
(14). The importance of miRNAs in
the regulation of cancer signaling pathways has attracted
increasing attention to the mechanisms involved in the regulation
of these small non-coding RNAs. It has been revealed that
epigenetic alterations, such as DNA methylation, can alter the
expression levels of miRNAs (15). In
a study conducted by Botla et al, it was reported that the
addition of a methyl group into the miR-192 promoter decreased the
expression of this miRNA in cancer tissue, leading to the
progression of pancreatic cancer (16). Bioanformatic tools (miRWalk) suggest
that miR-192 is able to target several genes, for example, cyclin
T2, epiregulin and plexin B2(14).
Although multiple lines of evidence emphasize the significant role
of miRNAs in different human cancers, to the best of our knowledge,
there are no studies available to date on the role of miR-9 and
miR-192 in breast cancer. In the present study, we aimed to examine
the expression levels of these miRNAs in breast cancer tissues and
serum.
Subjects and methods
Tissue samples
The present study was approved by the Shahid
Beheshti University of Medical Sciences (IR.SBMU. MSP.REC.1397.505,
grant no. 13756). To evaluate and compare the expression levels of
miR-9 and miR-192 in serum and cancerous tissue, 38 breast cancer
tissues and their adjacent normal tissues were collected from
Taleghani Hospital between 2017 and 2019 (Table I). Informed consent was obtained from
the patients prior to enrolment in this study. All tissues were
stored in RNAlater (Qiagen GmbH) at -20˚C and all serum samples
were stored in -70˚C. An expert pathologist confirmed the stage of
breast cancer and all patients’ information was recorded. Of note,
the samples of those patients who had been treated with
chemotherapy were excluded from the study. The inclusion criteria
in this study were no treatment with chemotherapy or
radiotherapy.
 | Table INucleotide sequences of primers used
for RT-qPCR. |
Table I
Nucleotide sequences of primers used
for RT-qPCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| U6 |
GAGAAGATTAGCATGGCCCCT |
ATATGGAACGCTTCACGAATTTGC |
| miR-9 |
CTTTGGTTATCTAGCTGTATGAGTCGT |
ATCCAGTGCAGGGTCCGA |
| miR-192 |
CTGACCTATGAATTGACAGCCGT |
ATCCAGTGCAGGGTCCGA |
RNA extraction and cDNA synthesis
To extract RNA from breast tissues and serum, all
samples were digested with RNX-plus solution (Cinnagen). Following
the addition of chloroform, propanol was used to precipitate the
RNA samples. The purity and quality of the extracted RNA were
confirmed by electrophoresis and using a Nanodrop spectrophotometer
(Eppendorf). Subsequently, 10 µl of RNA from each sample were added
to the reaction containing 0.5 µl primer of RT miRNA-9, 0.5 µl
primer of RT miRNA-192, 0.5 µl of the U6 reverse primer and 9 µl of
reverse transcriptase (BioFACT) to synthesize the cDNA. The PCR
(Bio Intellectica PCR) schedule was as follows: 5 min at 95˚C and
40 min at 50˚C. In order to ensure a suitable concentration, the
cDNA was diluted twice sterile water.
Quantitative PCR (qPCR)
To evaluate the expression level of miR-9 and
miR-192 in the breast cancer tissue and serum, qPCR was performed
using Rotor-Gene 6000 (Corbett Life Science) in 36-well Gene Discs,
using a final volume of 20 µl. We then combined 10 µl of BIOFACT™
2X real-time PCR master mix (for SYBR-Green I; BioFACT), 1 µl (10
pmol) of forward primer, 1 µl of (10 pmol) reverse primer, 2 µl of
1/2 diluted cDNA and 6 μl of sterile water to evaluate the
expression of miR-9 and miR-192. All reactions were performed in
triplicate, simultaneously, to confirm the results. The sequences
of the primers used in this study are presented in Table I. The thermal cycling conditions were
95˚C for 10 min followed by 40 cycles of 95˚C for 20 sec, 55˚C for
20 sec and 72˚C for 20 sec. The values for the relative
quantification were calculated using the 2-ΔΔCq
expression formula (17).
Statistical analysis
To analysis the results of miRNA expression,
Graph-PadPrism software (version 5.1) was used. The experimental
data are expressed as the means ± standard deviation of 3
independent assays. The unpaired, two-tailed Student's t-test was
used to analyze the statistical differences between groups using
Graph-Pad Prism software. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients with
breast cancer
In the present study, 38 breast cancer tissues and
their adjacent normal tissues coupled with serum samples were
collected from breast cancer patients hospitalized at Taleghani
Hospital. Following the extraction of RNA from both tissue and
serum samples, the expression levels of miR-9 and miR-192 were
examined by RT-qPCR analysis. It is worth noting that 52% of the
patients presented with stage III-IV of the disease. The
characteristics of each patient, including their tumor size, tumor
location, family history, and the genetic status of the tumors, are
summarized in Table II.
 | Table IIClinical characteristics of the 38
patients with breast cancer. |
Table II
Clinical characteristics of the 38
patients with breast cancer.
| Characteristics | Percentage of
patients |
|---|
| Age of >60
years | 69% |
| Tumor
localization | Left, 45%; right,
55% |
| Family history of
breast cancer | Absent, 88.1%;
present, 11.9% |
| Lymph node
metastasis | Negative, 48%;
positive, 52% |
| Tumor size | >2 cm, 62.8%;
<2 cm, 37.2% |
| Tumor stage | I-II, 48%; III-IV,
52% |
| Estrogen receptor
(ER) status | Negative, 54.3%;
positive, 45.7% |
| Progesterone receptor
(PR) status | Negative, 46.6%;
positive, 53.4% |
Expression level of miR-9 in breast
cancer tissues and serum samples
To evaluate the expression level of miR-9 in both
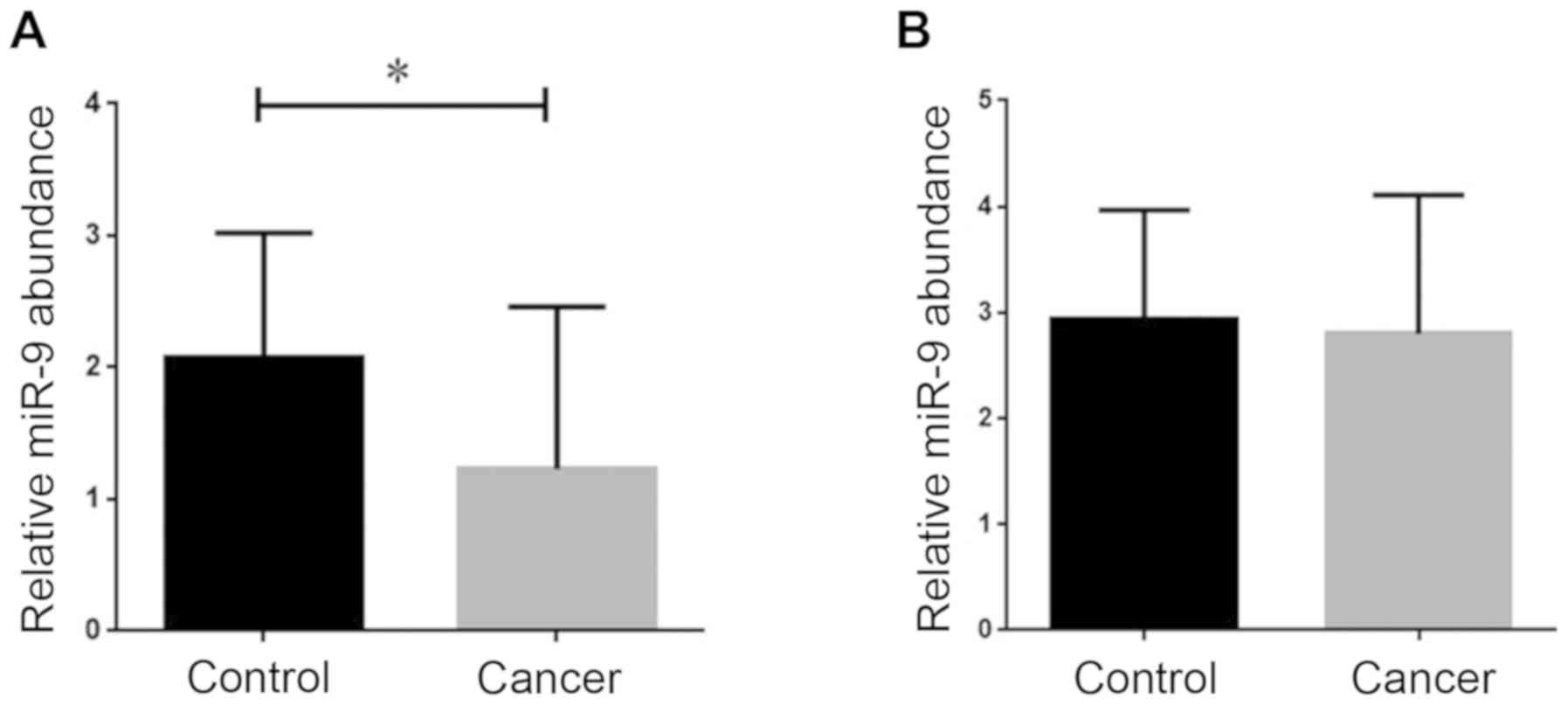
breast cancer tissues and serum, we performed RT-qPCR. As presented
in Fig. 1A, the results of RT-qPCR
revealed in the breast cancer tissue samples, the expression of
miR-9 was significantly downregulated compared with the normal
tissue samples (P<0.05). However, the results regarding miRNA-9
expression in serum from the breast cancer patients revealed that
while the expression level of this miRNA was slightly decreased in
the breast cancer samples, this alteration in gene expression was
not considered meaningful according to the statistical analysis
(Fig. 1B).
Evaluation of the expression level of
miR-192 in breast cancer tissues and serum samples
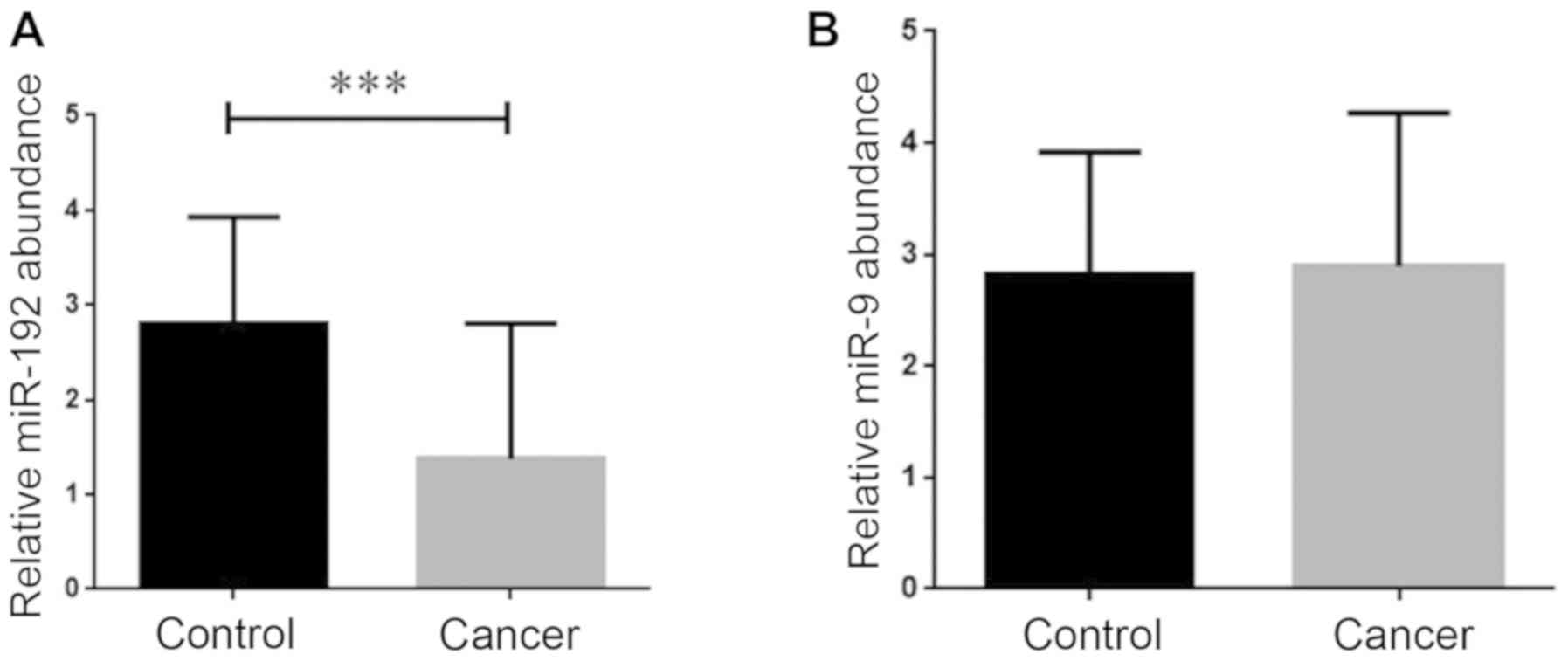
Given the critical participation of miR-192 in the
progression of different types of human cancer, it was of
particular interest to evaluate whether there is an alteration in
the expression of this miRNA in the breast cancer patients tissues
and serum samples. We found that the mRNA expression level of
miR-192 was significantly decreased in the breast cancer tissues
(P<0.001; Fig. 2A). After
analyzing the expression of miRNA-192 in the serum from the breast
cancer patients, we failed to find any noticeable changes in its
expression in the cancerous serum samples in comparison with the
healthy counterparts (Fig. 2B).
Discussion
Since miRNAs are strongly considered to be
responsible for the regulation of the main mechanisms of in cancer
pathogenesis, exploiting this small non-coding RNAs has been
currently under intense investigation for future cancer management
strategies. By bifurcating at several signaling pathways, miRNAs
have a noticeable impact on the diverse intracellular process, and
it has been suggested that epigenetic alterations, such as
methylation are the main mechanisms through which miRNA expression
is regulated (16,18). Among the panoply of miRNAs involved in
tumor progression, metastasis and drug resistance, miR-9 and
miR-192 are the most important ones.
In the present study, we compared the expression
levels of miR-9 and miR-192 in both tissue and serum samples
collected from breast cancer patients and healthy counterparts. In
this study, there were some limitations of collecting serum and
tissue samples. The expression levels of miRNA-9 and -192 were
assessed by RT-qPCR method; however, northern blotting could have
been performed to validate the results. The results of this study
revealed that while the expression level of miR-9 was significantly
diminished in the cancerous tissue, there was no distinct
difference between the transcriptional activity of this miRNA in
the serum of breast cancer patients and the healthy individuals. In
accordance with this finding, Orangi and Motovali-Bashi claimed
that the downregulation in the expression level of miR-9 in breast
cancer patients was only restricted to the cancerous tissues and
not to the patient plasma (19).
Moreover, in another study, it was demonstrated that the miR-9
expression pattern was dependent on the stage of breast cancer. In
spite of the fact that the expression of miR-9 was decreased in
benign breast tumors, its expression level displayed an elevated
profile in more advanced stages of the disease. In addition,
Hasanzadeh et al demonstrated that in benign and malignant
breast tumors, there was a downregulation compared to healthy
breast tissues (20). This
association between the expression of miR-9 and cancer stage has
also been reported in gastric cancer, at least partially, due to
the varied hypermethylation status in different phases of the
malignancy (21). The reduction in
the expression level of miR-9 has also been reported in other types
of human cancer, including gastric and renal cell carcinoma
(21,22). In the study conducted by Cheng et
al, it was demonstrated that miR-9 and MicroRNA-221enhance the
generation of breast cancer stem cells (23). Ma et al demonstrated that
miRNA-9 was able to downregulate E-cadherin; therefore, metastasis
in breast tumors occurred (24).
However, another study delineated that the up-regulation of miR-9
provided an opportunity for breast cancer cells to invade to
distant organs through the down-regulation of FOXO1(25). In line with these oncogenic properties
of miR-9, several investigations have suggested that the expression
of miR-9 can be used as a prognostic biomarker in triple-negative
breast cancer (TNBC) and estrogen receptor (ER)-positive breast
cancer (26,27).
Another miRNA, whose participation in different
types of human tumors has been well-established in numerous studies
is miR-192 (28-30).
Notably, in the present study, we found that the expression of
miR-192 was significantly decreased only in the breast cancer
tissues, but not in the serum. Consistently, Hu et al
introduced miR-192 as a tumor suppressor miRNA which, coupled with
bone morphogenetic protein-6 (BMP-6), were downregulated in breast
cancer, proposing these two molecules as novel therapeutic targets
for breast cancer treatment (31).
Likewise, the results of another study demonstrated that both the
expression of miRNA-192-3p and miRNA-192-5p were decreased in stage
IIIB colon cancer as compared to healthy tissues (32). The reduction in the expression level
of this tumor suppressor miRNA has been reported in osteosarcoma,
lung cancer and pancreatic cancer, where it has been reported that
miR-192 exhibits a tight association with the regulation of cell
proliferation and apoptosis (33-34). Moreover, it has
been reported that the downregulation of miR-192 in hepatocellular
carcinoma (HCC) may provide a signal that upregulates
SLC39A6/SNAIL, a molecule involved in cell invasion, which in turn
deteriorates patient outcome (35).
In conclusion, the findings of the present study
suggested that the expression of both miR-9 and miR-192 was
downregulated in breast cancer tissues, suggesting that these
miRNAs could serve as effective biomarkers for the diagnosis of
breast cancer.
Acknowledgements
The present study was conducted at the School of
Medicine, Shahid Beheshti University of Medical Sciences.
Funding
The present article was financially supported by the
Research Department of the School of Medicine Shahid Beheshti
University of Medical Sciences. (IR. SBMU. MSP.REC.1397.505, grant
no. 13756).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
EF and ST designed and performed the cell culture
and molecular experiments. HG and FT performed the statistical
analyses. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
This study was approved by the Shahid Beheshti
University of Medical Sciences (IR.SBMU. MSP.REC.1397.505, grant
no. 13756). Informed consent was obtained from the patients prior
to enrolment in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davies B, Miles DW, Happerfield LC, Naylor
MS, Bobrow LG, Rubens RD and Balkwill FR: Activity of type IV
collagenases in benign and malignant breast disease. Br J Cancer.
67:1126–1131. 1993.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Greaney ML, Sprunck-Harrild K, Ruddy KJ,
Ligibel J, Barry WT, Baker E, Meyer M, Emmons KM and Partridge AH:
Study protocol for Young & Strong: A cluster randomized design
to increase attention to unique issues faced by young women with
newly diagnosed breast cancer. BMC Public Health.
15(37)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Villarreal-Garza C, Aguila C,
Magallanes-Hoyos MC, Mohar A, Bargalló E, Meneses A, Cazap E, Gomez
H, López-Carrillo L, et al: Breast cancer in young women in Latin
America: An unmet, growing burden. Oncologist. 18:26–34.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Knaul F, Bustreo F, Ha E and Langer A:
Breast cancer: why link early detection to reproductive health
interventions in developing countries? Salud Publica Mex.
51:220–227. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Faghihloo E, Saremi MR, Mahabadi M, Akbari
H and Saberfar E: Prevalence and characteristics of Epstein-Barr
virus-associated gastric cancer in Iran. Arch Iran Med. 17:767–770.
2014.PubMed/NCBI
|
|
6
|
Mirzaei H, Goudarzi H, Eslami G and
Faghihloo E: Role of viruses in gastrointestinal cancer. J Cell
Physiol. 233:4000–4014. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pormohammad A, Azimi T, Falah F and
Faghihloo E: Relationship of human herpes virus 6 and multiple
sclerosis: A systematic review and meta-analysis. J Cell Physiol.
233:2850–2862. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vaezjalali M, Rashidpour S, Rezaee H,
Hajibeigi B, Zeidi M, Gachkar L, Aghamohamad S, Najafi R and
Goudarzi H: Hepatitis B viral DNA among HBs antigen negative
healthy blood donors. Hepat Mon. 13(e6590)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lai EC: microRNAs: Runts of the genome
assert themselves. Curr Biol. 13:R925–R936. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: Bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tavakolian S, Goudarzi H, Eslami G and
Faghihloo E: Transcriptional regulation of epithelial to
mesenchymal transition related genes by lipopolysaccharide in human
cervical cancer cell line HeLa. Asian Pac J Cancer Prev.
20:2455–2461. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pourbagheri-Sigaroodi A, Bashash D,
Safaroghli-Azar A, Farshi-Paraasghari M, Momeny M, Mansoor FN and
Ghaffari SH: Contributory role of microRNAs in anti-cancer effects
of small molecule inhibitor of telomerase (BIBR1532) on acute
promyelocytic leukemia cell line. Eur J Pharmacol. 846:49–62.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dweep H and Gretz N: miRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12(697)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ortiz IMDP, Barros-Filho MC, Dos Reis MB,
Beltrami CM, Marchi FA, Kuasne H, do Canto LM, de Mello JBH,
Abildgaard C, Pinto CAL, et al: Loss of DNA methylation is related
to increased expression of miR-21 and miR-146b in papillary thyroid
carcinoma. Clin Epigenetics. 10(144)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Botla SK, Savant S, Jandaghi P, Bauer AS,
Mücke O, Moskalev EA, Neoptolemos JP, Costello E, Greenhalf W,
Scarpa A, et al: Early epigenetic downregulation of microRNA-192
expression promotes pancreatic cancer progression. Cancer Res.
76:4149–4159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Faghihloo E, Sadeghizadeh M, Shahmahmoodi
S and Mokhtari-Azad T: Cdc6 expression is induced by HPV16 E6 and
E7 oncogenes and represses E-cadherin expression. Cancer Gene Ther.
Nov 11. 2016.doi.org/10.1038/cgt.2016.51. PubMed/NCBI View Article : Google Scholar
|
|
19
|
Orangi E and Motovali-Bashi M: Evaluation
of miRNA-9 and miRNA-34a as potential biomarkers for diagnosis of
breast cancer in Iranian women. Gene. 687:272–279. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hasanzadeh A, Mesrian Tanha H, Ghaedi K
and Madani M: Aberrant expression of miR-9 in benign and malignant
breast tumors. Mol Cell Probes. 30:279–284. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Luo H, Zhang H, Zhang Z, Zhang X, Ning B,
Guo J, Nie N, Liu B and Wu X: Down-regulated miR-9 and miR-433 in
human gastric carcinoma. J Exp Clin Cancer Res.
28(82)2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hildebrandt MA, Gu J, Lin J, Ye Y, Tan W,
Tamboli P, Wood CG and Wu X: Hsa-miR-9 methylation status is
associated with cancer development and metastatic recurrence in
patients with clear cell renal cell carcinoma. Oncogene.
29:5724–5728. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cheng CW, Yu JC, Hsieh YH, Liao WL, Shieh
JC, Yao CC, Lee HJ, Chen PM, Wu PE and Shen CY: Increased cellular
levels of microRNA-9 and microRNA-221 correlate with cancer
stemness and predict poor outcome in human breast cancer. Cell
Physiol Biochem. 48:2205–2218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ma L, Young J, Prabhala H, Pan E, Mestdagh
P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S,
et al: miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin
and cancer metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Liu DZ, Chang B, Li XD, Zhang QH and Zou
YH: MicroRNA-9 promotes the proliferation, migration, and invasion
of breast cancer cells via down-regulating FOXO1. Clin Transl
Oncol. 19:1133–1140. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jang MH, Kim HJ, Gwak JM, Chung YR and
Park SY: Prognostic value of microRNA-9 and microRNA-155 expression
in triple-negative breast cancer. Hum Pathol. 68:69–78.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou X, Marian C, Makambi KH, Kosti O,
Kallakury BV, Loffredo CA and Zheng YL: MicroRNA-9 as potential
biomarker for breast cancer local recurrence and tumor estrogen
receptor status. PLoS One. 7(e39011)2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Filipska M, Skrzypski M, Czetyrbok K,
Stokowy T, Stasiłojć G, Supernat A, Jassem J, Żaczek AJ and Bigda
J: MiR-192 and miR-662 enhance chemoresistance and invasiveness of
squamous cell lung carcinoma. Lung Cancer. 118:111–118.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen ZJ, Yan YJ, Shen H, Zhou JJ, Yang GH,
Liao YX, Zeng JM and Yang T: miR-192 is overexpressed and promotes
cell proliferation in prostate cancer. Med Princ Pract. 28:124–132.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Y, Jia LS, Yuan W, Wu Z, Wang HB, Xu
T, Sun JC, Cheng KF and Shi JG: Low miR-34a and miR-192 are
associated with unfavorable prognosis in patients suffering from
osteosarcoma. Am J Transl Res. 7:111–119. 2015.PubMed/NCBI
|
|
31
|
Hu F, Meng X, Tong Q, Liang L, Xiang R,
Zhu T and Yang S: BMP-6 inhibits cell proliferation by targeting
microRNA-192 in breast cancer. Biochim Biophys Acta.
1832:2379–2390. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li P, Ou Q, Braciak TA, Chen G and Oduncu
FS: MicroRNA-192-5p is a predictive biomarker of survival for Stage
IIIB colon cancer patients. Jpn J Clin Oncol. 48:619–624.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shang G, Mi Y, Mei Y, Wang G, Wang Y, Li
X, Wang Y, Li Y and Zhao G: MicroRNA-192 inhibits the
proliferation, migration and invasion of osteosarcoma cells and
promotes apoptosis by targeting matrix metalloproteinase-11. Oncol
Lett. 15:7265–7272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Feng S, Cong S, Zhang X, Bao X, Wang W, Li
H, Wang Z, Wang G, Xu J, Du B, et al: MicroRNA-192 targeting
retinoblastoma 1 inhibits cell proliferation and induces cell
apoptosis in lung cancer cells. Nucleic Acids Res. 39:6669–6678.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lian J, Jing Y, Dong Q, Huan L, Chen D,
Bao C, Wang Q, Zhao F, Li J, Yao M, et al: miR-192, a prognostic
indicator, targets the SLC39A6/SNAIL pathway to reduce tumor
metastasis in human hepatocellular carcinoma. Oncotarget.
7:2672–2683. 2016.PubMed/NCBI View Article : Google Scholar
|